Preparation and Characterization of a Novel Sulfonated Titanium Oxide Incorporated Chitosan Nanocomposite Membranes for Fuel Cell Application
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Chemicals
2.2. Sulfonation of Nano-TiO2
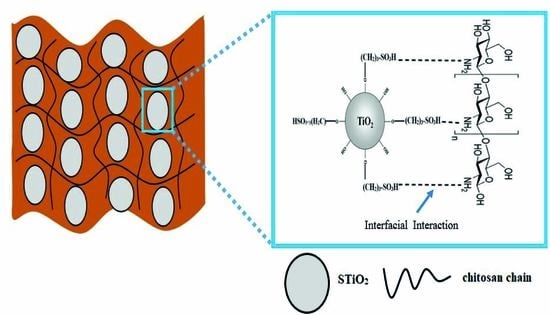
2.3. Preparation of CS/STiO2 Nanocomposite Membranes
2.4. Characterization
2.5. Measurement of Water Uptake and Swelling in Dimension
2.6. Calculation of Ion Exchange Capacity (IEC)
2.7. Measurement of Proton Conductivity
3. Results and Discussions
3.1. Characterization of STiO2
3.2. Structural Characterization of CS/STiO2 Nanocomposite Membranes
3.3. Thermal and Mechanical Stability of CS/STiO2 Nanocomposite Membranes
3.4. Water and Methanol Uptake, Dimensional Stability and IEC of CS/STiO2 Nanocomposite Membranes
3.5. Electrochemical Characteristics of CS/STiO2 Nanocomposite Membranes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, S.; Jia, T.; Jia, J.; Wang, N.; Song, D.; Zhao, J.; Jin, J.; Che, Q. Constructing anhydrous proton exchange membranes through alternate depositing graphene oxide and chitosan on sulfonated poly (vinylidenefluoride) or sulfonated poly (vinylidene fluoride-co-hexafluoropropylene) membranes. Eur. Polym. J. 2021, 142, 110160. [Google Scholar] [CrossRef]
- Swaghatha, A.A.K.; Cindrella, L. Enhanced self-humidification and proton conductivity in magnetically aligned NiO-Co3O4/chitosan nanocomposite membranes for high-temperature PEMFCS. Polym. J. 2021, 1–15. [Google Scholar]
- Bahar, T. Development of reasonably stable chitosan based proton exchange membranes for a glucose oxidase based enzymatic biofuel cell. Electroanalis 2020, 32, 536–545. [Google Scholar] [CrossRef]
- Hamid, A.; Khan, M.; Hussain, F.; Zada, A.; Li, T.; Alei, D.; Ali, A. Synthesis and physiochemical performances of PVC-sodium polyacrylate and PVC-sodium polyacrylate-graphite composite polymer membrane. Z. Phys. Chem. 2021. [Google Scholar] [CrossRef]
- Tsen, W.-C. Composite proton exchange membranes based on chitosan and phosphotungstic acid immobilized one-dimensional attapulgite for direct methanol fuel cells. Nanomaterials 2020, 10, 1641. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Wang, W.; Shan, B.; Liu, C.; Xie, C.; Zhu, L.; Chen, X.; Li, N. Enhanced performance of the chitosan proton exchange membrane via anatase titania anchored go and sodium ligninsulfonate constructing proton transport channels. Energy Fuels 2020, 34, 3867–3876. [Google Scholar] [CrossRef]
- Wang, J.; Gong, C.; Wen, S.; Liu, H.; Qin, C.; Xiong, C.; Dong, L. A facile approach of fabricating proton exchange membranes by incorporating polydopamine-functionalized carbon nanotubes into chitosan. Int. J. Hydrog. Energy 2019, 44, 6909–6918. [Google Scholar] [CrossRef]
- Ahmed, S.; Ali, M.; Cai, Y.; Lu, Y.; Ahmad, Z.; Khannal, S.; Xu, S. Novel sulfonated multi-walled carbon nanotubes filled chitosan composite membrane for fuel-cell applications. J. Appl. Polym. Sci. 2019, 136, 47603. [Google Scholar] [CrossRef]
- Ahmed, S.; Cai, Y.; Ali, M.; Khanal, S.; Xu, S. Preparation and performance of nanoparticle-reinforced chitosan proton-exchange membranes for fuel-cell applications. J. Appl. Polym. Sci. 2019, 136. [Google Scholar] [CrossRef] [Green Version]
- Sanij, F.D.; Balakrishnan, P.; Leung, P.; Shah, A.; Su, H.; Xu, Q. Advanced Pd-based nanomaterials for electro-catalytic oxygen reduction in fuel cells: A review. Int. J. Hydrog. Energy 2021. [Google Scholar] [CrossRef]
