Vaccination with Combination DNA and Virus-Like Particles Enhances Humoral and Cellular Immune Responses upon Boost with Recombinant Modified Vaccinia Virus Ankara Expressing Human Immunodeficiency Virus Envelope Proteins
Abstract
:1. Introduction
2. Materials and Methods
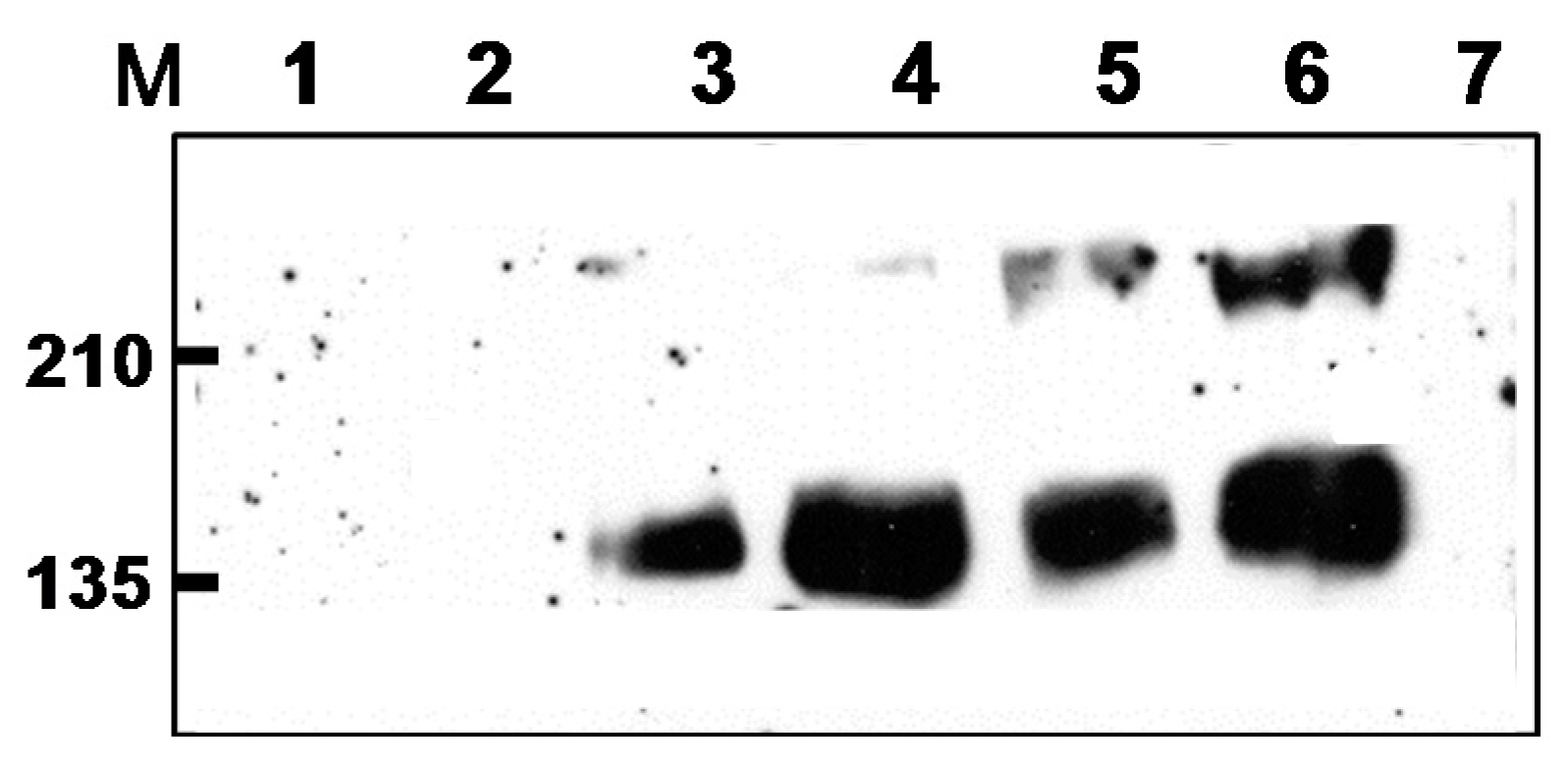
2.1. Preparation of DNA, rMVA Expressing tPAhFLex-gp120, and VLP Vaccines
2.2. Mice and Immunizations
2.3. Preparation of Peripheral Blood Mononuclear Cells (PBMCs) and Intracellular Cytokine Staining (ICC) Assay
2.4. DC Preparation and CD4+ T Cell Isolation
2.5. Lymphoproliferation and Cytokine Production In Vitro
2.6. ELISA (Enzyme-Linked Immunosorbent Assay)
2.7. ELISPOT (Enzyme-Linked ImmunoSpot)
2.8. Statistical Analysis
3. Results
3.1. Boost with Combination VLP+DNA Vaccines Prior to rMVA Enhances HIV Env gp120-Specific Antibody Responses
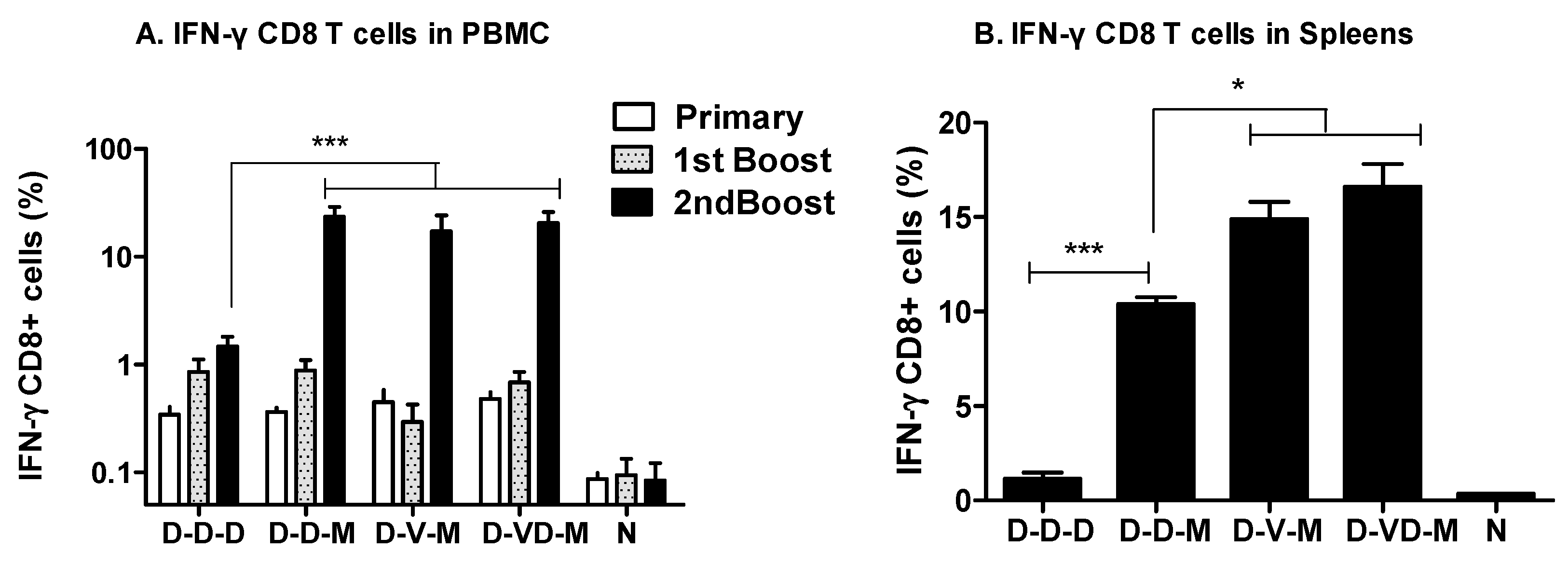
3.2. Boosting with Combination VLP + DNA Vaccines Prior to rMVA Enhances IFN-γ CD8 T Cells in Spleens
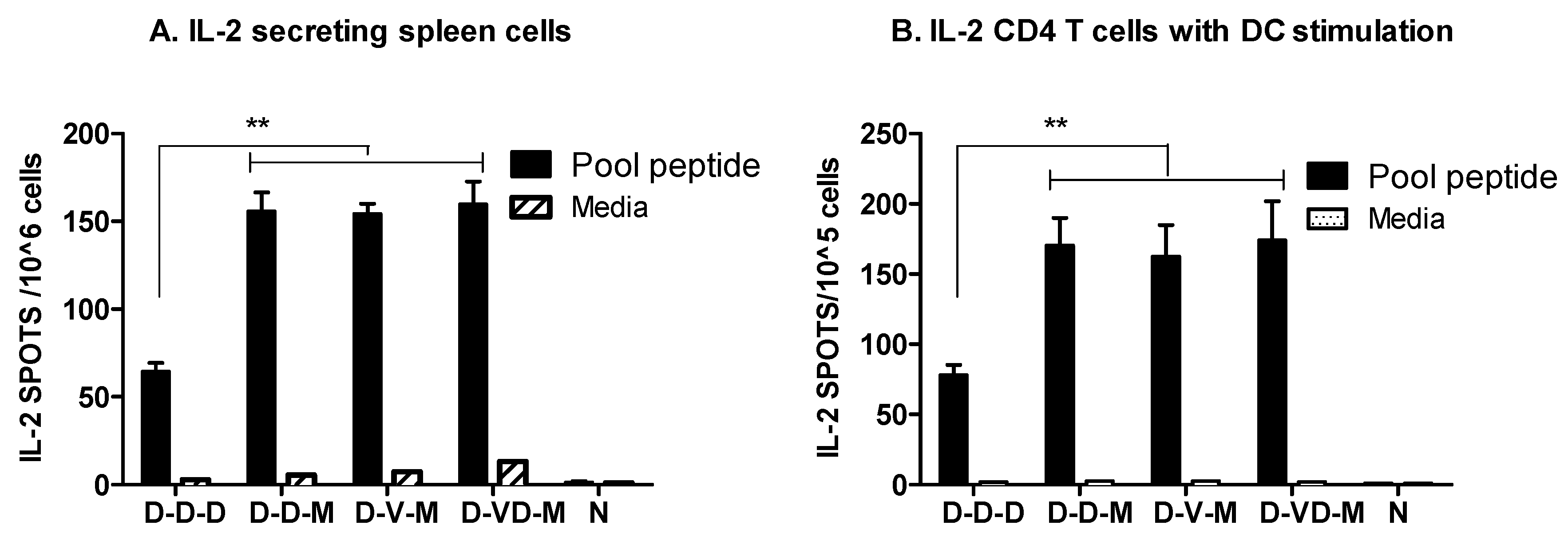
3.3. Boost with a Combination of VLP and DNA Vaccine Prior to rMVA Boost Effectively Induces IFN-γ CD4+ T Cells
3.4. Combination of VLP and DNA Vaccination Effectively Induces Splenocyte Proliferation Responses
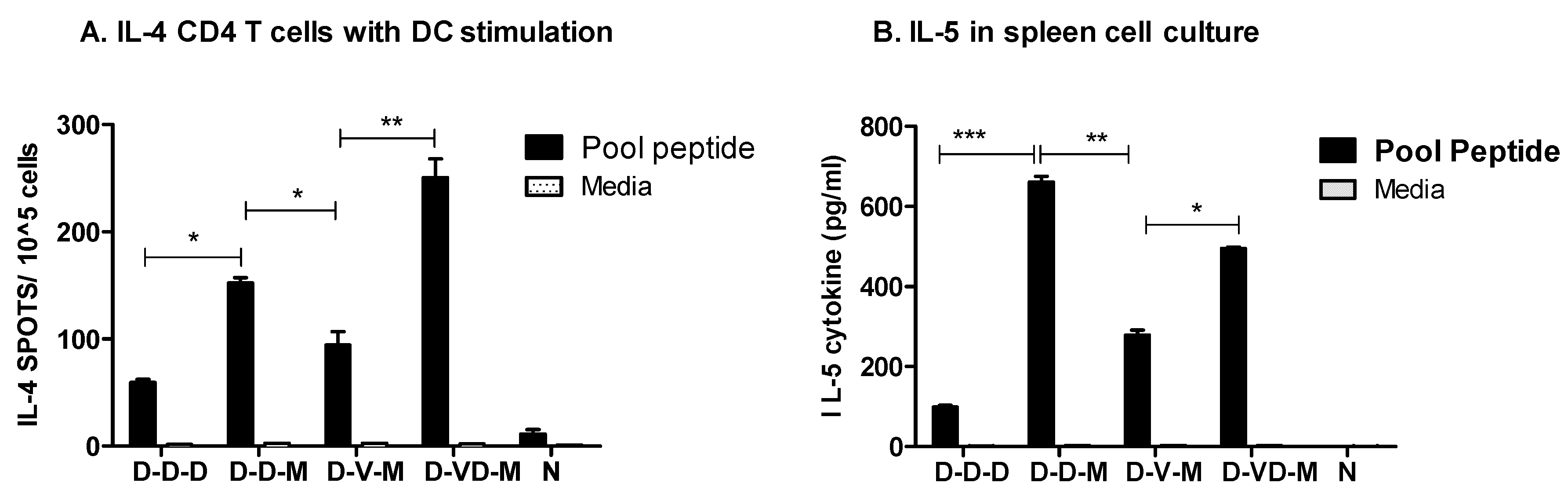
3.5. Boost with Combination VLP + DNA Vaccines Prior to rMVA Is Effective in Induceing IL-4 CD4+ T Cells
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Desrosiers, R.C. Prospects for an AIDS vaccine. Nat. Med. 2004, 10, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Baba, T.W.; Liska, V.; Khimani, A.H.; Ray, N.B.; Dailey, P.J.; Penninck, D.; Bronson, R.; Greene, M.F.; McClure, H.M.; Martin, L.N.; et al. Live attenuated, multiply deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat. Med. 1999, 5, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Sekaly, R.P. The failed HIV Merck vaccine study: A step back or a launching point for future vaccine development? J. Exp. Med. 2008, 205, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Valentine, L.E.; Watkins, D.I. Relevance of studying T cell responses in SIV-infected rhesus macaques. Trends Microbiol. 