Equine Vaccines: How, When and Why? Report of the Vaccinology Session, French Equine Veterinarians Association, 2016, Reims
Abstract
:1. Introduction
2. What Are the Usefulness and Efficacy of Emergency Vaccination?
2.1. Equine Influenza (EI)
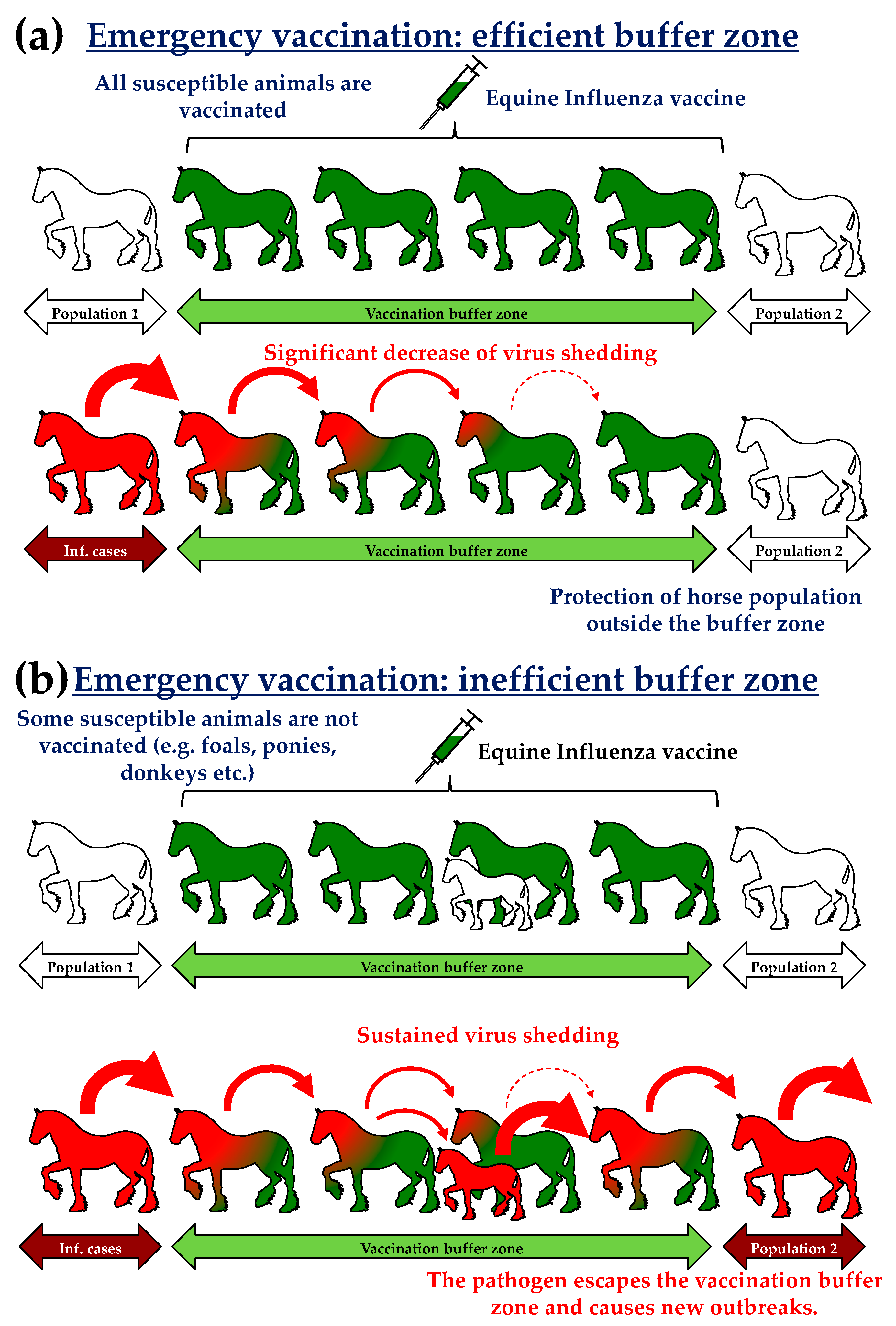
- The establishment of vaccination zones (10 km wide buffer zone; Figure 1) surrounding infectious areas (i.e., zones where infected horses have been detected). Subsequent epidemiological models and analysis revealed that the « suppressive » immunisation (i.e., 1 to 3 km radius) induced the best results with a marked reduction of new cases (−64%) and affected areas (−9%) [7,8]. The Australian state of Queensland was compartmentalised by vaccination corridors separated from each other by 25 to 35 km. Buffer zones were also used to protect 5 to 8000 wild equids in the state of New South Wales in order to avoid an endemic establishment of the disease. By the end of November, between 80% and 90% of horses in Queensland and New South Wales were vaccinated. Such levels of vaccination were above the levels usually recognised and described to provide herd immunity against infection [9].
