Maternal Vaccination. Immunization of Sows during Pregnancy against ETEC Infections
Abstract
:1. The Immunodeficient Mother and Child; Just a Matter of Evolution
2. Protection by Maternal Immunity
3. Milk-Derived Immunity
4. Maternal Antibodies and Leukocytes in the Suckling Infant
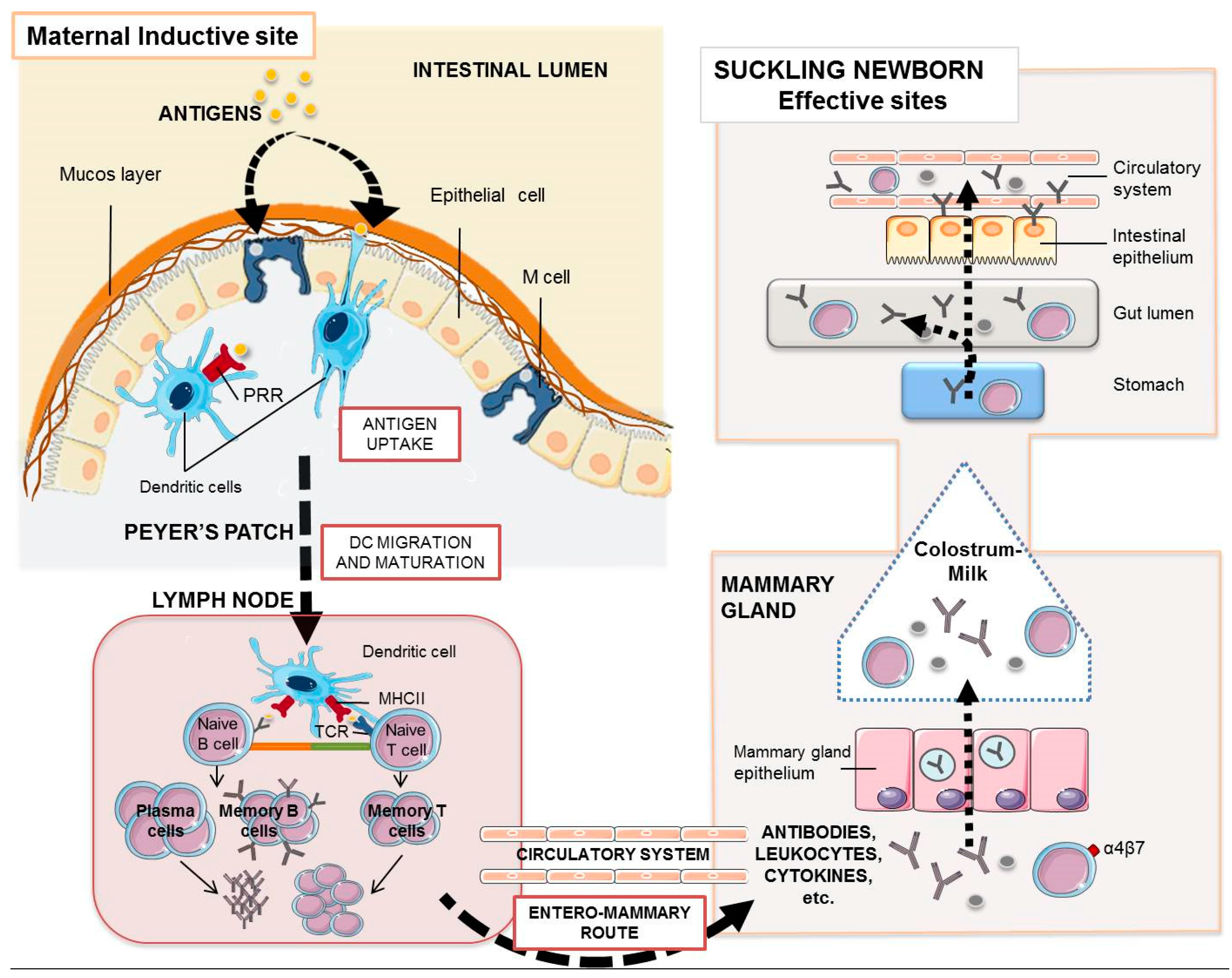
5. Acquired Specific Piglet Immunity through the Sow
6. Maternal Vaccines. Targeting the Mucosal Immune System
7. Different Types of Vaccines
8. Final Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mor, G.; Cardenas, I. The immune system in pregnancy: A unique complexity. Am. J. Reprod. Immunol. 2010, 63, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Racicot, K.; Kwon, J.Y.; Aldo, P.; Silasi, M.; Mor, G. Understanding the complexity of the immune system during pregnancy. Am. J. Reprod. Immunol. 2014, 72, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.E.; Sinkora, M.; Wertz, N.; Holtmeier, W.; Lemke, C.D. Development of the neonatal B and T cell repertoire in swine: Implications for comparative and veterinary immunology. Vet. Res. 2006, 37, 417–441. [Google Scholar] [CrossRef] [PubMed]
- Hanson, L.A.; Korotkova, M.; Lundin, S.; Håversen, L.; Silfverdal, S.A.; Mattsby-Baltzer, I.; Strandvik, B.; Telemo, E. The transfer of immunity from mother to child. Ann. N. Y. Acad. Sci. 2003, 987, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Rooke, J.A.; Bland, I.M. The acquisition of passive immunity in the new-born piglet. Livest. Prod. Sci. 2002, 78, 13–23. [Google Scholar] [CrossRef]
- Vaerman, J.P.; Langendries, A.; Pabst, R.; Rothkotter, H.J. Contribution of serum IgA to intestinal lymph IgA, and vice versa, in minipigs. Vet. Immunol. Immunopathol. 1997, 58, 301–308. [Google Scholar] [CrossRef]
- Markowska-Daniel, I.; Pomorska-mól, M.; Pejsak, Z. Dynamic changes of immunoglobulin concentrations in pig colostrum and serum around parturition. Pol. J. Vet. Sci. 2010, 13, 21–27. [Google Scholar] [PubMed]
- Markowska-Daniel, I.; Pomorska-mól, M. Shifts in immunoglobulins levels in the porcine mammary secretions during whole lactation period. Bull. Vet. Inst. Pulawy. 2010, 54, 345–349. [Google Scholar]
- Pacha, J. Development of intestinal transport function in mammals. Physiol. Rev. 2000, 80, 1633–1667. [Google Scholar] [PubMed]
- Ward, L.A.; Rich, E.D.; Besser, T.E. Role of maternally derived circulating antibodies in protection of neonatal swine against porcine group a rotavirus. J. Infect. Dis. 1996, 174, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Bradley, P.A.; Bourne, F.J.; Brown, P.J. The respiratory tract immune system in the pig. Vet. Pathol. 1976, 132, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.P. Immunomodulating effects of intestinal absorbed maternal colostral leukocytes by neonatal pigs. Can. J. Vet. Res. 1993, 57, 1–8. [Google Scholar] [PubMed]
- Schollenberger, A.; Degorski, A.; Frymus, T.; Schollenberger, A. Cells of sow mammary secretions. I. Morphology and differential counts during lactation. Zentralbl. Veterinarmed. A 1986, 33, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Schollenberger, A.; Frymus, T.; Degorski, A.; Schollenberger, A. Cells of sow mammary secretions. II. Characterization of lymphocyte populations. Zentralbl. Veterinarmed. A 1986, 33, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Salmon, H.; Berri, M.; Gerdts, V.; Meurens, F. Humoral and cellular factors of maternal immunity in swine. Dev. Comp. Immunol. 2009, 333, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Eglinton, B.A.; Roberton, D.M.; Cummins, A.G. Phenotype of T cells, their soluble receptor levels, and cytokine profile of human breast milk. Immunol. Cell Biol. 1994, 724, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Rodewald, R.; Kraehenbuhl, J.P. Receptor-mediated transport of IgG. J. Cell Biol. 1984, 99, 159s–164s. [Google Scholar] [CrossRef] [PubMed]