2008, 16, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Watkins, D.I.; Burton, D.R.; Kallas, E.G.; Moore, J.P.; Koff, W.C. Nonhuman primate models and the failure of the Merck HIV-1 vaccine in humans. Nat. Med. 2008, 14, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Rerks-Ngarm, S.; Pitisuttithum, P.; Nitayaphan, S.; Kaewkungwal, J.; Chiu, J.; Paris, R.; Premsri, N.; Namwat, C.; de Souza, M.; Adams, E.; et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 2009, 361, 2209–2220. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, R.S.; Paradis, T.; Hartshorn, K.L.; Vogt, M.; Ho, D.D.; Hirsch, M.S.; Leban, J.; Sato, V.L.; Schooley, R.T. Antibody-dependent cell-mediated cytotoxicity against cells infected with the human immunodeficiency virus. J. Infect. Dis. 1987, 156, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.A.; Thomson-Honnebier, G.; Steimer, K.; Paoletti, E.; Perkus, M.E.; Hollander, H.; Levy, J.A. Antibody-dependent cellular cytotoxicity is directed against both the gp120 and gp41 envelope proteins of HIV. AIDS 1989, 3, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Blue, C.E.; Spiller, O.B.; Blackbourn, D.J. The relevance of complement to virus biology. Virology 2004, 319, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Jayasekera, J.P.; Moseman, E.A.; Carroll, M.C. Natural antibody and complement mediate neutralization of influenza virus in the absence of prior immunity. J. Virol. 2007, 81, 3487–3494. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, C.; Jia, L.; Wen, C.; Liu, H.; Wang, Y.; Sun, Y.; Huang, L.; Zhou, Y.; Song, H. A novel approach to inhibit HIV-1 infection and enhance lysis of HIV by a targeted activator of complement. Virol. J. 2009, 6. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; Amara, R.R. DNA/MVA Vaccines for HIV/AIDS. Vaccines (Basel) 2014, 2, 160–178. [Google Scholar] [CrossRef] [PubMed]
- Hanke, T.; McMichael, A.J.; Mwau, M.; Wee, E.G.; Ceberej, I.; Patel, S.; Sutton, J.; Tomlinson, M.; Samuel, R.V. Development of a DNA-MVA/HIVA vaccine for Kenya. Vaccine 2002, 20, 1995–1998. [Google Scholar] [CrossRef]
