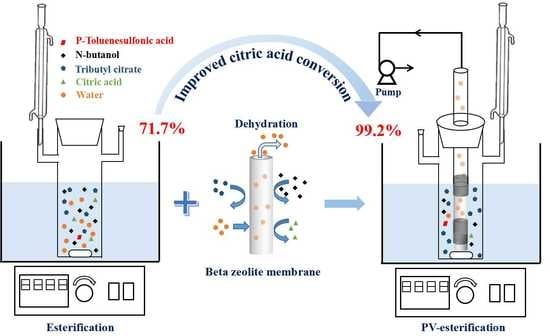
Improved Esterification of Citric Acid and n-Butanol Using a Dense and Acid-Resistant Beta Zeolite Membrane
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Esterification with PV
2.3. Characterization and PV Test
3. Results and Discussion
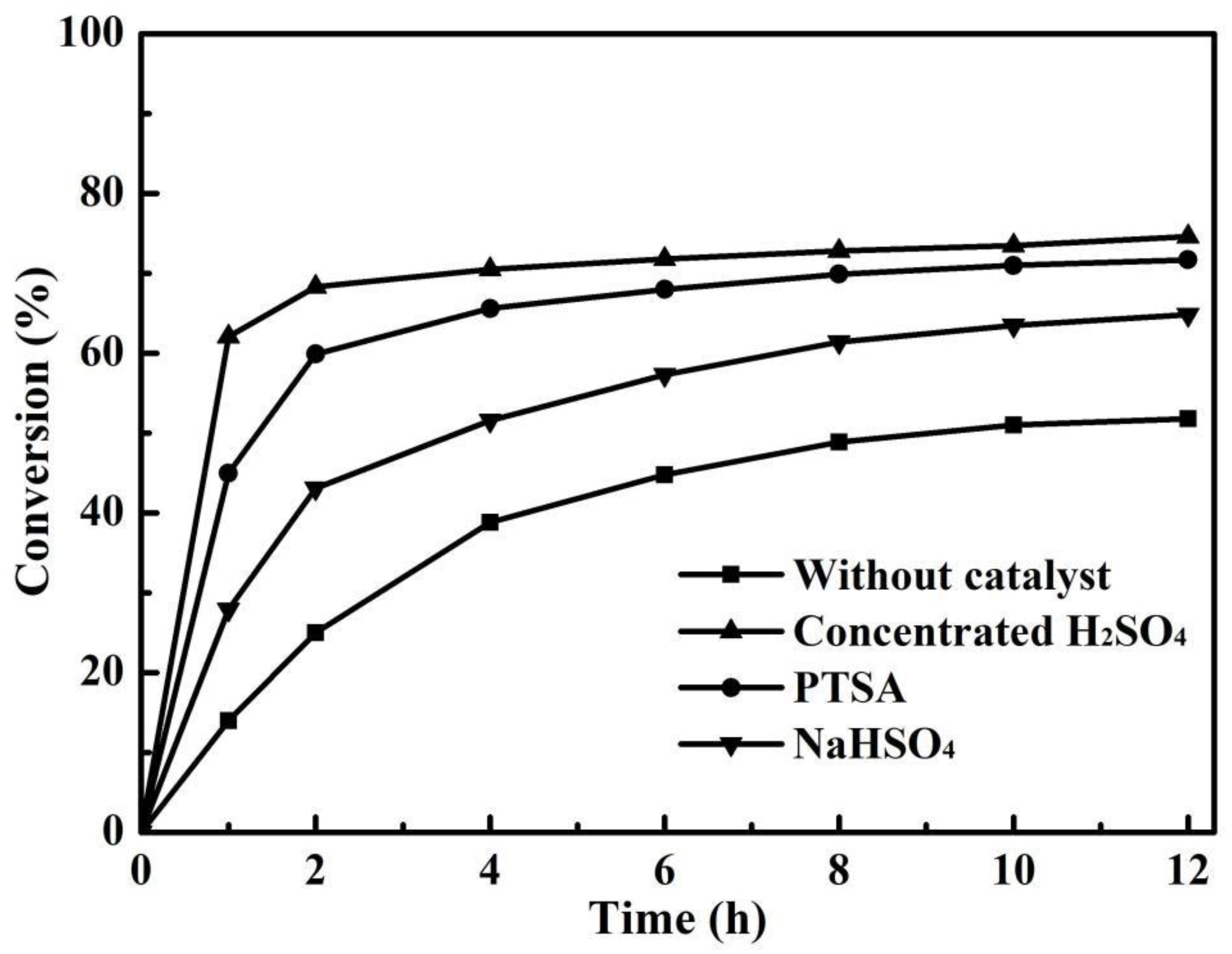
3.1. Effect of Catalyst on Esterification without PV
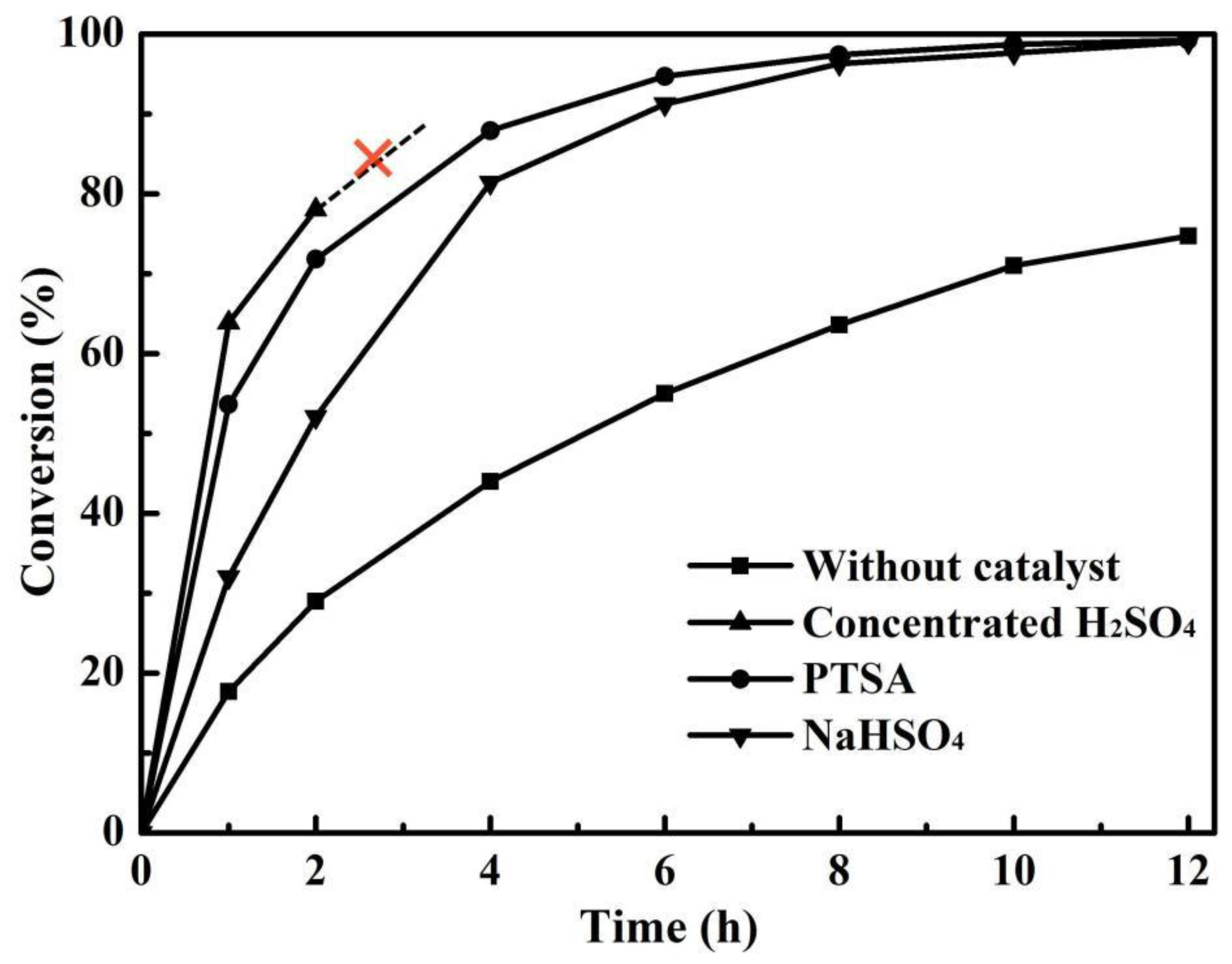
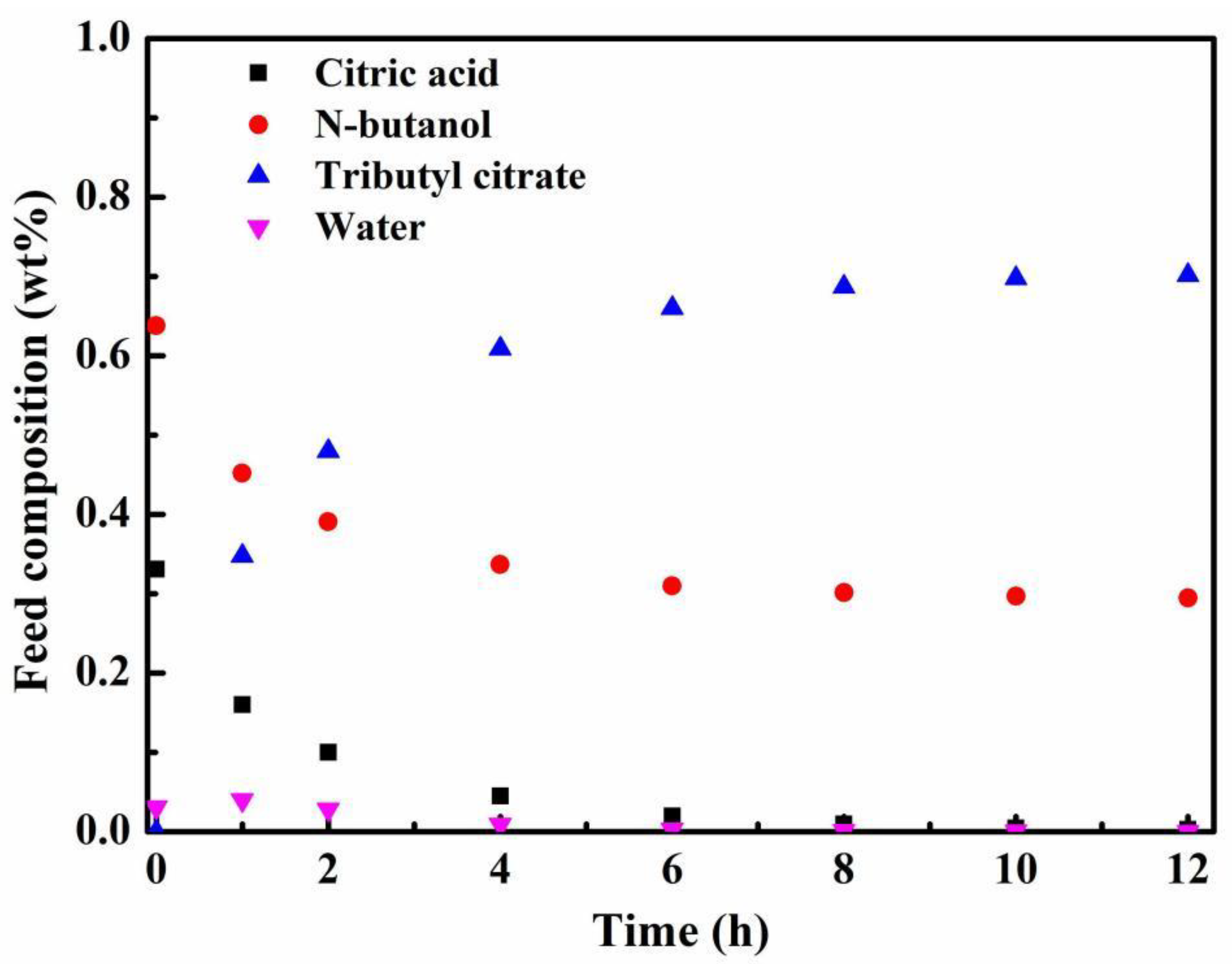
3.2. Effect of Catalyst on Esterification with PV
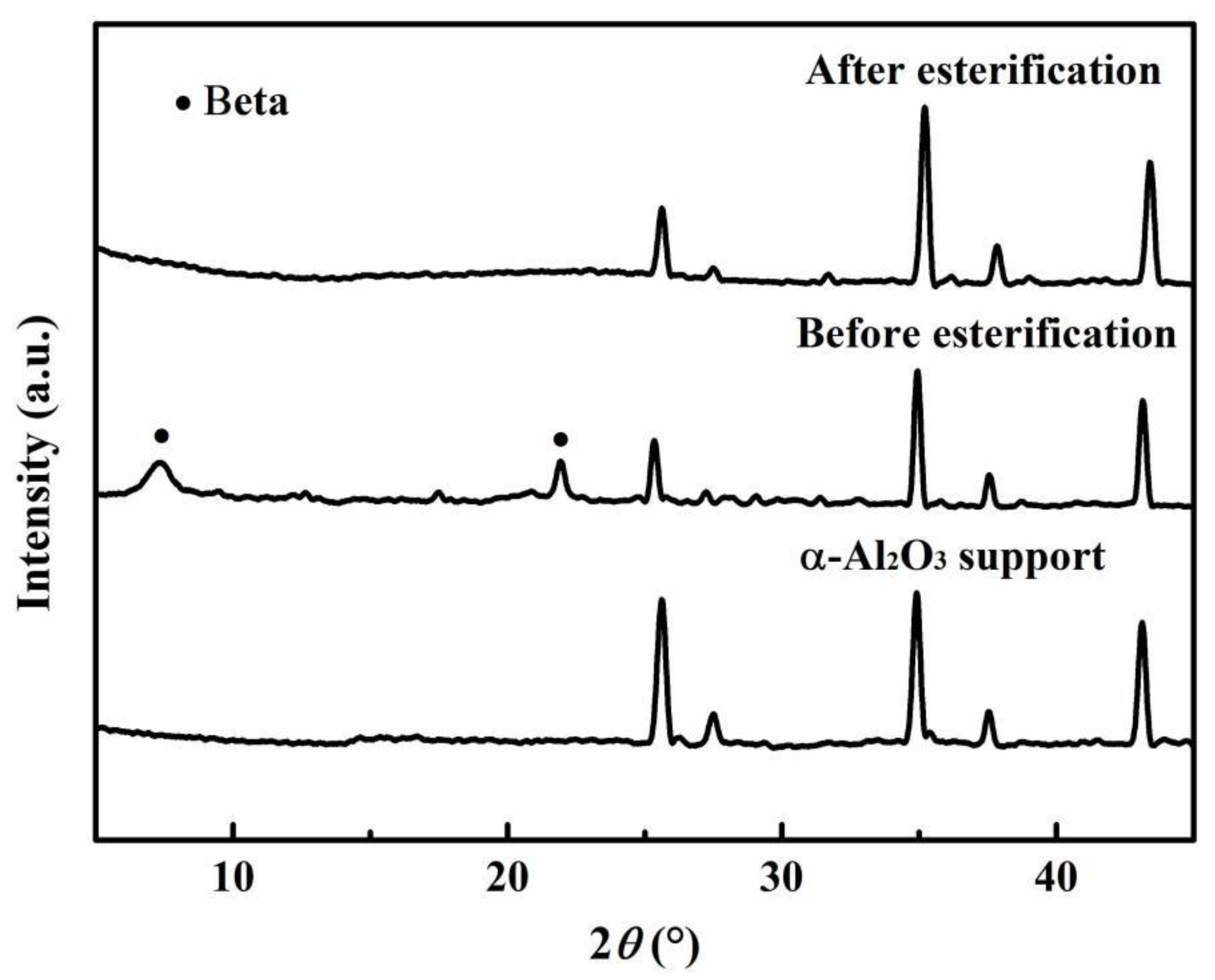
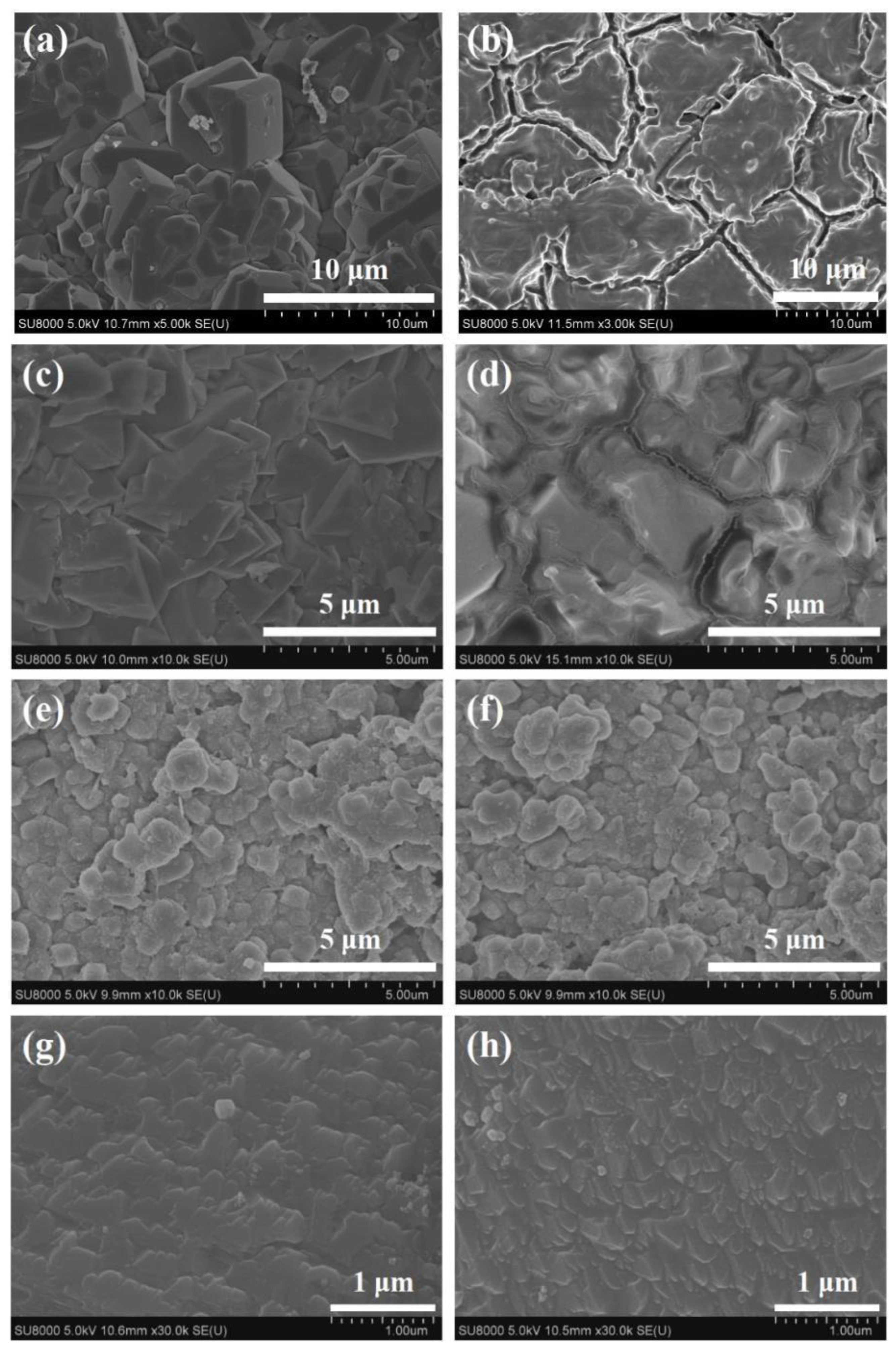
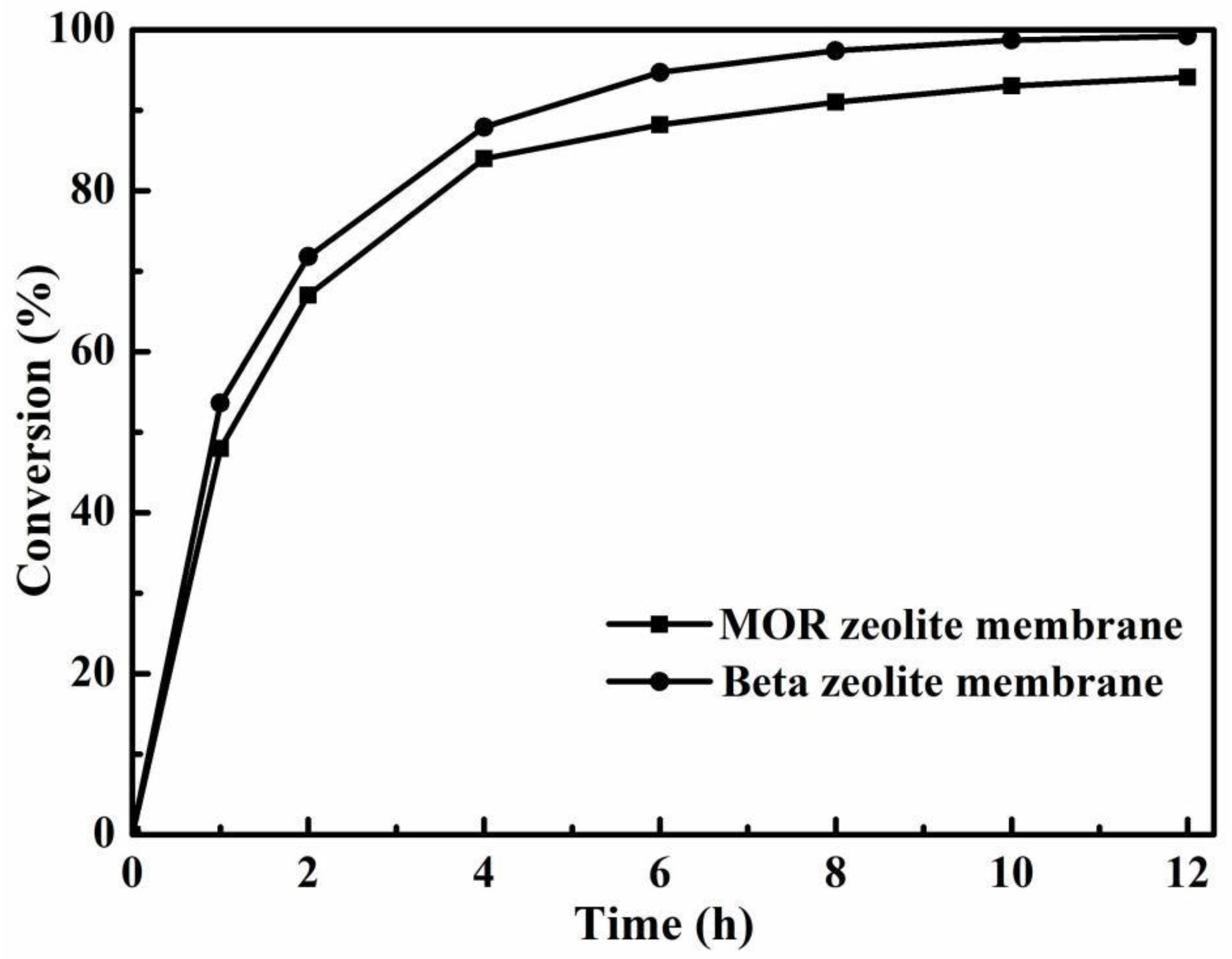
3.3. Effect of Zeolite Membrane Type
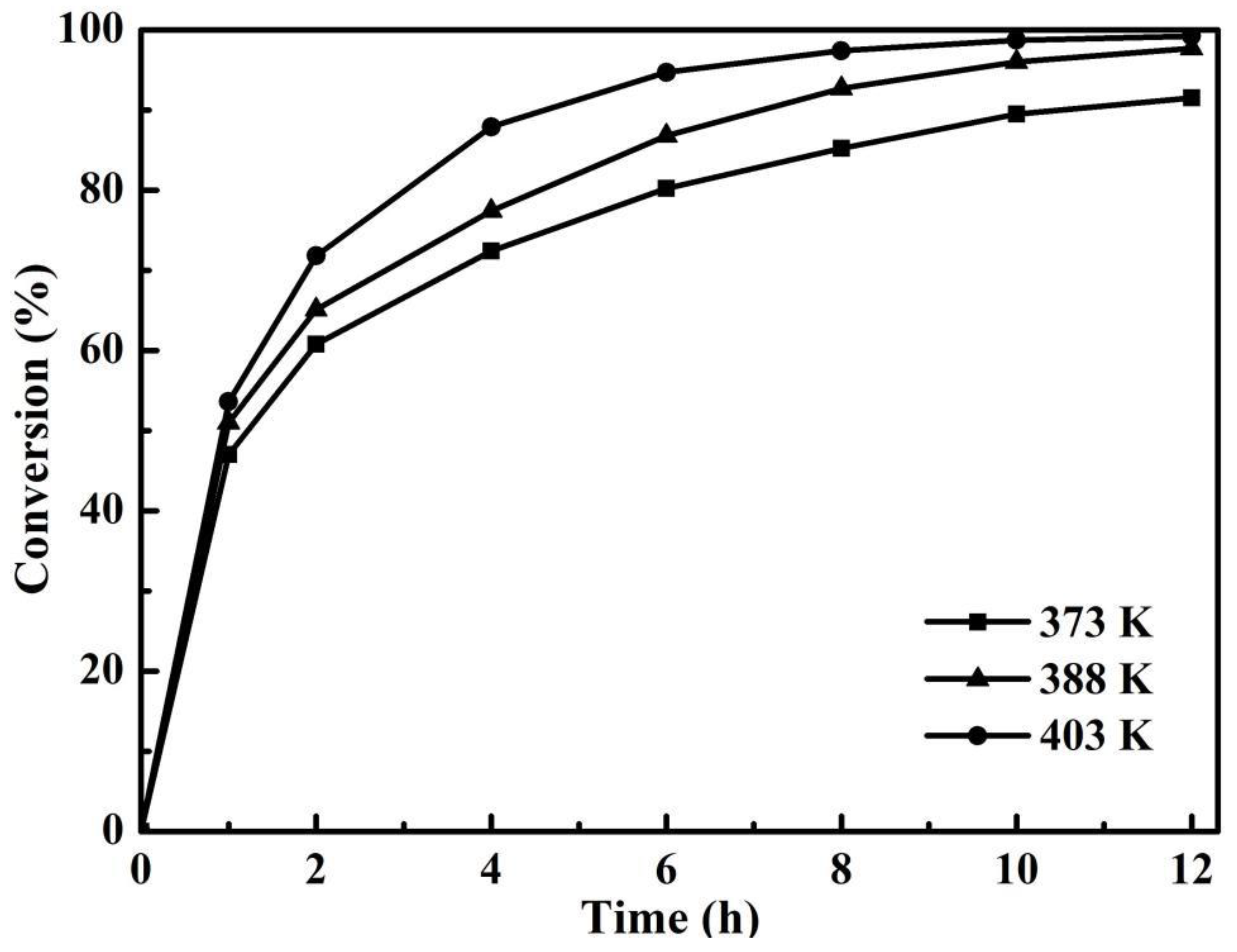
3.4. Effect of Temperature
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bui, T.T.; Giovanoulis, G.; Cousins, A.P.; Magner, J.; Cousins, I.T.; de Wit, C.A. Human exposure, hazard and risk of alternative plasticizers to phthalate esters. Sci. Total. Environ. 2016, 541, 451–467. [Google Scholar] [CrossRef] [PubMed]
- Lemmouchi, Y.; Murariu, M.; Santos, A.M.D.; Amass, A.J.; Schacht, E.; Dubois, P. Plasticization of poly (lactide) with blends of tributyl citrate and low molecular weight poly (D,L-lactide)-b-poly (ethylene glycol) copolymers. Eur. Polym. J. 2009, 45, 2839–2848. [Google Scholar] [CrossRef]