- Hurley, W.L.; Theil, P.K. Perspectives on immunoglobulins in colostrum and milk. Nutrients 2011, 34, 442–474. [Google Scholar] [CrossRef] [PubMed]
- Bourne, F.J.; Curtis, J. The transfer of immunoglobins IgG, IgA and IgM from serum to colostrum and milk in the sow. Immunology 1973, 241, 157–162. [Google Scholar]
- Stirling, C.M.A.; Charleston, B.; Takamatsu, H.; Claypool, S.; Lencer, W.; Blumberg, R.S.; Wileman, T.E. Characterization of the porcine neonatal Fc receptor—Potential use for trans-epithelial protein delivery. Immunology 2005, 114, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Cervenak, J.; Kacskovics, I. The neonatal Fc receptor plays a crucial role in the metabolism of IgG in livestock animals. Vet. Immunol. Immunopathol. 2009, 128, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Snoeck, V.; Peters, I.R.; Cox, E. The IgA system: A comparison of structure and function in different species. Vet. Res. 2006, 373, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Möller, R.; Hansen, G.H.; Danielsen, E.M. IgG trafficking in the adult pig small intestine: One- or bidirectional transfer across the enterocyte brush border? Histochem. Cell Biol. 2017, 1473, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Bautista, E.R.; Ramirez-Estudillo, M.C.; Rojas-Gomez, O.I.; Vega-Lopez, M.A. Tracheal and bronchial polymeric immunoglobulin secretory immune system (PISIS) development in a porcine model. Dev. Comp. Immunol. 2015, 532, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Le Jan, C. Cellular components of mammary secretions and neonatal immunity: A review. Vet. Res. 1996, 27, 403–417. [Google Scholar] [PubMed]
- Tuboly, S.; Bernath, S.; Glavits, R.; Medveczky, I. Intestinal absorption of colostral lymphoid cells in newborn piglets. Vet. Immunol. Immunopathol. 1988, 20, 75–85. [Google Scholar] [CrossRef]
- Tizard, I.R. Veterinary Immunology: An Introduction, 7th ed.; Saunders: Philadelphia, PA, USA; London, UK, 2004. [Google Scholar]
- Tanaka, Y.; Morita, C.T.; Tanaka, Y.; Nieves, E.; Brenner, M.B.; Bloom, B.R. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature 1995, 375, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Luissint, A.C.; Parkos, C.A.; Nusrat, A. Inflammation and the intestinal barrier: Leukocyte–epithelial cell interactions, cell junction remodeling, and mucosal repair. Gastroenterology 2016, 1514, 616–632. [Google Scholar] [CrossRef] [PubMed]
- Cabinian, A.; Sinsimer, D.; Tang, M.; Zumba, O.; Mehta, H.; Toma, A.; Sant’Angelo, D.; Laouar, Y.; Laouar, A. Transfer of maternal immune cells by breastfeeding: Maternal cytotoxic T lymphocytes present in breast milk localize in the peyer’s patches of the nursed infant. PLoS ONE 2016, 116, e0156762. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, L.; Muñoz, C.; Arevalo, M.; Arredondo, S.; Mendez, G. Functional capacity of colostral leukocytes from women delivering prematurely. J. Pediatr. Gastroenterol. Nutr. 1989, 81, 89–94. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Food Outlook: Biannual Report on Global Food Markets; FAO: Rome, Italy, 2017. [Google Scholar]
- Pappas, G. Socio-economic, industrial and cultural parameters of pig-borne Infections. Clin. Microbiol. Infect. 2013, 19, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Rocadembosch, J.; Amador, J.; Bernaus, J.; Font, J.; Fraile, L. Production parameters and pig production cost: Temporal evolution 2010–2014. Porcine Health Manag. 2016, 2. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A. Escherichia coli: A brief review of diarrheagenic pathotypes and their role in diarrheal diseases in Iran. Iran. J. Microbiol. 2012, 4, 102–117. [Google Scholar] [PubMed]