- Goepfert, P.A.; Elizaga, M.L.; Sato, A.; Qin, L.; Cardinali, M.; Hay, C.M.; Hural, J.; DeRosa, S.C.; DeFawe, O.D.; Tomaras, G.D.; et al. Phase 1 safety and immunogenicity testing of DNA and recombinant modified vaccinia Ankara vaccines expressing HIV-1 virus-like particles. J. Infect. Dis. 2011, 203, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Goulder, P.J.; Rowland-Jones, S.L.; McMichael, A.J.; Walker, B.D. Anti-HIV cellular immunity: Recent advances towards vaccine design. AIDS 1999, 13, S121–S136. [Google Scholar] [PubMed]
- Schmitz, J.E.; Kuroda, M.J.; Santra, S.; Sasseville, V.G.; Simon, M.A.; Lifton, M.A.; Racz, P.; Tenner-Racz, K.; Dalesandro, M.; Scallon, B.J.; et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 1999, 283, 857–860. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.A.; Mackewicz, C.E.; Barker, E. Controlling HIV pathogenesis: The role of the noncytotoxic anti-HIV response of CD8+ T cells. Immunol. Today 1996, 17, 217–224. [Google Scholar] [CrossRef]
- Lai, L.; Vodros, D.; Kozlowski, P.A.; Montefiori, D.C.; Wilson, R.L.; Akerstrom, V.L.; Chennareddi, L.; Yu, T.; Kannanganat, S.; Ofielu, L.; et al. GM-CSF DNA: An adjuvant for higher avidity IgG, rectal IgA, and increased protection against the acute phase of a SHIV-89.6P challenge by a DNA/MVA immunodeficiency virus vaccine. Virology 2007, 369, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Robinson, H.L.; Montefiori, D.C.; Villinger, F.; Robinson, J.E.; Sharma, S.; Wyatt, L.S.; Earl, P.L.; McClure, H.M.; Moss, B.; Amara, R.R. Studies on GM-CSF DNA as an adjuvant for neutralizing Ab elicited by a DNA/MVA immunodeficiency virus vaccine. Virology 2006, 352, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lai, L.; Amara, R.R.; Montefiori, D.C.; Villinger, F.; Chennareddi, L.; Wyatt, L.S.; Moss, B.; Robinson, H.L. Preclinical studies of human immunodeficiency virus/AIDS vaccines: Inverse correlation between avidity of anti-Env antibodies and peak postchallenge viremia. J. Virol. 2009, 83, 4102–4111. [Google Scholar] [CrossRef] [PubMed]