- A targeted vaccination (or predictive) limited to specific horse populations of strategic and/or economic importance (thoroughbreds, police horses, competition horses, as examples).
- In some regions, vaccination was mandatory.
2.2. Rhinopneumonitis and Secondary Forms of the Disease
2.3. Tetanus
3. What Is the Immunological Impact of Using/Mixing Vaccines from Different Brands and Manufacturers?
3.1. Equine Influenza Vaccines
- Equine influenza outbreak in Newmarket (United Kingdom), 2003: the epidemiological analysis revealed that risk of infection with EIV was significantly reduced in animals with a mixed vaccine history. A whole inactivated EI vaccine adjuvanted with hydroxide aluminum seems to have provided limited protection during this outbreak, which in part explains the benefit of mixed vaccination (the limited efficacy induced by this vaccine was balanced when other EI vaccines were used) (Table 1) [26]. However, the time since last vaccination was one of the most important factor associated with risk of infection, explaining some of the counter-intuitive associations of infection with increased age and increased number of immunization/types of EI vaccines administered (i.e., ≥4 types in Table 1). Older horses (likely to have received multiple vaccinations and different types of EI vaccines) are likely to be immunized on an annual basis or to have lapse vaccination due to a lack of requirement. In this condition, the gap between immunisations received may be greater in older horses when compared to two-year old horses (identified as the less affected group of horses in the epidemiological study) that have recently completed their primary course of immunization (i.e., V1, V2, and V3; cf. Section 4). The limited number of horses in this specific category (i.e., ≥4 types) (n = 21) when compared with the other groups (n = 116, 161 and 103, respectively) may have also weakened the statistical analysis.
- Mandatory EI vaccination in Hong Kong (preliminary result from a current study): around 30% of the horse population in Hong Kong is renewed every year (through importation). Equine influenza vaccination is mandatory for horses that are being exported to Hong Kong (in the weeks prior to exportation). Upon arrival, horses receive a fresh primary course of EI immunization with a unique EI vaccine. Due to numerous countries of origin and the diversity of EI vaccines used worldwide, the mix of EI vaccine in recently imported Hong Kong horses is inevitable. Preliminary results demonstrate that differences between pre- and post-importation EI vaccines has no measurable impact on EIV-specific SRH antibody response [27].
3.2. Rhinopneumonitis Vaccines (Equine Herpes Virus Type 1 and 4)
3.3. Tetanus Vaccines
4. Lapse in Vaccination History and Vaccine Shortage
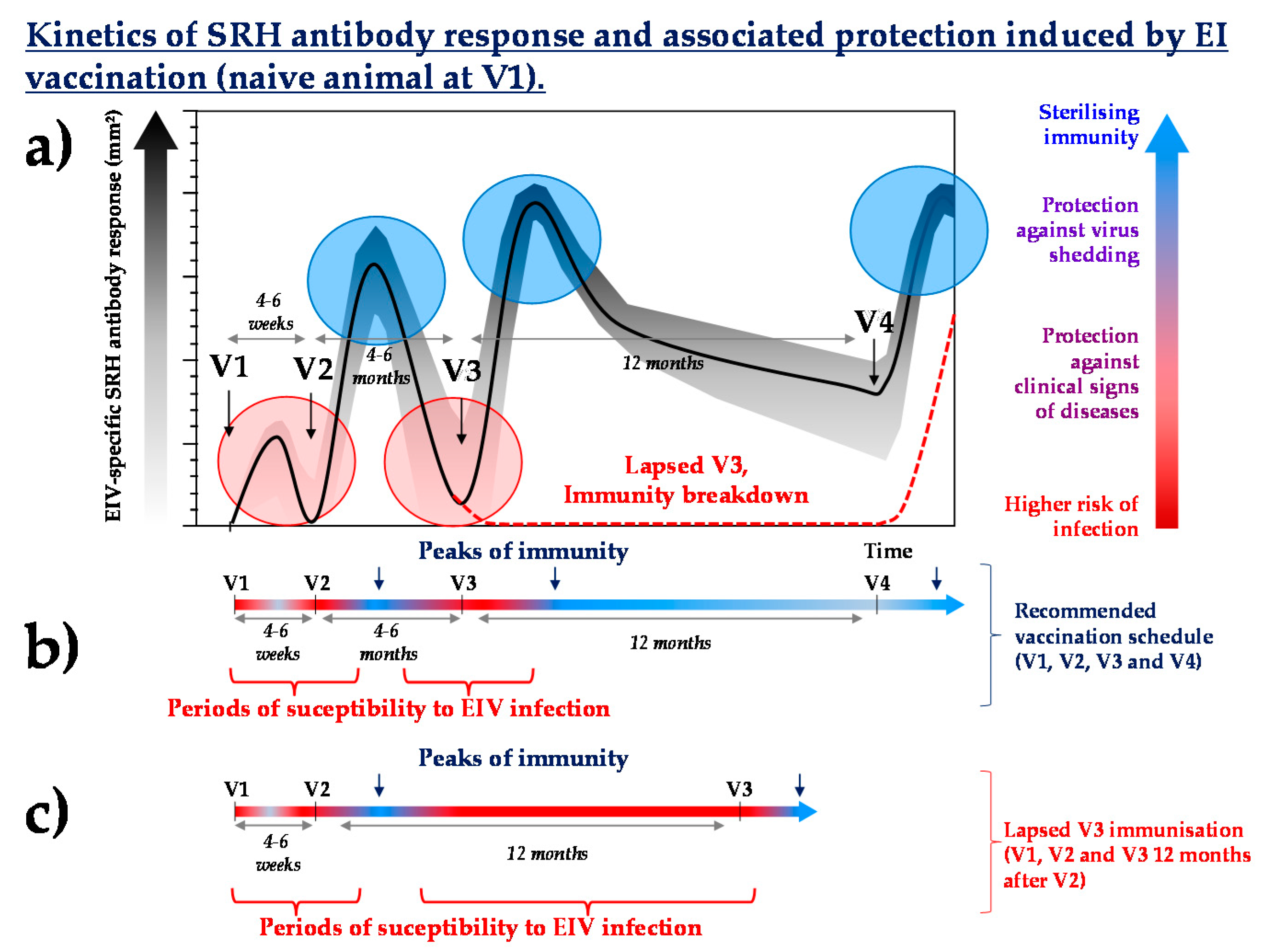
- What is the immunological history (vaccination and/or infection) of the individual? Lapsed vaccination could be a concern if it happens during the primary course of immunisation or shorty after. It is likely to be less problematic in adult horses that have been frequently and repeatedly vaccinated. For example, the absence of a third immunisation (V3) against EI (Figure 3) may increase the susceptibility to EIV infection by several months (until the next boost immunisation, usually scheduled 12 months after V3, 16 to 18 months after V2) and compromise the duration of immunity induced by the last vaccination.
