Cytokines IL-17, TNF and IFN-γ Alter the Expression of Antimicrobial Peptides and Proteins Disparately: A Targeted Proteomics Analysis using SOMAscan Technology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Reagents and Antibodies
2.3. Slow Off-Rate Modified Aptamer (SOMAmer®)-based Protein Array
2.4. Western Blots
2.5. ELISA
2.6. Statistical Analyses
3. Results
3.1. Chemokine Production in Response to IL-17, TNF and IFN-γ
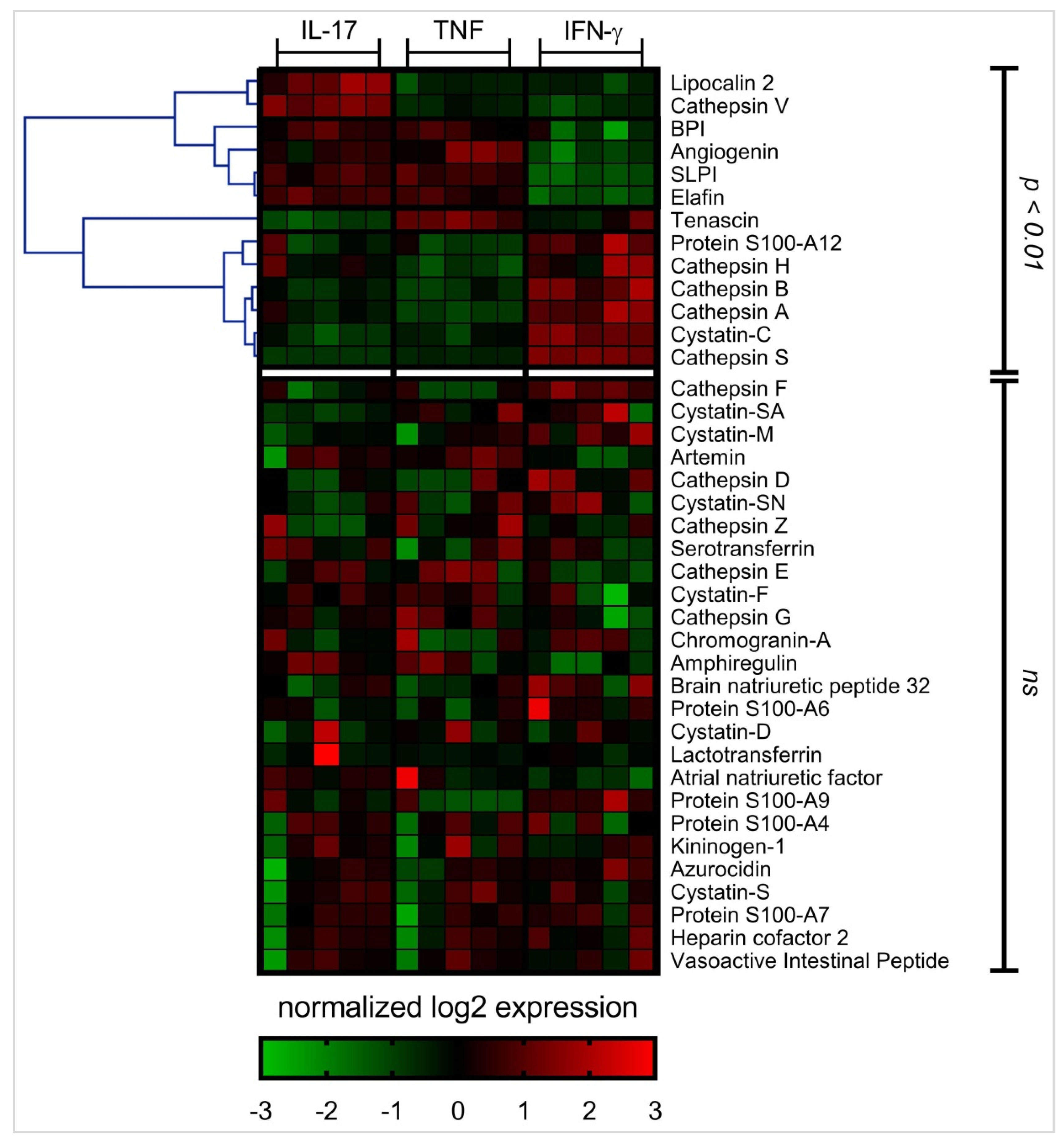
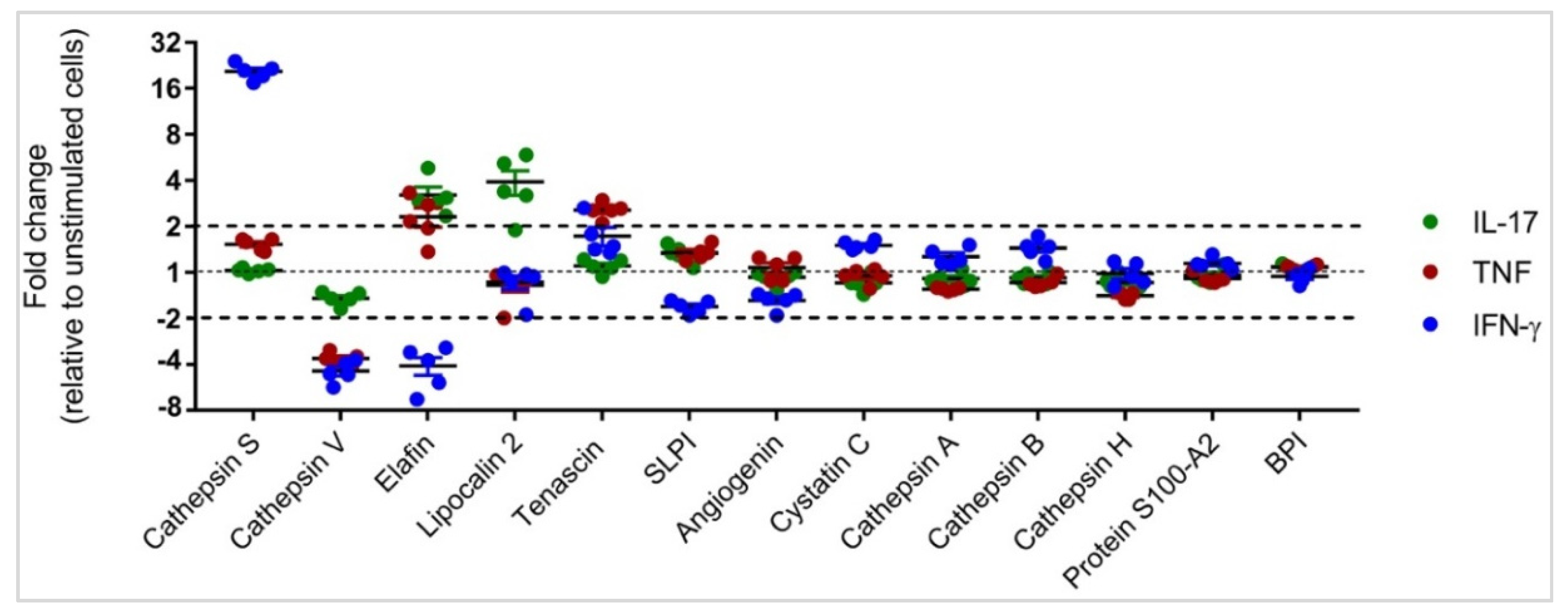
3.2. Differential APP Expression Profiles in Response to IL-17, TNF and IFN-γ
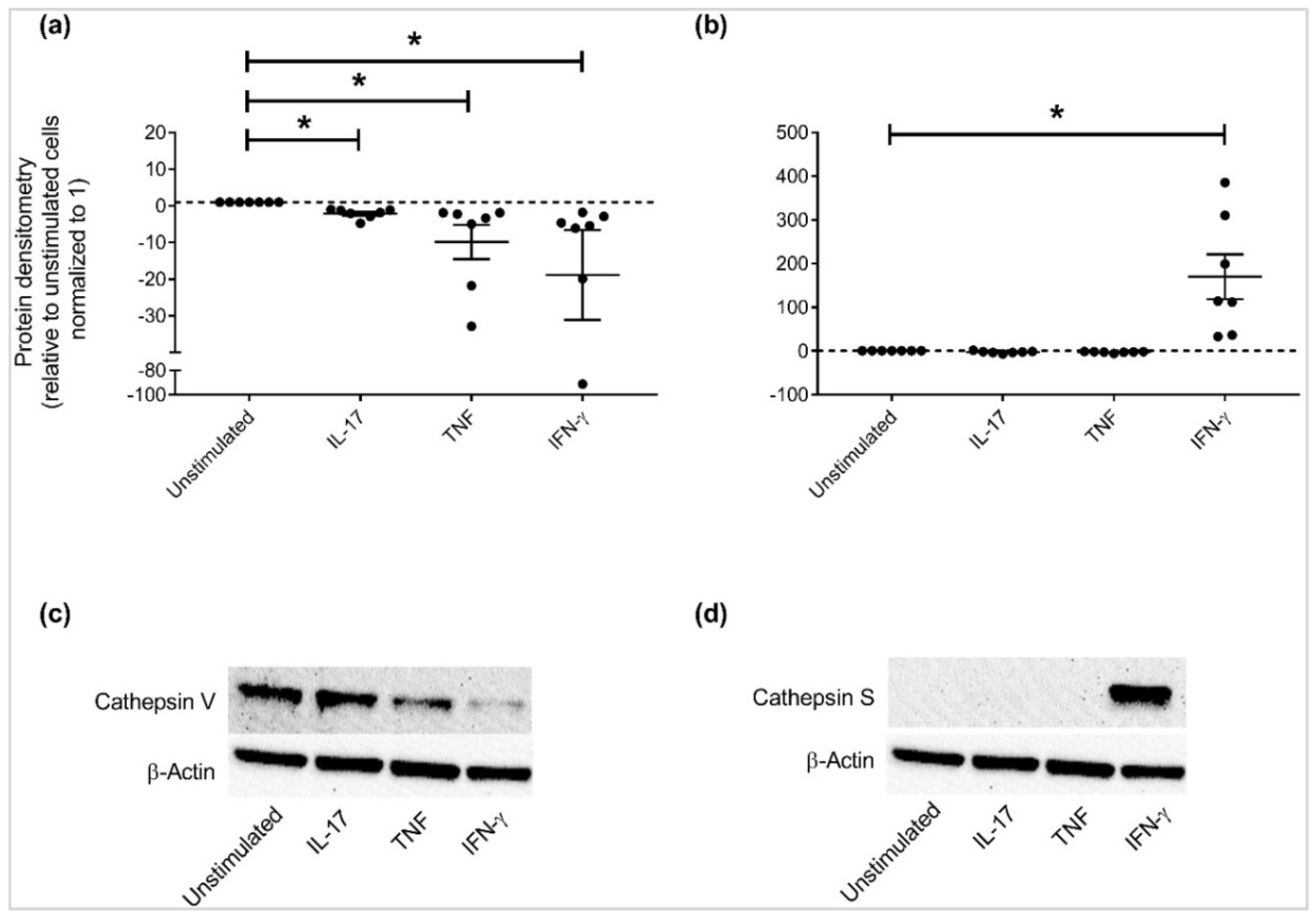
3.3. Independent Validation of Specific APP Production in HBEC
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Levy, O. Antimicrobial proteins and peptides: Anti-infective molecules of mammalian leukocytes. J. Leukoc. Biol. 2004, 76, 909–925. [Google Scholar] [CrossRef] [PubMed]
- Rogan, M.P.; Geraghty, P.; Greene, C.M.; O’Neill, S.J.; Taggart, C.C.; McElvaney, N.G. Antimicrobial proteins and polypeptides in pulmonary innate defence. Respir. Res. 2006, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.; Haney, E.F.; Gill, E.E. The immunology of host defence peptides: Beyond antimicrobial activity. Nat. Rev. Immunol. 2016, 16, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Battersby, A.J.; Khara, J.; Wright, V.J.; Levy, O.; Kampmann, B. Antimicrobial proteins and peptides in early life: Ontogeny and translational opportunities. Front. Immunol. 2016, 7, 309. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Fjell, C.D.; Hiss, J.A.; Hancock, R.E.; Schneider, G. Designing antimicrobial peptides: Form follows function. Nat. Rev. Drug Discov. 2011, 11, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Huttner, K.M.; Bevins, C.L. Antimicrobial peptides as mediators of epithelial host defense. Pediatr. Res. 1999, 45, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Allaker, R.P. Host defence peptides-a bridge between the innate and adaptive immune responses. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Mookherjee, N.; Brown, K.L.; Bowdish, D.M.; Doria, S.; Falsafi, R.; Hokamp, K.; Roche, F.M.; Mu, R.; Doho, G.H.; Pistolic, J.; et al. Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J. Immunol. 2006, 176, 2455–2464. [Google Scholar] [CrossRef] [PubMed]