- Sailaja, G.; Husain, S.; Nayak, B.P.; Jabbar, A.M. Long-term maintenance of gp120-specific immune responses by genetic vaccination with the HIV-1 envelope genes linked to the gene encoding Flt-3 ligand. J. Immunol. 2003, 170, 2496–2507. [Google Scholar] [CrossRef] [PubMed]
- Kwa, S.; Sadagopal, S.; Shen, X.; Hong, J.J.; Gangadhara, S.; Basu, R.; Victor, B.; Iyer, S.S.; LaBranche, C.C.; Montefiori, D.C.; et al. CD40L-adjuvanted DNA/modified vaccinia virus Ankara simian immunodeficiency virus (SIV) vaccine enhances protection against neutralization-resistant mucosal SIV infection. J. Virol. 2015, 89, 4690–4695. [Google Scholar] [CrossRef] [PubMed]
- Kannanganat, S.; Wyatt, L.S.; Gangadhara, S.; Chamcha, V.; Chea, L.S.; Kozlowski, P.A.; LaBranche, C.C.; Chennareddi, L.; Lawson, B.; Reddy, P.B.; et al. High Doses of GM-CSF Inhibit Antibody Responses in Rectal Secretions and Diminish Modified Vaccinia Ankara/Simian Immunodeficiency Virus Vaccine Protection in TRIM5alpha-Restrictive Macaques. J. Immunol. 2016, 197, 3586–3596. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; Gangadhara, S.; Victor, B.; Shen, X.; Chen, X.; Nabi, R.; Kasturi, S.P.; Sabula, M.J.; Labranche, C.C.; Reddy, P.B.; et al. Virus-Like Particles Displaying Trimeric Simian Immunodeficiency Virus (SIV) Envelope gp160 Enhance the Breadth of DNA/Modified Vaccinia Virus Ankara SIV Vaccine-Induced Antibody Responses in Rhesus Macaques. J. Virol. 2016, 90, 8842–8854. [Google Scholar] [CrossRef] [PubMed]
- Quan, F.S.; Sailaja, G.; Skountzou, I.; Huang, C.; Vzorov, A.; Compans, R.W.; Kang, S.M. Immunogenicity of virus-like particles containing modified human immunodeficiency virus envelope proteins. Vaccine 2007, 25, 3841–3850. [Google Scholar] [CrossRef] [PubMed]