- Viviana, T.L.; Alicia Zon, M.; García Ovando, H.; Roberto Vettorazzi, N.; Javier Arévalo, F.; Fernández, H. Electrochemical Magneto Immunosensor Based on Endogenous B-Galactosidase Enzyme to Determine Enterotoxicogenic Escherichia coli F4 (K88) In Swine Feces Using Square Wave Voltammetry. Talanta 2017, 174, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Luppi, A.; Gibellini, M.; Gin, T.; Vangroenweghe, F.; Vandenbroucke, V.; Bauerfeind, R.; Bonilauri, P.; Labarque, G.; Hidalgo, Á. Prevalence of virulence factors in enterotoxigenic Escherichia coli isolated from pigs with post-weaning diarrhoea in Europe. Porcine Health Manag. 2016, 2. [Google Scholar] [CrossRef] [PubMed]
- Luppi, A. Swine enteric colibacillosis: Diagnosis, therapy and antimicrobial resistance. Porcine Health Manag. 2017, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- Amezcua, R.; Friendship, R.M.; Dewey, C.E.; Gyles, C.; Fairbrother, J.M. Presentation of postweaning Escherichia coli diarrhea in southern Ontario, prevalence of hemolytic E. coli serogroups involved, and their antimicrobial resistance patterns. Can. J. Vet. Res. 2002, 662, 73–78. [Google Scholar]
- Shahriar, F.; Ngeleka, M.; Gordon, J.; Simko, E. Identification by mass spectroscopy of F4ac-fimbrial-binding proteins in porcine milk and characterization of lactadherin as an inhibitor of F4ac-positive Escherichia coli attachment to intestinal villi in vitro. Dev. Comp. Immunol. 2006, 308, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Niewold, T.A.; van Dijk, A.J.; Geenen, P.L.; Roodink, H.; Margry, R.; van der Meulen, J. Dietary specific antibodies in spray-dried immune plasma prevent enterotoxigenic Escherichia coli F4 (ETEC) post weaning diarrhoea in piglets. Vet. Microbiol. 2007, 124, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.; Silva, C.; Ono, M.; Vidotto, O.; Vidotto, M. Humoral Immune Response of Immunized Sows with Recombinant Proteins of Enterotoxigenic Escherichia coli. World J. Vaccines 2015, 5, 60–68. [Google Scholar] [CrossRef]
- Fleckenstein, J.M.; Sheikh, A.; Qadri, F. Novel antigens for enterotoxigenic Escherichia coli vaccines. Expert Rev. Vaccines 2014, 135, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Gamazo, C.; Martín-Arbella, N.; Brotons, A.; Camacho, A.; Irache, J. Mimicking microbial strategies for the design of mucus-permeating nanoparticles for oral immunization. Eur. J. Pharm. Biopharm. 2015, 96, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Luo, Q.; Vickers, T.; Sheikh, A.; Lewis, W.; Fleckenstein, J.; Payne, S. Eata, an immunogenic protective antigen of enterotoxigenic Escherichia coli, degrades intestinal mucin. Infect. Immun. 2013, 82, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Vickers, T.; Fleckenstein, J. Immunogenicity and protective efficacy against enterotoxigenic Escherichia coli colonization following intradermal, sublingual, or oral vaccination with Etpa adhesin. Clin. Vaccine Immunol. 2016, 23, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.; Bartels, S.; Qadri, F.; Fleckenstein, J. Enterotoxigenic Escherichia coli Elicits Immune Responses to Multiple Surface Proteins. Infect. Immun. 2010, 78, 3027–3035. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.; Hamilton, D.; Ostmann, M.M.; Fleckenstein, J.M. Vaccination with EtpA glycoprotein or flagellin protects against colonization with enterotoxigenic Escherichia coli in a murine model. Vaccine 2009, 27, 4601–4608. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.; Hilliard, G.; Hamilton, D.; Luo, J.; Ostmann, M.; Fleckenstein, J. Enterotoxigenic Escherichia coli Etpa Mediates Adhesion Between Flagella and Host Cells. Nature 2008, 457, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.; Luo, Q.; Roy, K.; Shabaan, S.; Kumar, P.; Qadri, F.; Fleckenstein, J.M. Contribution of the Highly Conserved EaeH Surface Protein to Enterotoxigenic Escherichia coli Pathogenesis. Infect. Immun. 2014, 82, 3657–3666. [Google Scholar] [CrossRef] [PubMed]