- Schaller-Bals, S.; Schulze, A.; Bals, R. Increased levels of antimicrobial peptides in tracheal aspirates of newborn infants during infection. Am. J. Respir. Crit. Care Med. 2002, 165, 992–995. [Google Scholar] [CrossRef] [PubMed]
- Beisswenger, C.; Kandler, K.; Hess, C.; Garn, H.; Felgentreff, K.; Wegmann, M.; Renz, H.; Vogelmeier, C.; Bals, R. Allergic airway inflammation inhibits pulmonary antibacterial host defense. J. Immunol. 2006, 177, 1833–1837. [Google Scholar] [CrossRef] [PubMed]
- Andresen, E.; Gunther, G.; Bullwinkel, J.; Lange, C.; Heine, H. Increased expression of beta-defensin 1 (DEFB1) in chronic obstructive pulmonary disease. PLoS ONE 2011, 6, e21898. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.L.; Hiemstra, P.S.; Ward, C.; Forrest, I.A.; Murphy, D.; Proud, D.; Lordan, J.; Corris, P.A.; Fisher, A.J. Antimicrobial peptides in lung transplant recipients with bronchiolitis obliterans syndrome. Eur. Respir. J. 2008, 32, 670–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.; Jin, Y.; Carlsten, C. Inflammatory health effects of indoor and outdoor particulate matter. J. Allergy Clin. Immunol. 2018, 141, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Mookherjee, N.; Piyadasa, H.; Ryu, M.H.; Rider, C.F.; Ezzati, P.; Spicer, V.; Carlsten, C. Inhaled diesel exhaust alters the allergen-induced bronchial secretome in humans. Eur. Respir. J. 2018, 51. [Google Scholar] [CrossRef] [PubMed]
- Piyadasa, H.; Hemshekhar, M.; Carlsten, C.; Mookherjee, N. Inhaled diesel exhaust decreases the antimicrobial peptides alpha-defensin and S100A7 in human bronchial secretions. Am. J. Respir. Crit. Care Med. 2018, 197, 1358–1361. [Google Scholar] [CrossRef] [PubMed]
- Diamond, G.; Beckloff, N.; Ryan, L.K. Host defense peptides in the oral cavity and the lung: Similarities and differences. J. Dent. Res. 2008, 87, 915–927. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Nanzer, A.M.; Pfeffer, P.E.; Richards, D.F.; Timms, P.M.; Martineau, A.R.; Griffiths, C.J.; Corrigan, C.J.; Hawrylowicz, C.M. Distinct endotypes of steroid-resistant asthma characterized by IL-17A(high) and IFN-gamma(high) immunophenotypes: Potential benefits of calcitriol. J. Allergy Clin. Immunol. 