- Peera, S.G.; Maiyalagan, T.; Liu, C.; Ashmath, S.; Lee, T.G.; Jiang, Z.; Mao, S. A review on carbon and non-precious metal based cathode catalysts in microbial fuel cells. Int. J. Hydrog. Energy 2021, 46, 3056–3089. [Google Scholar] [CrossRef]
- Lv, X.W.; Weng, C.C.; Zhu, Y.P.; Yuan, Z.Y. Nanoporous metal phosphonate hybrid materials as a novel platform for emerging applications: A critical review. Small 2021, 2005304. [Google Scholar] [CrossRef] [PubMed]
- Shaari, N.; Kamarudin, S.K.; Bahru, R.; Osman, S.H.; Md Ishak, N.A.I. Progress and challenges: Review for direct liquid fuel cell. Int. J. Energy Res. 2021, 45, 6644–6688. [Google Scholar] [CrossRef]
- Nunes, S.P.; Ruffmann, B.; Rikowski, E.; Vetter, S.; Richau, K. Inorganic modification of proton conductive polymer membranes for direct methanol fuel cells. J. Membr. Sci. 2002, 203, 215–225. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, Z.; Wu, L.; Xu, T. Novel sulfonated polyimides proton-exchange membranes via a facile polyacylation approach of imide monomers. J. Membr. Sci. 2014, 455, 1–6. [Google Scholar] [CrossRef]
- Liao, H.; Zhang, K.; Xiao, G.; Yan, D. High performance sulfonated poly (phthalazinone ether phosphine oxide)s for proton exchange membranes. J. Membr. Sci. 2013, 447, 43–49. [Google Scholar] [CrossRef]
- Ahmed, S.; Cai, Y.; Ali, M.; Khannal, S.; Ahmad, Z.; Lu, Y.; Wang, S.; Xu, S. One-step phosphorylation of graphene oxide for the fabrication of nanocomposite membranes with enhanced proton conductivity for fuel cell applications. J. Mater. Sci. Mater. Electron. 2019, 30, 13056–13066. [Google Scholar] [CrossRef]
- Hamid, A.; Khan, M.; Hayat, A.; Raza, J.; Zada, A.; Ullah, A.; Raziq, F.; Li, T.; Hussain, F. Probing the physio-chemical appraisal of green synthesized PbO nanoparticles in PbO-PVC nanocomposite polymer membranes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 235, 118303. [Google Scholar] [CrossRef]
- Jafari Sanjari, A.; Asghari, M. A review on chitosan utilization in membrane synthesis. ChemBioEng Rev. 2016, 3, 134–158. [Google Scholar] [CrossRef]
- Ahmed, S.; Cai, Y.; Ali, M.; Khannal, S.; Xu, S. Preparation and properties of alkyl benzene sulfonic acid coated boehmite/chitosan nanocomposite membranes with enhanced proton conductivity for proton exchange membrane fuel cells. Mater. Res. Express 2019, 9, 42–50. [Google Scholar] [CrossRef]
- Xiao, Y.; Xiang, Y.; Xiu, R.; Lu, S. Development of cesium phosphotungstate salt and chitosan composite membrane for direct methanol fuel cells. Carbohydr. Polym. 2013, 98, 233–240. [Google Scholar] [CrossRef]
- Zada, A.; Khan, M.; Hussain, Z.; Shah, M.I.A.; Ateeq, M.; Ullah, M.; Ali, N.; Shaheen, S.; Yasmeen, H.; Shah, S.N.A.; et al. Extended visible light driven photocatalytic hydrogen generation by electron induction from g-C3N4 nanosheets to ZnO through the proper heterojunction. Z. Phys. Chem. 2021. [Google Scholar] [CrossRef]
- Huang, Z.; Guan, H.-m.; Tan, W.l.; Qiao, X.-Y.; Kulprathipanja, S. Pervaporation study of aqueous ethanol solution through zeolite-incorporated multilayer poly (vinyl alcohol) membranes: Effect of zeolites. J. Membr. Sci. 2006, 276, 260–271. [Google Scholar] [CrossRef]
- Shaheer Akhtar, M.; Chun, J.-M.; Yang, O.B. Advanced composite gel electrolytes prepared with titania nanotube fillers in polyethylene glycol for the solid-state dye-sensitized solar cell. Electrochem. Commun. 2007, 9, 2833–2837. [Google Scholar] [CrossRef]