- Sailaja, G.; Skountzou, I.; Quan, F.S.; Compans, R.W.; Kang, S.M. Human immunodeficiency virus-like particles activate multiple types of immune cells. Virology 2007, 362, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Belyakov, I.M.; Wyatt, L.S.; Ahlers, J.D.; Earl, P.; Pendleton, C.D.; Kelsall, B.L.; Strober, W.; Moss, B.; Berzofsky, J.A. Induction of a mucosal cytotoxic T-lymphocyte response by intrarectal immunization with a replication-deficient recombinant vaccinia virus expressing human immunodeficiency virus 89.6 envelope protein. J. Virol. 1998, 72, 8264–8272. [Google Scholar] [PubMed]
- Takahashi, H.; Cohen, J.; Hosmalin, A.; Cease, K.B.; Houghten, R.; Cornette, J.L.; DeLisi, C.; Moss, B.; Germain, R.N.; Berzofsky, J.A. An immunodominant epitope of the human immunodeficiency virus envelope glycoprotein gp160 recognized by class I major histocompatibility complex molecule-restricted murine cytotoxic T lymphocytes. Proc. Natl. Acad. Sci. USA 1988, 85, 3105–3109. [Google Scholar] [CrossRef] [PubMed]
- Chamcha, V.; Kannanganat, S.; Gangadhara, S.; Nabi, R.; Kozlowski, P.A.; Montefiori, D.C.; LaBranche, C.C.; Wrammert, J.; Keele, B.F.; Balachandran, H.; et al. Strong, but Age-Dependent, Protection Elicited by a Deoxyribonucleic Acid/Modified Vaccinia Ankara Simian Immunodeficiency Virus Vaccine. Open Forum Infect. Dis. 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Nigam, P.; Earl, P.L.; Americo, J.L.; Sharma, S.; Wyatt, L.S.; Edghill-Spano, Y.; Chennareddi, L.S.; Silvera, P.; Moss, B.; Robinson, H.L.; et al. DNA/MVA HIV-1/AIDS vaccine elicits long-lived vaccinia virus-specific immunity and confers protection against a lethal monkeypox challenge. Virology 2007, 366, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.M.; Amara, R.R.; Campbell, D.; Xu, Y.; Patel, M.; Sharma, S.; Butera, S.T.; Ellenberger, D.L.; Yi, H.; Chennareddi, L.; et al. DNA/MVA vaccine for HIV type 1: Effects of codon-optimization and the expression of aggregates or virus-like particles on the immunogenicity of the DNA prime. AIDS Res. Hum. Retrovir. 2004, 20, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Amara, R.R.; Villinger, F.; Staprans, S.I.; Altman, J.D.; Montefiori, D.C.; Kozyr, N.L.; Xu, Y.; Wyatt, L.S.; Earl, P.L.; Herndon, J.G.; et al. Different patterns of immune responses but similar control of a simian-human immunodeficiency virus 89.6P mucosal challenge by modified vaccinia virus Ankara (MVA) and DNA/MVA vaccines. J. Virol. 2002, 76, 7625–7631. [Google Scholar] [CrossRef] [PubMed]