2015, 136, 628–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, M.; Brightling, C.; Pavord, I.; Wardlaw, A. TNF-alpha in asthma. Curr. Opin. Pharmacol. 2007, 7, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.A.; Hargadon, B.; Shelley, M.; Parker, D.; Shaw, D.E.; Green, R.H.; Bradding, P.; Brightling, C.E.; Wardlaw, A.J.; Pavord, I.D. Evidence of a role of tumor necrosis factor alpha in refractory asthma. N. Engl. J. Med. 2006, 354, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Immunology of asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 2008, 8, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.E. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat. Med. 2012, 18, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Gaffen, S.L. Structure and signalling in the IL-17 receptor family. Nat. Rev. Immunol. 2009, 9, 556–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKinley, L.; Alcorn, J.F.; Peterson, A.; Dupont, R.B.; Kapadia, S.; Logar, A.; Henry, A.; Irvin, C.G.; Piganelli, J.D.; Ray, A.; et al. TH17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J. Immunol. 2008, 181, 4089–4097. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.S.; Yates, D.H.; Barnes, P.J. Tumor necrosis factor-alpha increases airway responsiveness and sputum neutrophilia in normal human subjects. Am. J. Respir. Crit. Care Med. 1995, 152, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Borish, L.; Steinke, J.W. Interleukin-33 in asthma: How big of a role does it play? Curr. Allergy Asthma Rep. 2011, 11, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Tworoger, S.S.; Stampfer, M.J.; Dillon, S.T.; Gu, X.; Sawyer, S.J.; Chan, A.T.; Libermann, T.A.; Eliassen, A.H. Stability and reproducibility of proteomic profiles measured with an aptamer-based platform. Sci. Rep. 2018, 8, 8382. [Google Scholar] [CrossRef] [PubMed]
- Webber, J.; Stone, T.C.; Katilius, E.; Smith, B.C.; Gordon, B.; Mason, M.D.; Tabi, Z.; Brewis, I.A.; Clayton, A. Proteomics analysis of cancer exosomes using a novel modified aptamer-based array (SOMAscan) platform. Mol. Cell Proteom. 2014, 13, 1050–1064. [Google Scholar] [CrossRef] [PubMed]