- Bonderer, L.J.; Studart, A.R.; Gauckler, L.J. Bioinspired design and assembly of platelet reinforced polymer films. Science 2008, 319, 1069–1073. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Dhayal, M. Electrochemical studies of novel chitosan/TiO2 bioactive electrode for biosensing application. Electrochem. Commun. 2008, 10, 263–267. [Google Scholar] [CrossRef]
- De Sitter, K.; Winberg, P.; D’Haen, J.; Dotremont, C.; Leiden, R.; Martens, J.A.; Mullens, S.; Maurer, F.H.J.; Vankelecom, I.F.J. Silica filled poly(1-trimethylsilyl-1-propyne) nanocomposite membranes: Relation between the transport of gases and structural characteristics. J. Membr. Sci. 2006, 278, 83–91. [Google Scholar] [CrossRef]
- Yasmeen, H.; Zada, A.; Ali, S.; Khan, I.; Ali, W.; Khan, W.; Khan, M.; Anwar, N.; Ali, A.; Huerta-Flores, A.M.; et al. Visible light-excited surface plasmon resonance charge transfer significantly improves the photocatalytic activities of ZnO semiconductor for pollutants degradation. J. Chin. Chem. Soc. 2020, 67, 1611–1617. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Zhang, H.; Ma, C.; Liu, J.; Cao, S.; Zhang, X. Enhancement of proton conductivity of chitosan membrane enabled by sulfonated graphene oxide under both hydrated and anhydrous conditions. J. Power Sources 2014, 269, 898–911. [Google Scholar] [CrossRef]
- Bai, H.; Zhang, H.; He, Y.; Liu, J.; Zhang, B.; Wang, J. Enhanced proton conduction of chitosan membrane enabled by halloysite nanotubes bearing sulfonate polyelectrolyte brushes. J. Membr. Sci. 2014, 454, 220–232. [Google Scholar] [CrossRef]
- Wu, H.; Hou, W.; Wang, J.; Xiao, L.; Jiang, Z. Preparation and properties of hybrid direct methanol fuel cell membranes by embedding organophosphorylated titania submicrospheres into a chitosan polymer matrix. J. Power Sources 2010, 195, 4104–4113. [Google Scholar] [CrossRef]
- Santamaria, M.; Pecoraro, C.M.; Di Quarto, F.; Bocchetta, P. Chitosan–phosphotungstic acid complex as membranes for low temperature h2–o2 fuel cell. J. Power Sources 2015, 276, 189–194. [Google Scholar] [CrossRef]
- Geng, J.; Jiang, Z.; Wang, J.; Shi, Y.; Yang, D.; Xiao, L. Chitosan/titanate nanotube hybrid membrane with low methanol crossover for direct methanol fuel cells. Chem. Eng. Technol. 2010, 33, 244–250. [Google Scholar] [CrossRef]
- Wu, H.; Zheng, B.; Zheng, X.; Wang, J.; Yuan, W.; Jiang, Z. Surface-modified y zeolite-filled chitosan membrane for direct methanol fuel cell. J. Power Sources 2007, 173, 842–852. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Jiang, Z.; Yang, X.; Xiao, L. Tuning the performance of direct methanol fuel cell membranes by embedding multifunctional inorganic submicrospheres into polymer matrix. J. Power Sources 2009, 188, 64–74. [Google Scholar] [CrossRef]
- Wang, K.; McDermid, S.; Li, J.; Kremliakova, N.; Kozak, P.; Song, C.; Tang, Y.; Zhang, J.; Zhang, J. Preparation and performance of nano silica/nafion composite membrane for proton exchange membrane fuel cells. J. Power Sources 2008, 184, 99–103. [Google Scholar] [CrossRef]
- Bai, H.; Li, Y.; Zhang, H.; Chen, H.; Wu, W.; Wang, J.; Liu, J. Anhydrous proton exchange membranes comprising of chitosan and phosphorylated graphene oxide for elevated temperature fuel cells. J. Membr. Sci. 2015, 495, 48–60. [Google Scholar] [CrossRef]
- Jin, Y.G.; Qiao, S.Z.; da Costa, J.C.D.; Wood, B.J.; Ladewig, B.P.; Lu, G.Q. Hydrolytically stable phosphorylated hybrid silica for proton conduction. Adv. Funct. Mater. 2007, 17, 3304–3311. [Google Scholar] [CrossRef]
- Khan, M.; Hamid, A.; Tiehu, L.; Zada, A.; Attique, F.; Ahmad, N.; Ullah, A.; Hayat, A.; Mahmood, I.; Hussain, A.; et al. Surface optimization of detonation nanodiamonds for the enhanced mechanical properties of polymer/nanodiamond composites. Diam. Relat. Mater. 2020, 107, 107897. [Google Scholar] [CrossRef]