- Goepfert, P.A.; Elizaga, M.L.; Seaton, K.; Tomaras, G.D.; Montefiori, D.C.; Sato, A.; Hural, J.; DeRosa, S.C.; Kalams, S.A.; McElrath, M.J.; et al. Specificity and 6-month durability of immune responses induced by DNA and recombinant modified vaccinia Ankara vaccines expressing HIV-1 virus-like particles. J. Infect. Dis. 2014, 210, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Wen, Z.; Dong, K.; Pan, L.; Bu, Z.; Compans, R.W.; Zhang, H.; Yang, C. Immunization with a Mixture of HIV Env DNA and VLP Vaccines Augments Induction of CD8 T Cell Responses. J. Biomed. Biotechnol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Buonaguro, L.; Devito, C.; Tornesello, M.L.; Schroder, U.; Wahren, B.; Hinkula, J.; Buonaguro, F.M. DNA-VLP prime-boost intra-nasal immunization induces cellular and humoral anti-HIV-1 systemic and mucosal immunity with cross-clade neutralizing activity. Vaccine 2007, 25, 5968–5977. [Google Scholar] [CrossRef] [PubMed]
- Forsell, M.N.; Li, Y.; Sundback, M.; Svehla, K.; Liljestrom, P.; Mascola, J.R.; Wyatt, R.; Karlsson Hedestam, G.B. Biochemical and immunogenic characterization of soluble human immunodeficiency virus type 1 envelope glycoprotein trimers expressed by semliki forest virus. J. Virol. 2005, 79, 10902–10914. [Google Scholar] [CrossRef] [PubMed]
- Vasan, S.; Schlesinger, S.J.; Chen, Z.; Hurley, A.; Lombardo, A.; Than, S.; Adesanya, P.; Bunce, C.; Boaz, M.; Boyle, R.; et al. Phase 1 safety and immunogenicity evaluation of ADMVA, a multigenic, modified vaccinia Ankara-HIV-1 B’/C candidate vaccine. PLoS ONE 2010, 5, e8816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemachandra, A.; Puls, R.L.; Sirivichayakul, S.; Kerr, S.; Thantiworasit, P.; Ubolyam, S.; Cooper, D.A.; Emery, S.; Phanuphak, P.; Kelleher, A.; et al. An HIV-1 clade A/E DNA prime, recombinant fowlpox virus boost vaccine is safe, but non-immunogenic in a randomized phase 1/11a trial in Thai volunteers at low risk of HIV infection. Hum. Vaccines 2010, 6. [Google Scholar] [CrossRef] [PubMed]