- Râpă, M.; Miteluţ, A.C.; Tănase, E.E.; Grosu, E.; Popescu, P.; Popa, M.E.; Rosnes, J.T.; Sivertsvik, M.; Darie-Niţă, R.N.; Vasile, C. Influence of chitosan on mechanical, thermal, barrier and antimicrobial properties of PLA-biocomposites for food packaging. Compos. Part B Eng. 2016, 102, 112–121. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, J.; Wei, L.V.; Gao, Y. Synthesis of tributyl citrate using solid acid as a catalyst. Chem. Eng. Commun. 2011, 198, 474–482. [Google Scholar] [CrossRef]
- Kolah, A.K.; Asthana, N.S.; Vu, D.T.; Lira, C.T.; Miller, D.J. Reaction kinetics of the catalytic esterification of citric acid with ethanol. Ind. Eng. Chem. Res. 2007, 46, 3180–3187. [Google Scholar] [CrossRef]
- Kolah, A.K.; Asthana, N.S.; Vu, D.T.; Lira, C.T.; Miller, D.J. Triethyl citrate synthesis by reactive distillation. Ind. Eng. Chem. Res. 2008, 47, 1017–1025. [Google Scholar] [CrossRef]
- Khan, Z.; Javed, F.; Shamair, Z.; Hafeez, A.; Fazal, T.; Aslam, A.; Zimmerman, W.B.; Rehman, F. Current developments in esterification reaction: A review on process and parameters. J. Ind. Eng. Chem. 2021, 103, 80–101. [Google Scholar] [CrossRef]
- Aqar, D.Y.; Mujtaba, I.M. Economic feasibility of an integrated semi-batch reactive distillation operation for the production of methyl decanoate. Sep. Purif. Technol. 2021, 257, 117871. [Google Scholar] [CrossRef]