- Lee, W.S.; Kang, T.; Kim, S.-H.; Jeong, J. An antibody-immobilized silica inverse opal nanostructure for label-free optical biosensors. Sensors 2018, 18, 307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.P.; Lin, C.W.; Li, S.S.; Tsai, Y.H.; Wen, C.Y.; Lin, W.J.; Hsiao, F.M.; Chiu, Y.P.; Tsukagoshi, K.; Osada, M. Self-assembly atomic stacking transport layer of 2d layered titania for perovskite solar cells with extended UV stability. Adv. Energy Mater. 2018, 8. [Google Scholar] [CrossRef]
- Kharel, P.L.; Zamborini, F.P.; Alphenaar, B.W. Enhancing the photovoltaic performance of dye-sensitized solar cells with rare-earth metal oxide nanoparticles. J. Electrochem. Soc. 2018, 165, H52–H56. [Google Scholar] [CrossRef]
- Khan, W.A.; Arain, M.B.; Bibi, H.; Tuzen, M.; Shah, N.; Zada, A. Selective electromembrane extraction and sensitive colorimetric detection of copper(II). Z. Phys. Chem. 2020. [Google Scholar] [CrossRef]
- Yagizatli, Y.; Ulas, B.; Cali, A.; Sahin, A.; Ar, I. Improved fuel cell properties of nano-TiO2 doped poly (vinylidene fluoride) and phosphonated poly (vinyl alcohol) composite blend membranes for PEM fuel cells. Int. J. Hydrog. Energy 2020, 45, 35130–35138. [Google Scholar] [CrossRef]
- Li, J.-F.; Xu, Z.-L.; Yang, H.; Yu, L.-Y.; Liu, M. Effect of TiO2 nanoparticles on the surface morphology and performance of microporous pes membrane. Appl. Surf. Sci. 2009, 255, 4725–4732. [Google Scholar] [CrossRef]
- Slade, S.; Smith, J.; Campbell, S.; Ralph, T.; de Leon, C.P.; Walsh, F. Characterisation of a re-cast composite nafion® 1100 series of proton exchange membranes incorporating inert inorganic oxide particles. Electrochim. Acta 2010, 55, 6818–6829. [Google Scholar] [CrossRef] [Green Version]
- Tahrim, A.; Amin, I. Advancement in phosphoric acid doped polybenzimidazole membrane for high temperature pem fuel cells: A review. J. Appl. Memb. Sci. Technol. 2019, 23. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.P.; Kulshrestha, V. Synthesis of highly stable and high water retentive functionalized biopolymer-graphene oxide modified cation exchange membranes. RSC Adv. 2015, 5, 56498–56506. [Google Scholar] [CrossRef]
- Khan, M.; Zada, A.; Hayat, A.; Ali, T.; Uddin, I.; Hayat, A.; Khan, M.; Ullah, A.; Hussain, A.; Li, T.; et al. A concise review on the elastomeric behavior of electroactive polymer materials. Int. J. Energy Res. 2021, 1–32. [Google Scholar]
- Hassan, M.; Afzal, A.; Tariq, M.; Ahmed, S. Synthesis of the hyper-branched polyamides and their effective utilization in adsorption and equilibrium isothermal study for cadmium ion uptake. J. Polym. Res. 2021, 28, 1–11. [Google Scholar] [CrossRef]
- Vijayalekshmi, V.; Khastgir, D. Eco-friendly methanesulfonic acid and sodium salt of dodecylbenzene sulfonic acid doped cross-linked chitosan based green polymer electrolyte membranes for fuel cell applications. J. Membr. Sci. 2017, 523, 45–59. [Google Scholar] [CrossRef]
- Liu, H.; Gong, C.; Wang, J.; Liu, X.; Liu, H.; Cheng, F.; Wang, G.; Zheng, G.; Qin, C.; Wen, S. Chitosan/silica coated carbon nanotubes composite proton exchange membranes for fuel cell applications. Carbohydr. Polym. 2016, 136, 1379–1385. [Google Scholar] [CrossRef]
- Ioelovich, M. Crystallinity and hydrophility of chitin and chitosan. J. Chem. 2014, 3, 7–14. [Google Scholar]