- Quan, F.S.; Kim, Y.C.; Yoo, D.G.; Compans, R.W.; Prausnitz, M.R.; Kang, S.M. Stabilization of influenza vaccine enhances protection by microneedle delivery in the mouse skin. PLoS ONE 2009, 4, e7152. [Google Scholar] [CrossRef] [PubMed]
- Song, J.M.; Hossain, J.; Yoo, D.G.; Lipatov, A.S.; Davis, C.T.; Quan, F.S.; Chen, L.M.; Hogan, R.J.; Donis, R.O.; Compans, R.W.; et al. Protective immunity against H5N1 influenza virus by a single dose vaccination with virus-like particles. Virology 2010, 405, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Bosio, C.M.; Moore, B.D.; Warfield, K.L.; Ruthel, G.; Mohamadzadeh, M.; Aman, M.J.; Bavari, S. Ebola and Marburg virus-like particles activate human myeloid dendritic cells. Virology 2004, 326, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Buonaguro, L.; Tornesello, M.L.; Tagliamonte, M.; Gallo, R.C.; Wang, L.X.; Kamin-Lewis, R.; Abdelwahab, S.; Lewis, G.K.; Buonaguro, F.M. Baculovirus-derived human immunodeficiency virus type 1 virus-like particles activate dendritic cells and induce ex vivo T-cell responses. J. Virol. 2006, 80, 9134–9143. [Google Scholar] [CrossRef] [PubMed]
- Lenz, P.; Day, P.M.; Pang, Y.Y.; Frye, S.A.; Jensen, P.N.; Lowy, D.R.; Schiller, J.T. Papillomavirus-like particles induce acute activation of dendritic cells. J. Immunol. 2001, 166, 5346–5355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, M.; Chen, C.; Yao, Q. SHIV virus-like particles bind and activate human dendritic cells. Vaccine 2004, 23, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Moron, V.G.; Rueda, P.; Sedlik, C.; Leclerc, C. In vivo, dendritic cells can cross-present virus-like particles using an endosome-to-cytosol pathway. J. Immunol. 2003, 171, 2242–2250. [Google Scholar] [CrossRef] [PubMed]
- Takamura, S.; Niikura, M.; Li, T.C.; Takeda, N.; Kusagawa, S.; Takebe, Y.; Miyamura, T.; Yasutomi, Y. DNA vaccine-encapsulated virus-like particles derived from an orally transmissible virus stimulate mucosal and systemic immune responses by oral administration. Gene Ther. 2004, 11, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Klinman, D.M.; Seder, R.A. DNA vaccines: Immunology, application, and optimization. Annu. Rev. Immunol. 2000, 18, 927–974. [Google Scholar] [CrossRef] [PubMed]