- Haaz, E.; Toth, A.J. Methanol dehydration with pervaporation: Experiments and modelling. Sep. Purif. Technol. 2018, 205, 121–129. [Google Scholar] [CrossRef]
- Jyothi, M.S.; Reddy, K.R.; Soontarapa, K.; Naveen, S.; Raghu, A.V.; Kulkarni, R.V.; Suhas, D.P.; Shetti, N.P.; Nadagouda, M.N.; Aminabhavi, T.M. Membranes for dehydration of alcohols via pervaporation. J. Environ. Manag. 2019, 242, 415–429. [Google Scholar] [CrossRef]
- Li, G.; Ma, S.; Ye, F.; Luo, Y.; Fan, S.; Lang, X.; Wang, Y.; Zhou, L. Permeation characteristics of a T-type zeolite membrane for bio-oil pervaporation dehydration. Microporous Mesoporous Mater. 2021, 315, 110884. [Google Scholar] [CrossRef]
- Qiu, H.; Jiang, J.; Peng, L.; Liu, H.; Gu, X. Choline chloride templated CHA zeolite membranes for solvents dehydration with improved acid stability. Microporous Mesoporous Mater. 2019, 284, 170–176. [Google Scholar] [CrossRef]
- Xu, Y.M.; Chung, T.-S. High-performance UiO-66/polyimide mixed matrix membranes for ethanol, isopropanol and n-butanol dehydration via pervaporation. J. Membrane Sci. 2017, 531, 16–26. [Google Scholar] [CrossRef]
- Van Gestel, T.; Velterop, F.; Meulenberg, W.A. Zirconia-supported hybrid organosilica microporous membranes for CO2 separation and pervaporation. Sep. Purif. Technol. 2021, 259, 118114. [Google Scholar] [CrossRef]