- Facchinatto, W.M.; Dos Santos, D.M.; Fiamingo, A.; Bernardes-Filho, R.; Campana-Filho, S.P.; de Azevedo, E.R.; Colnago, L.A. Evaluation of chitosan crystallinity: A high-resolution solid-state nmr spectroscopy approach. Carbohydr. Polym. 2020, 250, 116891. [Google Scholar] [CrossRef]
- Nguyen, C.-C.; Nguyen, D.T.; Do, T.-O. A novel route to synthesize C/Pt/TiO2 phase tunable anatase–rutile TiO2 for efficient sunlight-driven photocatalytic applications. Appl. Catal. B 2018, 226, 46–52. [Google Scholar] [CrossRef]
- Seo, J.A.; Koh, J.H.; Roh, D.K.; Kim, J.H. Preparation and characterization of crosslinked proton conducting membranes based on chitosan and pssa-ma copolymer. Solid State Ion. 2009, 180, 998–1002. [Google Scholar] [CrossRef]
- Zada, A.; Ali, N.; Ateeq, M.; Huerta-Flores, A.M.; Hussain, Z.; Shaheen, S.; Ullah, M.; Ali, S.; Khan, I.; Ali, W.; et al. Surface plasmon resonance excited electron induction greatly extends H2 evolution and pollutant degradation activity of g-C3N4 under visible light irradiation. J. Chin. Chem. Soc. 2020, 67, 983–989. [Google Scholar] [CrossRef]
- Kim, S.H.; Mehmood, A.; Ahn, Y.; Kim, H.-S.; Ha, H.Y.; Kim, D.; Han, O.H. Proton conductivity improvement of polymer electrolyte membrane using nano-scale explosion of water in the membrane. J. Electroanal. Chem. 2016, 782, 32–35. [Google Scholar] [CrossRef]
- Peighambardoust, S.; Rowshanzamir, S.; Amjadi, M. Review of the proton exchange membranes for fuel cell applications. Int. J. Hydrog. Energy 2010, 35, 9349–9384. [Google Scholar] [CrossRef]
- Vilčiauskas, L.; Tuckerman, M.E.; Bester, G.; Paddison, S.J.; Kreuer, K.-D. The mechanism of proton conduction in phosphoric acid. Nat. Chem. 2012, 4, 461. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, J.; Wen, S.; Gong, C.; Cheng, F.; Wang, G.; Zheng, G.; Qin, C. Composite membranes of chitosan and titania-coated carbon nanotubes as promising materials for new proton-exchange membranes. J. Appl. Polym. Sci. 2016, 133, 43365. [Google Scholar] [CrossRef]








| Samples | T5% °C | Char Yield (wt. %) | |||
|---|---|---|---|---|---|
| 500 °C | 600 °C | 700 °C | 800 °C | ||
| TiO2 | 420 | 92 | 91 | 91 | 90 |
| STiO2 | 256 | 89 | 88 | 88 | 88 |
| Serial No. | Ti | O | S |
|---|---|---|---|
| TiO2 | 22.71 | 77.29 | -- |
| STiO2 | 23.43 | 74.57 | 2.00 |
| Membranes | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|
| CS/STiO2–0 | 13.05 ± 1.03 | 15.61 ± 6.98 |
| CS/STiO2–1 | 17.84 ± 2.02 | 23.54 ± 2.22 |
| CS/STiO2–3 | 21.32 ± 3.33 | 19.57 ± 4.20 |
| CS/STiO2–5 | 23.27 ± 0.92 | 21.59 ± 5.70 |
| CS/STiO2–7 | 25.30 ± 2.69 | 19.34 ± 4.35 |
| Nafion 117 | 27 | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, S.; Arshad, T.; Zada, A.; Afzal, A.; Khan, M.; Hussain, A.; Hassan, M.; Ali, M.; Xu, S. Preparation and Characterization of a Novel Sulfonated Titanium Oxide Incorporated Chitosan Nanocomposite Membranes for Fuel Cell Application. Membranes 2021, 11, 450. https://doi.org/10.3390/membranes11060450
Ahmed S, Arshad T, Zada A, Afzal A, Khan M, Hussain A, Hassan M, Ali M, Xu S. Preparation and Characterization of a Novel Sulfonated Titanium Oxide Incorporated Chitosan Nanocomposite Membranes for Fuel Cell Application. Membranes. 2021; 11(6):450. https://doi.org/10.3390/membranes11060450
Chicago/Turabian StyleAhmed, Saad, Tasleem Arshad, Amir Zada, Annum Afzal, Muhammad Khan, Amjad Hussain, Muhammad Hassan, Muhammad Ali, and Shiai Xu. 2021. "Preparation and Characterization of a Novel Sulfonated Titanium Oxide Incorporated Chitosan Nanocomposite Membranes for Fuel Cell Application" Membranes 11, no. 6: 450. https://doi.org/10.3390/membranes11060450