- Quan, F.S.; Yoo, D.G.; Song, J.M.; Clements, J.D.; Compans, R.W.; Kang, S.M. Kinetics of immune responses to influenza virus-like particles and dose-dependence of protection with a single vaccination. J. Virol. 2009, 83, 4489–4497. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hellerstein, M.; McDonnel, M.; Amara, R.R.; Wyatt, L.S.; Moss, B.; Robinson, H.L. Dose-response studies for the elicitation of CD8 T cells by a DNA vaccine, used alone or as the prime for a modified vaccinia Ankara boost. Vaccine 2007, 25, 2951–2958. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gangadhara, S.; Kwon, Y.-M.; Jeeva, S.; Quan, F.-S.; Wang, B.; Moss, B.; Compans, R.W.; Amara, R.R.; Jabbar, M.A.; Kang, S.-M. Vaccination with Combination DNA and Virus-Like Particles Enhances Humoral and Cellular Immune Responses upon Boost with Recombinant Modified Vaccinia Virus Ankara Expressing Human Immunodeficiency Virus Envelope Proteins. Vaccines 2017, 5, 52. https://doi.org/10.3390/vaccines5040052
Gangadhara S, Kwon Y-M, Jeeva S, Quan F-S, Wang B, Moss B, Compans RW, Amara RR, Jabbar MA, Kang S-M. Vaccination with Combination DNA and Virus-Like Particles Enhances Humoral and Cellular Immune Responses upon Boost with Recombinant Modified Vaccinia Virus Ankara Expressing Human Immunodeficiency Virus Envelope Proteins. Vaccines. 2017; 5(4):52. https://doi.org/10.3390/vaccines5040052
Chicago/Turabian StyleGangadhara, Sailaja, Young-Man Kwon, Subbiah Jeeva, Fu-Shi Quan, Baozhong Wang, Bernard Moss, Richard W. Compans, Rama Rao Amara, M. Abdul Jabbar, and Sang-Moo Kang. 2017. "Vaccination with Combination DNA and Virus-Like Particles Enhances Humoral and Cellular Immune Responses upon Boost with Recombinant Modified Vaccinia Virus Ankara Expressing Human Immunodeficiency Virus Envelope Proteins" Vaccines 5, no. 4: 52. https://doi.org/10.3390/vaccines5040052