- Vorotyntsev, V.M.; Drozdov, P.N.; Kolotilov, E.Y. Gas mixtures separation by an absorbing pervaporation method. Desalination 2002, 149, 23–27. [Google Scholar] [CrossRef]
- Prihatiningtyas, I.; Al-Kebsi, A.-H.A.H.; Hartanto, Y.; Zewdie, T.M.; Van der Bruggen, B. Techno-economic assessment of pervaporation desalination of hypersaline water. Desalination 2022, 527, 115538. [Google Scholar] [CrossRef]
- Sun, H.; Sun, D.; Shi, X.; Li, B.; Yue, D.; Xiao, R.; Ren, P.; Zhang, J. PVA/SO42--AAO difunctional catalytic-pervaporation membranes: Preparation and characterization. Sep. Purif. Technol. 2020, 241, 116739. [Google Scholar] [CrossRef]
- Lim, S.Y.; Park, B.; Hung, F.; Sahimi, M.; Tsotsis, T.T. Design issues of pervaporation membrane reactors for esterification. Chem. Eng. Sci. 2002, 57, 4933–4946. [Google Scholar] [CrossRef]
- Han, Y.; Lv, E.; Ma, L.; Lu, J.; Chen, K.; Ding, J. Coupling membrane pervaporation with a fixed-bed reactor for enhanced esterification of oleic acid with ethanol. Energy. Convers. Manag. 2015, 106, 1379–1386. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Abe, C.; Mizukami, F.; Kowata, Y.; Hanaoka, T. Application of a CHA-type zeolite membrane to the esterification of adipic acid with isopropyl alcohol using sulfuric acid catalyst. J. Membr. Sci. 2012, 415–416, 368–374. [Google Scholar] [CrossRef]