- Van den Broeck, W.; Cox, E.; Oudega, B.; Goddeeris, B. The F4 Fimbrial Antigen of Escherichia coli and Its Receptors. Vet. Microbiol. 2000, 71, 223–244. [Google Scholar] [CrossRef]
- Verdonck, F. Oral Immunization of Piglets with Recombinant F4 Fimbrial Adhesin Faeg Monomers Induces a Mucosal and Systemic F4-Specific Immune Response. Vaccine 2004, 22, 4291–4299. [Google Scholar] [CrossRef] [PubMed]
- Nagy, B.; Fekete, P. Enterotoxigenic Escherichia coli in Veterinary Medicine. Int. J. Med. Microbiol. 2005, 295, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.; Vidotto, M.; Nascimento, K.; Santos, A.; Mechler, M.; Oliveira, L. Virulence Factors of Escherichia coli In Relation To The Importance Of Vaccination In Pigs. Ciência. Rural 2016, 46, 1430–1437. [Google Scholar] [CrossRef]
- Azegami, T.; Yuki, Y.; Kiyono, H. Challenges in mucosal vaccines for the control of infectious diseases. Int. Immunol. 2014, 269, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Pineiro, A.M.; Bergstrom, J.H.; Ermund, A.; Gustafsson, J.K.; Schutte, A.; Johansson, M.E.V.; Hansson, G.C. Studies of mucus in mouse stomach, small intestine, and colon. II. Gastrointestinal mucus proteome reveals Muc2 and Muc5ac accompanied by a set of core proteins. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 3055, G348–G356. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Gowda, D.V.; Madhunapantula, S.V.; Shinde, C.G.; Iyer, M. Mucosal vaccines: A paradigm shift in the development of mucosal adjuvants and delivery vehicles. APMIS 2015, 1234, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Salmon, H. Immunophysiology of the mammary gland and transmission of immunity to the young. Reprod. Nutr. Dev. 2003, 435, 471–475. [Google Scholar] [CrossRef]
- Vela Ramirez, J.E.; Sharpe, L.A.; Peppas, N.A. Current state and challenges in developing oral vaccines. Adv. Drug Deliv. Rev. 2017, 114, 116–131. [Google Scholar] [CrossRef] [PubMed]
- Di Pasquale, A.; Preiss, S.; Tavares da Silva, F.; Garçon, N. Vaccine adjuvants: From 1920 to 2015 and beyond. Vaccines 2015, 32, 320–343. [Google Scholar] [CrossRef] [PubMed]
- Keller-Stanislawski, B.; Englund, J.A.; Kang, G.; Mangtani, P.; Neuzil, K.; Nohynek, H.; Pless, R.; Lambach, P.; Zuber, P. Safety of immunization during pregnancy: A review of the evidence of selected inactivated and live attenuated vaccines. Vaccine 2014, 3252, 7057–7064. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.I. An assessment of enterotoxigenic Escherichia coli and Shigella vaccine candidates for infants and children. Vaccine 2015, 338, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Fairbrothera, J.M.; Nadeau, E.; Bélanger, L.; Tremblay, C.L.; Tremblay, D.; Brunelle, M.; Wolf, R.; Hellmann, K.; Hidalgo, A. Immunogenicity and protective efficacy of a single-dose live non-pathogenic Escherichia coli oral vaccine against F4-positive enterotoxigenic Escherichia coli challenge in pigs. Vaccine 2017, 352, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, É.; Fairbrother, J.M.; Zentek, J.; Bélanger, L.; Tremblay, D.; Tremblay, C.L.; Röhe, I.; Vahjen, W.; Brunelle, M.; Hellmann, K.; et al. Efficacy of a single oral dose of a live bivalent E. coli vaccine against post-weaning diarrhea due to F4 and F18-positive enterotoxigenic E. coli. Vet. J. 2017, 226, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Cox, E.; Melkebeek, V.; Devriendt, B.; Goddeeris, B.; Vanrompay, D. Vaccines against enteric E. coli infections in animals. In Pathogenic Escherichia coli: Molecular and Cellular Microbiology; Stefano, M., Ed.; Caister Academic Press: Poole, UK, 2014; pp. 255–270. [Google Scholar]