- Carlsten, C.; Blomberg, A.; Pui, M.; Sandstrom, T.; Wong, S.W.; Alexis, N.; Hirota, J. Diesel exhaust augments allergen-induced lower airway inflammation in allergic individuals: A controlled human exposure study. Thorax 2016, 71, 35–44. [Google Scholar] [CrossRef] [PubMed]
- McAllister, F.; Henry, A.; Kreindler, J.L.; Dubin, P.J.; Ulrich, L.; Steele, C.; Finder, J.D.; Pilewski, J.M.; Carreno, B.M.; Goldman, S.J.; et al. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony-stimulating factor in bronchial epithelium: Implications for airway inflammation in cystic fibrosis. J. Immunol. 2005, 175, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Piyadasa, H.; Hemshekhar, M.; Altieri, A.; Basu, S.; van der Does, A.M.; Halayko, A.J.; Hiemstra, P.S.; Mookherjee, N. Immunomodulatory innate defence regulator (IDR) peptide alleviates airway inflammation and hyper-responsiveness. Thorax 2018. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.E.; Chan, K. Interleukin-17 stimulates the expression of interleukin-8, growth-related oncogene-alpha, and granulocyte-colony-stimulating factor by human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2002, 26, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Van der Velden, V.H.; Verheggen, M.M.; Bernasconi, S.; Sozzani, S.; Naber, B.A.; van der Linden-van Beurden, C.A.; Hoogsteden, H.C.; Mantovani, A.; Versnel, M. Interleukin-1beta and interferon-gamma differentially regulate release of monocyte chemotactic protein-1 and interleukin-8 by human bronchial epithelial cells. Eur. Cytokine Netw. 1998, 9, 269–277. [Google Scholar] [PubMed]
- Wouters, E.F.; Reynaert, N.L.; Dentener, M.A.; Vernooy, J.H. Systemic and local inflammation in asthma and chronic obstructive pulmonary disease: Is there a connection? Proc. Am. Thorac. Soc. 2009, 6, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Manni, M.L.; Robinson, K.M.; Alcorn, J.F. A tale of two cytokines: IL-17 and IL-22 in asthma and infection. Expert Rev. Respir. Med. 2014, 8, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wong, C.K.; Yin, Y.; Lam, C.W. Activation of human bronchial epithelial cells by inflammatory cytokines IL-27 and TNF-alpha: Implications for immunopathophysiology of airway inflammation. J. Cell Physiol. 2010, 223, 788–797. [Google Scholar] [PubMed]