- Itoh, N.; Ishida, J.; Sato, T.; Hasegawa, Y. Vapor phase esterification using a CHA type of zeolite membrane. Catal. Today 2016, 268, 79–84. [Google Scholar] [CrossRef]
- Li, W.; Liu, W.; Xing, W.; Xu, N. Esterification of acetic acid and n-propanol with vapor permeation using NaA zeolite membrane. Ind. Eng. Chem. Res. 2013, 52, 6336–6342. [Google Scholar] [CrossRef]
- Li, X.; Kita, H.; Zhu, H.; Zhang, Z.; Tanaka, K. Synthesis of long-term acid-stable zeolite membranes and their potential application to esterification reactions. J. Membr. Sci. 2009, 339, 224–232. [Google Scholar] [CrossRef]
- Zhang, W.; Na, S.; Li, W.; Xing, W. Kinetic modeling of pervaporation aided esterification of propionic acid and ethanol using T-type zeolite membrane. Ind. Eng. Chem. Res. 2015, 54, 4940–4946. [Google Scholar] [CrossRef]
- Yan, Z.; Wu, X.; Zhu, B.; Li, Y.; Gui, T.; Li, Y.; Zhu, M.; Chen, X.; Kita, H. Improvement of esterification conversion by rapid pervaporation dehydration using a high-flux and acid-resistant MOR zeolite membrane. Sep. Purif. Technol. 2022, 286, 120415. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, X.; Nai, S.; Zhang, B. Template-free synthesis of beta zeolite membranes on porous α-Al2O3 supports. Chem. Commun. 2014, 50, 8834–8837. [Google Scholar] [CrossRef]