- Baxter, D. Active and Passive Immunity, Vaccine Types, Excipients and Licensing. Occup. Med. 2007, 57, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Choi, J. Tetanus–Diphtheria–Acellular Pertussis Vaccination for Adults: An Update. Clin. Exp. Vaccine Res. 2017, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.; Friberg, M.; Clements, J. LT(R192G), A non-toxic mutant of the heat-labile enterotoxin of Escherichia coli, elicits enhanced humoral and cellular immune responses associated with protection against lethal oral challenge with Salmonella Spp. Vaccine 1998, 16, 732–740. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, C.; Francis, D.; Fang, Y.; Knudsen, D.; Nataro, J.; Robertson, D. Genetic fusions of heat-labile (LT) and heat-stable (ST) toxoids of porcine enterotoxigenic Escherichia coli elicit neutralizing anti-LT and anti-STA antibodies. Infect. Immun. 2009, 78, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Snoeck, V.; Huyghebaert, N.; Cox, E.; Vermeire, A.; Vancaeneghem, S.; Remon, J.; Goddeeris, B. Enteric-coated pellets of F4 fimbriae for oral vaccination of suckling piglets against enterotoxigenic Escherichia coli infections. Vet. Immunol. Immunopathol. 2003, 96, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Irache, J.M.; Esparza, I.; Gamazo, C.; Agüeros, M.; Espuelas, S. Nanomedicine: Novel approaches in human and veterinary therapeutics. Vet. Parasitol. 2011, 180, 47–71. [Google Scholar] [CrossRef] [PubMed]
- Gamazo, C.; Gastaminza, G.; Ferrer, M.; Sanz, M.L.; Irache, J.M. Nanoparticle based-immunotherapy against allergy. Immunotherapy 2014, 67, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Gamazo, C.; Irache, J.M. Nanostructures for oral vaccine delivery. In Nanostructured Biomaterials for Overcoming Biological Barriers; Alonso, M.J., Csaba, N.S., Eds.; Royal Society of Chemistry: London, UK, 2012; Chapter 2.3; pp. 91–113. [Google Scholar]
- Kim, S.; Doh, H.; Jang, M.; Ha, Y.; Chung, S.; Park, H. Oral Immunization With Helicobacter Pylori-Loaded Poly(d,l-Lactide-co-Glycolide) Nanoparticles. Helicobacter 1999, 4, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Porporatto, C. Local and Systemic Activity of the Polysaccharide Chitosan at Lymphoid Tissues after Oral Administration. J. Leukoc. Biol. 2005, 78, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.; Ramer-Tait, A.; Broderick, S.; Kong, C.; Ulery, B.; Rajan, K.; Wannemuehler, M.; Narasimhan, B. Activation of innate immune responses in a pathogen-mimicking manner by amphiphilic polyanhydride nanoparticle adjuvants. Biomaterials 2011, 32, 6815–6822. [Google Scholar] [CrossRef] [PubMed]
- Ulery, B.; Kumar, D.; Ramer-Tait, A.; Metzger, D.; Wannemuehler, M.; Narasimhan, B. Design of a protective single-dose intranasal nanoparticle-based vaccine platform for respiratory infectious diseases. PLoS ONE 2011, 6, e17642. [Google Scholar] [CrossRef] [PubMed]
- Felder, C.; Vorlaender, N.; Gander, B.; Merkle, H.; Bertschinger, H. Microencapsulated enterotoxigenic Escherichia coli and detached fimbriae for peroral vaccination of pigs. Vaccine 2000, 19, 706–715. [Google Scholar] [CrossRef]