- Fujimoto, K.; Imaizumi, T.; Yoshida, H.; Takanashi, S.; Okumura, K.; Satoh, K. Interferon-gamma stimulates fractalkine expression in human bronchial epithelial cells and regulates mononuclear cell adherence. Am. J. Respir. Cell Mol. Biol. 2001, 25, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.S.; O’Toole, R.F. Convergence in the Epidemiology and Pathogenesis of COPD and Pneumonia. COPD 2016, 13, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Rohde, G.; Message, S.D.; Haas, J.J.; Kebadze, T.; Parker, H.; Laza-Stanca, V.; Khaitov, M.R.; Kon, O.M.; Stanciu, L.A.; Mallia, P.; et al. CXC chemokines and antimicrobial peptides in rhinovirus-induced experimental asthma exacerbations. Clin. Exp. Allergy 2014, 44, 930–999. [Google Scholar] [CrossRef] [PubMed]
- Wright, T.K.; Gibson, P.G.; Simpson, J.L.; McDonald, V.M.; Wood, L.G.; Baines, K.J. Neutrophil extracellular traps are associated with inflammation in chronic airway disease. Respirology 2016, 21, 467–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibata, Y.; Abe, S.; Inoue, S.; Takabatake, N.; Igarashi, A.; Takeishi, Y.; Sata, M.; Kubota, I. Altered expression of antimicrobial molecules in cigarette smoke-exposed emphysematous mice lungs. Respirology 2008, 13, 1061–1065. [Google Scholar] [CrossRef] [PubMed]
- Ruddy, M.J.; Wong, G.C.; Liu, X.K.; Yamamoto, H.; Kasayama, S.; Kirkwood, K.L.; Gaffen, S.L. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J. Biol. Chem. 2004, 279, 2559–2567. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, G.; Minton, J.E.; Ross, C.R.; Blecha, F. Regulation of cathelicidin gene expression: Induction by lipopolysaccharide, interleukin-6, retinoic acid, and Salmonella enterica serovar typhimurium infection. Infect. Immun. 2000, 68, 5552–5558. [Google Scholar] [CrossRef] [PubMed]
- Bando, M.; Zou, X.; Hiroshima, Y.; Kataoka, M.; Ross, K.F.; Shinohara, Y.; Nagata, T.; Herzberg, M.C.; Kido, J. Mechanism of interleukin-1alpha transcriptional regulation of S100A9 in a human epidermal keratinocyte cell line. Biochim. Biophys. Acta 2013, 1829, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Harkonen, E.; Virtanen, I.; Linnala, A.; Laitinen, L.L.; Kinnula, V.L. Modulation