- Ueno, K.; Yamada, S.; Negishi, H.; Okuno, T.; Tawarayama, H.; Ishikawa, S.; Miyamoto, M.; Uemiya, S.; Oumi, Y. Fabrication of pure-silica *BEA-type zeolite membranes on tubular silica supports coated with dilute synthesis gel via steam-assisted conversion. Sep. Purif. Technol. 2020, 247, 116934. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, M.; Hu, N.; Zhang, F.; Wu, T.; Chen, X.; Kita, H. Scale-up of high performance mordenite membranes for dehydration of water-acetic acid mixtures. J. Membr. Sci. 2018, 564, 174–183. [Google Scholar] [CrossRef]
- Wu, X.; Li, Y.; Chen, X.; Zhu, M.; Zhang, F.; Gui, T.; Hu, N.; Chen, X.; Kita, H. Preparation of chabazite zeolite membranes by a two-stage varying-temperature hydrothermal synthesis for water-ethanol separation. Sep. Purif. Technol. 2019, 234, 116055. [Google Scholar] [CrossRef]
- Wu, X.; Gui, T.; Yan, Z.; Zhu, B.; Li, Y.; Li, Y.; Zhu, M.; Zhang, F.; Chen, X.; Hidetoshi, K. Industrial-scale fabrication of mordenite membranes by dual heating method for production of ethyl acetate in an industrial VP-esterification plant. J. Membr. Sci. 2022, 647, 120335. [Google Scholar] [CrossRef]
- Wu, X.; Yan, Z.; Li, Y.; Zhu, B.; Gui, T.; Li, Y.; Zhu, M.; Zhang, F.; Chen, X.; Kita, H. Fabrication of low cost and high performance NaA zeolite membranes on 100-cm-long coarse macroporous supports for pervaporation dehydration of dimethoxymethane. Sep. Purif. Technol. 2021, 281, 119877. [Google Scholar] [CrossRef]