- Tamayo, I.; Irache, J.M.I.; Mansilla, C.; Ochoa-Reparaz, J.; Lasarte, J.; Gamazo, C. Poly(Anhydride) nanoparticles act as active Th1 adjuvants through toll-like receptor exploitation. Clin. Vaccine Immunol. 2010, 17, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Camacho, A.; Da Costa Martins, R.; Tamayo, I.; de Souza, J.; Lasarte, J.; Mansilla, C.; Esparza, I.; Irache, J.; Gamazo, C. Poly(Methyl Vinyl Ether-Co-Maleic Anhydride) nanoparticles as innate immune system activators. Vaccine 2011, 29, 7130–7135. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, K.; Melkebeek, V.; Cox, E.; Remon, J.; Vervaet, C. Erratum to “Adjuvant Effect of Gantrez®AN Nanoparticles during oral vaccination of piglets against F4+enterotoxigenic Escherichia coli. Vet. Immunol. Immunopathol. 2011, 139, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Salman, H.; Irache, J.; Gamazo, C. Immunoadjuvant Capacity of Flagellin And Mannosamine-Coated Poly(Anhydride) Nanoparticles in oral vaccination. Vaccine 2009, 27, 4784–4790. [Google Scholar] [CrossRef] [PubMed]
- Jose Matías, J.; Lasierra, T.; Pérez-Guzmán, I.; Cenoz, S.; Irache, J.M.I.; Gamazo, C. Nanoparticles Formulated from Protein Food-Born Polymers in the Development of a Mucosal Complex Vaccine against ETEC. Unpublishwed observation. 2016. [Google Scholar]
- Davis, H. Novel vaccines and adjuvant systems: The utility of animal models for predicting immunogenicity in humans. Hum. Vaccine 2008, 4, 246–250. [Google Scholar] [CrossRef]

| Vaccine | Composition | Route | Adjuvant | Weeks before Farrowing | Manufacturer |
|---|---|---|---|---|---|
| Porcilis® coli | LT toxoid | Parenteral | Unvaccinated gilts and sows | MSD Animal Health (Kenilworth, NJ, USA) | |
| Fimbriae (F4ab, F4ac, F5, F6) | 1st dose: 6–8 weeks 2nd dose: in the second half of pregnancy | ||||
| Porcilis® 2 * 4 * 3 | - ETEC bacterins: K88, K99, 987P, F4. | Parenteral | Unvaccinated gilts and sows | MSD Animal Health (Kenilworth, NJ, USA) | |
| - Inactivated Clostridium perfringens type C | 1st dose: 6–8 weeks 2nd dose: in the second half of pregnancy | ||||
| Suiseng® | - LT toxoid | Parenteral | Unvaccinated gilts | HIPRA (Gerona, Spain) | |
| Fimbriae (F4ab, F4ac, F5, F6) | 1st dose: 6 weeks | ||||
| - β-toxoid of Clostridium perfringens type C. | 2nd dose: 3 weeks | ||||
| Sows | |||||
| One dose: 2–3 weeks | |||||
| PILI SHIELD® | ETEC bacterins (K88, K99, 987P, F41 strains). | Parenteral | Unvaccinated gilts | Elanco (Greenfiled, IND, USA) | |
| 1st dose: 5 weeks | |||||
| 2nd dose: 2 weeks | |||||
| Sows | |||||
| One dose: 2 weeks before delivery | |||||
| SERKEL GASTRO RV® | - ETEC bacterins: (K88, 987P, K99, F41) | Parenteral | Unvaccinated gilts | Vencofarma (Paraná, Brazil) | |
| - Inactivated Rotavirus | 1st dose: 5 weeks | ||||
| - Toxoids from Clostridium perfringens type C and D. | 2nd dose: 2 weeks | ||||
| Sows | |||||
| One dose: 2 weeks | |||||
| Clostricol | - Escherichia coli bacterins K87, K88; O149: K91, K88; O101: K (A, K99, 987p. | Subcutaneous | Aluminium hydroxide | Sows | IDT Biologika GmbH (Dessau-Roßlau, Germany) |
| - Clostridium perfringens type C toxoid. | 1st dose: 5 weeks 2nd dose 2 weeks | ||||
| Colidex-C | - Escherichia coli bacterins K88, K99, F41, F18, P987. | Parenteral | Mineral oil | Unvaccinated gilts | CZ Veterinaria S.A. (Porriño, Spain) |
| - Clostridium perfringens type C toxoid. | 1st dose: 7 weeks | ||||
| 2nd dose 4 weeks | |||||
| Revaccinated Sows | |||||
| One dose: 4 weeks | |||||
| Coliporc PLUS | Escherichia coli bacterins O8; K87, K88 (F4); O149: K91, K88; O101 K99. | Subcutaneous | Aluminium hydroxide | Sows | IDT Biologika GmbH (Dessau-Roßlau, Germany) |
| 1st dose: 5 weeks 2nd dose: 2 weeks | |||||
| Colisuin-CL | - Escherichia coli fimbriae: 987P, K88ab, K88ac, K99 | Parenteral | Oil adjuvant | Unvaccinated gilts | HIPRA (Gerona, Spain) |
| - Clostridium perfringens type C toxoid. | - 1st dose: 8 weeks | ||||