of fibronectin and tenascin production in human bronchial epithelial cells by inflammatory cytokines in vitro. Am. J. Respir. Cell Mol. Biol. 1995, 13, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Van der Velden, J.L.; Ye, Y.; Nolin, J.D.; Hoffman, S.M.; Chapman, D.G.; Lahue, K.G.; Abdalla, S.; Chen, P.; Liu, Y.; Bennett, B.; et al. JNK inhibition reduces lung remodeling and pulmonary fibrotic systemic markers. Clin. Transl. Med. 2016, 5, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, H.; Endres, J.; Fox, D.A. The roles of IFN-gamma versus IL-17 in pathogenic effects of human Th17 cells on synovial fibroblasts. Mod. Rheumatol. 2013, 23, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, J.R.; Borregaard, N.; Cowland, J.B. Induction of neutrophil gelatinase-associated lipocalin expression by co-stimulation with interleukin-17 and tumor necrosis factor-alpha is controlled by IkappaB-zeta but neither by C/EBP-beta nor C/EBP-delta. J. Biol. Chem. 2010, 285, 14088–14100. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, A.M.; Meyer, H.A.; Hamelmann, E. The role of lipocalins in airway disease. Clin. Exp. Allergy 2013, 43, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Onishi, R.M.; Gaffen, S.L. Interleukin-17 and its target genes: Mechanisms of interleukin-17 function in disease. Immunology 2010, 129, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.; Weldon, S.; Taggart, C.C. SLPI and elafin: Multifunctional antiproteases of the WFDC family. Biochem. Soc. Trans. 2011, 39, 1437–1440. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.S.; Tseng, Y.T.; Chen, P.S.; Lin, M.C.; Wu, C.C.; Huang, M.S.; Wang, C.C.; Chen, K.S.; Lin, Y.C.; Wang, T.N.; et al. Protective effects of elafin against adult asthma. Allergy Asthma Proc. 2016, 37, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Storm van’s Gravesande, K.; Layne, M.D.; Ye, Q.; Le, L.; Baron, R.M.; Perrella, M.A.; Santambrogio, L.; Silverman, E.S.; Riese, R.J. IFN regulatory factor-1 regulates IFN-gamma-dependent cathepsin S expression. J. Immunol. 2002, 168, 4488–4494. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Kang, M.J.; Crothers, K.; Zhu, Z.; Liu, W.; Lee, C.G.; Rabach, L.A.; Chapman, H.A.; Homer, R.J.; Aldous, D.; et al. Role of cathepsin S-dependent epithelial cell apoptosis in IFN-gamma-induced alveolar remodeling and pulmonary emphysema. J. Immunol. 2005, 174, 8106–8115. [Google Scholar] [CrossRef] [PubMed]