- Wang, A.; Wang, J.; Lu, C.; Xu, M.; Lv, J.; Wu, X. Esterification for biofuel synthesis over an eco-friendly and efficient kaolinite-supported SO42−/ZnAl2O4 macroporous solid acid catalyst. Fuel 2018, 234, 430–440. [Google Scholar] [CrossRef]
- Zhu, B.; Li, Y.; Yan, Z.; Yang, Z.; Wu, X.; Gui, T.; Li, Y.; Zhang, F.; Chen, X.; Kita, H. Formation process of organic template-free chabazite zeolite membrane and its separation performance of water-rich mixtures. Microporous Mesoporous Mater. 2022, 341, 112085. [Google Scholar] [CrossRef]
- Wu, L.; Hu, X.; Wang, S.; Mahmudul Hasan, M.D.; Jiang, S.; Li, T.; Li, C.-Z. Acid-treatment of bio-oil in methanol: The distinct catalytic behaviours of a mineral acid catalyst and a solid acid catalyst. Fuel 2018, 212, 412–421. [Google Scholar] [CrossRef]
- Jin, B.; Duan, P.; Xu, Y.; Wang, B.; Wang, F.; Zhang, L. Lewis acid-catalyzed in situ transesterification/esterification of microalgae in supercritical ethanol. Bioresour. Technol. 2014, 162, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ding, B.; Zhang, M. Preparation supported heteropoly (acid)/polyaniline catalysts and catalytic synthesis of tributyl citrate. RSC Adv. 2019, 9, 33124–33129. [Google Scholar] [CrossRef] [Green Version]