| - Clostridium novyi toxoid. | - 2nd dose: 4 weeks | ||||
| Sows | |||||
| One dose: 4 weeks | |||||
| Colisuin-TP | Escherichia coli fimbriae: 987P, K88ab, K88ac, K99. | Parenteral | Liquid paraffin, Montanide 888 | Unvaccinated gilts | HIPRA (Gerona, Spain) |
| - 1st dose: 8 weeks | |||||
| - 2nd dose: 4 weeks | |||||
| Sows | |||||
| One dose: 4 weeks | |||||
| Combined Gastroenteritis, Rotavirus and E. coli | - Inactivated Rotavirus | Intranasal, intramuscular | Oil emulsion | The emulsified vaccine is administered twice: on the 5–6 weeks and 2–3 weeks. | Narvac (Moscow, Russia) |
| - Escherichia coli somatic 09, 078, 0141; capsular polysaccharides K80, K30, K87, K88 | - 1st dose: 13–14 weeks | ||||
| - 2nd dose: 10 weeks | |||||
| The dry vaccine is administered together with the emulsified one 10 weeks | |||||
| ECOvac E. coli | Escherichia coli bacterins: K88, K99, 987P | Intramuscular | Unvaccinated gilts | MSD Animal Health (Kenilworth, NJ, USA) | |
| - 1st dose: 7 weeks | |||||
| - 2nd dose: 3 weeks | |||||
| Sows | |||||
| One dose: 3 weeks | |||||
| Combined ECOvacLE | - Escherichia coli bacterins K88, K99, 987P. | Parenteral | Unvaccinated gilts | MSD Animal Health (Kenilworth, NJ, USA) | |
| - Leptospira interrogans bacterin. | - 1st dose: at selection | ||||
| - Erysipelothrix rhusiopathiae bacterin | - 2nd dose: 4–6 weeks later | ||||
| - 3rd: 3 weeks | |||||
| Sows with unknown vaccination history: | |||||
| - two vaccinations 4–6 weeks apart. | |||||
| Revaccination | |||||
| - booster dose at 3 weeks | |||||
| Kolierysin NEO | - Escherichia coli bacterins O147:K88 (F4) ab, O149:K88 (F4) ac, O101:K99 (F5), 987P (F6) and O101:K99:F41. | Parenteral | Oil emulsion | Sows and gilts | Bioveta, A.S. (Ivanovice na Hané, Czech Republic) |
| - LT toxoid | - not later than 5 weeks | ||||
| Revaccination with the single dose of the vaccine KOLISIN NEO: 10–14 days later; | |||||
| repeated 2–3 weeks before each next expected delivery. | |||||
| Kolisin NEO | - Escherichia coli bacterin O147:K88 (F4) ab, O149:K88 (F4) ac, O101:K99 (F5), 987P (F6) and O101:K99:F41. | Parenteral | Oil emulsion | Sows and gilts | Bioveta, A.S. (Ivanovice na Hané, Czech Republic) |
| - LT toxoid | - not later than 5 weeks | ||||
| Revaccination with the single dose of the vaccine KOLISIN NEO: 10–14 days later; | |||||
| repeated 2–3 weeks before each next expected delivery. | |||||
| LitterGuard | Escherichia coli bacterins K99, K88, 987P, F41 | Parenteral | Primary vaccination: | Zoetis [Pfizer; Fort Dodge Animal Health] (Gerona-Spain) | |
| - 1st dose: 2 weeks | |||||
| - 2nd dose: 2 weeks | |||||
| Revaccination: | |||||
| - One dose: 2 weeks before each subsequent farrowing. | |||||
| LitterGuard LT-C | - Escherichia coli bacterin K99, K88, 987P, F41. | Parenteral | Primary vaccination: | Zoetis [Pfizer; Fort Dodge Animal Health] (Gerona-Spain) | |
| - Clostridium perfringens type C toxoid | - 1st dose: 4 weeks | ||||
| - 2nd dose: 2 weeks | |||||
| Revaccination: | |||||
| One dose: 2 weeks before each subsequent farrowing | |||||
| Neocolipor | Escherichia coli fimbriae: F4 (F4ab, F4ac, F4ad), F5. F6. F41. | Parenteral | Aluminium hydroxide | Primary vaccination: | Boehringer Ingelheim (Duluth, Georgia, USA) |
| - 1st dose: 5–7 weeks | |||||
| - 2nd dose: 2 weeks | |||||
| Neumosan | - Escherichia coli bacterin K99 | Subcutaneous | Aluminium hydroxide | Primary vaccination: | Laboratorios Santa Elena S.A. [Virbac] (Montevideo, Uruguay) |
| - Mannheimia haemolytica bacterin | two doses with an interval of 3–4 weeks | ||||
| - Pasteurella multocida bacterin | Revaccinate annually. | ||||
| - Salmonella enterica Dublin bacterin | |||||