- Sukhova, G.K.; Shi, G.P.; Simon, D.I.; Chapman, H.A.; Libby, P. Expression of the elastolytic cathepsins S and K in human atheroma and regulation of their production in smooth muscle cells. J. Clin. Investig. 1998, 102, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Taggart, C.C.; Greene, C.M.; Smith, S.G.; Levine, R.L.; McCray, P.B., Jr.; O’Neill, S.; McElvaney, N.G. Inactivation of human beta-defensins 2 and 3 by elastolytic cathepsins. J. Immunol. 2003, 171, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Lecaille, F.; Naudin, C.; Sage, J.; Joulin-Giet, A.; Courty, A.; Andrault, P.M.; Veldhuizen, R.A.W.; Possmayer, F.; Lalmanach, G. Specific cleavage of the lung surfactant protein A by human cathepsin S may impair its antibacterial properties. Int. J. Biochem. Cell Biol. 2013, 45, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, Y.; Li, Z.; Greenbaum, D.; Bogyo, M.; Weber, E.; Bromme, D. Cathepsin V, a novel and potent elastolytic activity expressed in activated macrophages. J. Biol. Chem. 2004, 279, 36761–36770. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altieri, A.; Piyadasa, H.; Recksiedler, B.; Spicer, V.; Mookherjee, N. Cytokines IL-17, TNF and IFN-γ Alter the Expression of Antimicrobial Peptides and Proteins Disparately: A Targeted Proteomics Analysis using SOMAscan Technology. Vaccines 2018, 6, 51. https://doi.org/10.3390/vaccines6030051
Altieri A, Piyadasa H, Recksiedler B, Spicer V, Mookherjee N. Cytokines IL-17, TNF and IFN-γ Alter the Expression of Antimicrobial Peptides and Proteins Disparately: A Targeted Proteomics Analysis using SOMAscan Technology. Vaccines. 2018; 6(3):51. https://doi.org/10.3390/vaccines6030051
Chicago/Turabian StyleAltieri, Anthony, Hadeesha Piyadasa, Breann Recksiedler, Victor Spicer, and Neeloffer Mookherjee. 2018. "Cytokines IL-17, TNF and IFN-γ Alter the Expression of Antimicrobial Peptides and Proteins Disparately: A Targeted Proteomics Analysis using SOMAscan Technology" Vaccines 6, no. 3: 51. https://doi.org/10.3390/vaccines6030051