- Junming, X.U.; Jianchun, J.; Zhiyue, Z.; Jing, L. Synthesis of tributyl citrate using acid ionic liquid as catalyst. Process Saf. Environ. 2010, 88, 28–30. [Google Scholar] [CrossRef]
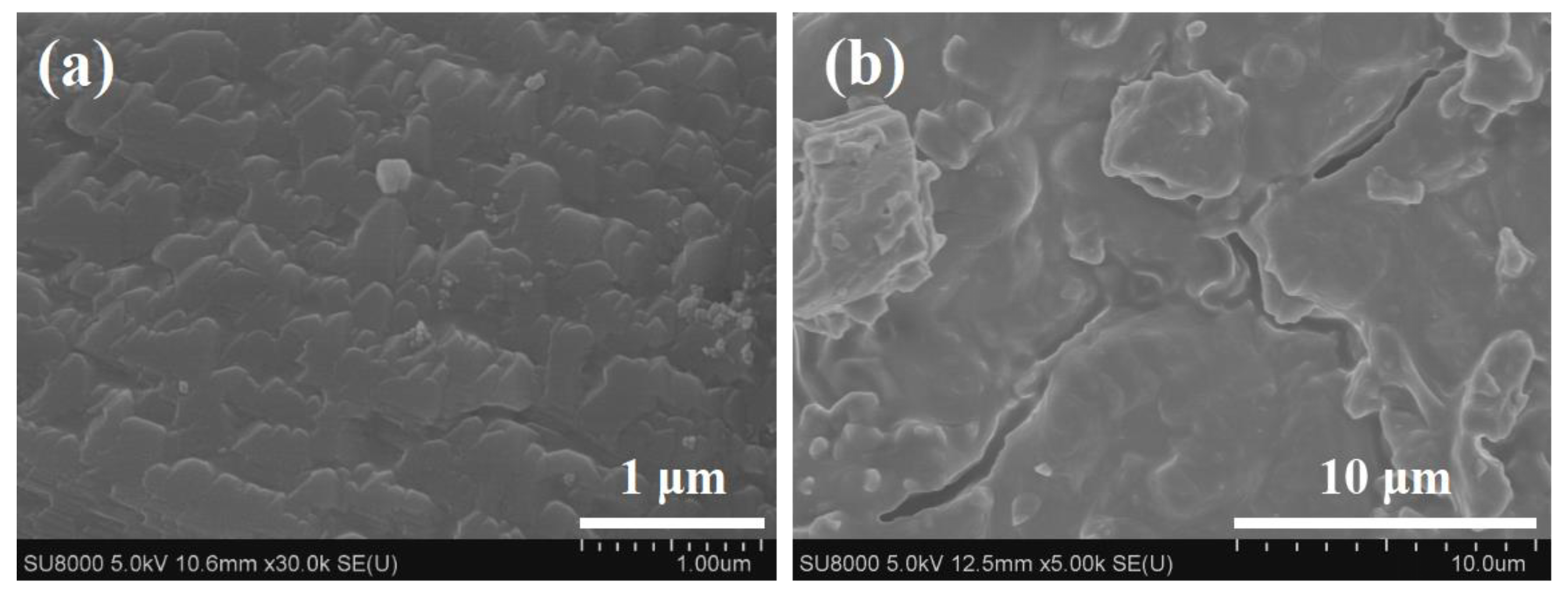

| Zeolite Membrane | J (kg m−2 h−1) | αH2O/n-BuOH | Water Content in Permeate (wt%) |
|---|---|---|---|
| beta | 2.83 | 15,000 | 99.94 |
| MOR | 1.85 | 5290 | 99.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Peng, M.; Li, Y.; Wu, X.; Gui, T.; Li, Y.; Zhang, F.; Chen, X.; Kita, H. Improved Esterification of Citric Acid and n-Butanol Using a Dense and Acid-Resistant Beta Zeolite Membrane. Membranes 2022, 12, 1269. https://doi.org/10.3390/membranes12121269
Yang Z, Peng M, Li Y, Wu X, Gui T, Li Y, Zhang F, Chen X, Kita H. Improved Esterification of Citric Acid and n-Butanol Using a Dense and Acid-Resistant Beta Zeolite Membrane. Membranes. 2022; 12(12):1269. https://doi.org/10.3390/membranes12121269
Chicago/Turabian StyleYang, Zhengquan, Mingyu Peng, Yu Li, Xiaowei Wu, Tian Gui, Yuqin Li, Fei Zhang, Xiangshu Chen, and Hidetoshi Kita. 2022. "Improved Esterification of Citric Acid and n-Butanol Using a Dense and Acid-Resistant Beta Zeolite Membrane" Membranes 12, no. 12: 1269. https://doi.org/10.3390/membranes12121269