| Polyvalent colibacteriosis | Escherichia coli bacterins 06, 09, 0138, 0139, 076, 0141, 0147, 0149 and K88 (optional) | Intramuscular | Aluminium hydroxide | 6–8 weeks | Diavak (Radovljica, Slovenia) |
| Porcine E. coli vaccine—Polyvalent | Escherichia coli bacterins K88, K99, 987P, F41. | Subcutaneous | Aluminium hydroxide gel | - 1st dose: 5–6 weeks | Green Cross Veterinary Products Co. Ltd. (Chungcheongnam-do, Korea) |
| - 2nd dose: 2–3 weeks | |||||
| Prefarrow Shield 9 | - Escherichia coli bacterins K88, K99, 987P, F41. | Intramuscular | Sows and gilts: | Elanco (Greenfiled, IND, USA) | |
| - Clostridium perfringens type C bacterin. | - 1st dose: 5 weeks | ||||
| - Bordetella bronchiseptica bacterin. | - 2nd dose: 2 weeks | ||||
| - Pasteurella multocida type C and D bacterin | Subsequent farrowing: | ||||
| - Erysipelothrix rhusiopathiae bacterin | One single dose | ||||
| ProSystem RCE | - Clostridium perfringens type C bacterin. | Parenteral | Primary vaccination: | MSD Animal Health (Kenilworth, NJ, USA) | |
| - Escherichia coli bacterins K88, K99, 987P, F41. | - 1st dose: 5 weeks | ||||
| - Porcine rotavirus attenuated. | - 2nd dose: 2 weeks | ||||
| In subsequent farrowings: | |||||
| One dose 2 weeks before farrowing. | |||||
| Rokovac NEO | Escherichia coli O101:K99 (F5); O147:K88 (F4); O149:K88 (F4); K85:987P (F6);O101:K99:F41 (F5, F41) | Parenteral | Oil emulsion | Primary vaccination: | Bioveta, A.S. (Ivanovice na Hané, Czech Republic) |
| - 1st dose: 4 weeks | |||||
| - 2nd dose: 2 weeks | |||||
| Revaccination: | |||||
| One dose 4–2 weeks prior to any other expected labor. | |||||
| Scourmune-C | - Escherichia coli bacterins K88, K99, 987P, F41. | Parenteral | Aluminium hydroxide | Primary vaccination: | MSD Animal Health (Kenilworth, NJ, USA) |
| - Clostridium perfringens type C bacterin. | - 1st dose: 6–7 weeks | ||||
| - 2nd dose: 3–4 weeks | |||||
| Subsequent farrowings: | |||||
| - one single dose 2–3 weeks prior to each subsequent farrowing. | |||||
| Suiven | - Escherichia coli bacterins K88, K99, 987P, F41. | Subcutaneous | Aluminum hydroxide gel | 4 weeks. | Vencofarma (Paraná, Brazil) |
| - Bordetella bronchiseptic bacterin. | |||||
| - Erysipelothrix rhusiopathiae bacterin. | |||||
| - Pasteurella multocida type A and D bacterins. | |||||
| - Salmonella enterica bacterin | |||||
| - Leptospira interrogans bacterin | |||||
| Anaerobic Enterotoxaemia and E. coli | - Escherichia coli bacterins 08, 09, 0138, 0139, 078, 0141, 0147, 0149, K88, K99. | Intramuscular | Aluminium hydroxide | - 1st dose: 5 weeks | FGUP Armavirskaja (Krasnodarskij Russia) |
| - Clostridium perfringens type C bacterin. | - 2nd dose: 3 weeks | ||||
| E. coli Inactivated | Escherichia coli bacterins KMIEV-40A, KMIEV-38, KMIEV-98, KMIEV-18 and K88, K99, F41, O18. | Intramuscular | Emulsified oil adjuvant | - 1st dose: 5–7 weeks | Institute for Experimental Veterinary-Medicine, (Kosice, Slovakia) |
| - 2nd dose: 2–3 weeks |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matías, J.; Berzosa, M.; Pastor, Y.; Irache, J.M.; Gamazo, C. Maternal Vaccination. Immunization of Sows during Pregnancy against ETEC Infections. Vaccines 2017, 5, 48. https://doi.org/10.3390/vaccines5040048
Matías J, Berzosa M, Pastor Y, Irache JM, Gamazo C. Maternal Vaccination. Immunization of Sows during Pregnancy against ETEC Infections. Vaccines. 2017; 5(4):48. https://doi.org/10.3390/vaccines5040048
Chicago/Turabian StyleMatías, Jose, Melibea Berzosa, Yadira Pastor, Juan M. Irache, and Carlos Gamazo. 2017. "Maternal Vaccination. Immunization of Sows during Pregnancy against ETEC Infections" Vaccines 5, no. 4: 48. https://doi.org/10.3390/vaccines5040048