- How efficacious and immunogenic is the vaccine used? Consequences of lapsed immunisation is likely to be inversely proportional to the duration of immunity of the last vaccine administered.
- What is the level of herd immunity? If the number of animals with lapsed immunisation is limited, then the risk of infection will be counterbalanced by the level of herd immunity of the surrounding population (i.e., the higher the herd immunity, the lower the risk of coming into contact with the pathogen).
- What is the risk of contact with the pathogen (i.e., isolated individual or numerous contacts with others equids)? Is there time for emergency vaccination? For most vaccines, reaching protective levels of immunity will take a few days only after boost immunisation if the horse has been frequently immunised (prior to lapse).
- In case of a vaccine shortage, veterinary practitioners have to face clients and horse owners discontent, which is justified by the concern of potential outbreaks (especially the risk of abortion in the case of EHV-1 infection). A vaccine shortage could induce a heavy financial loss (loss of commercial discounts and immobilisation of scheduled vaccine stocks).
- Both substitute products are monovalent EHV-1 vaccines. The number of doses available were limited (e.g., 150,000 doses of Bioequine H ND provided for 2016), which inevitably led numerous French veterinary clinics to prepare new stocks in order to cover the requirement. However, the use of these stockpiles of monovalent rhinopneumonitis vaccines was a concern, especially in case of the renewed availability of the bivalent EHV-1/4 vaccine.
- Financial loss also concerned horse owners, due to more expensive substitute vaccines, the absence of commercial discount for these new products and the necessity to administer new primary immunisation courses for horses with lapsed vaccination history (in order to comply with race and event regulations).
- The shortage of bivalent EHV-1/4 vaccine did not result in a notable increase of EHV-4 outbreaks being reported to the French Epidemiological Surveillance of Equine Pathologies (RESPE) between 2015 and 2016 (Figure 4). However, it had a negative impact on horse exportation due to the requirement for immunisation against both EHV-1 and EHV-4 in some countries and stud farms.
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- British Horseracing Authority. Economic Impact of British Racing, 2013; British Horseracing Authority: London, UK, 2013. [Google Scholar]
- Vial, C.; Bigot, G.; Heydemann, P.; Cordilhac, C. Les chiffres clés de la filière équine à l’international: Un essai de collecte d’informations. Equ'idée 2017. Available online: http://mediatheque.ifce.fr/doc_num.php?explnum_id=22144 (accessed on 3 November 2017). (In French).
- Paillot, R.; El-Hage, C.M. The use of a recombinant canarypox-based equine influenza vaccine during the 2007 australian outbreak: A systematic review and summary. Pathogens 2016, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Callinan, I. Equine Influenza, the August 2007 Outbreak in Australia. Available online: http://apo.org.au/files/Resource/commonwealthofaustralia_equineinfluenza_2008.pdf (accessed on 6 June 2016).
- Moloney, B.J. Overview of the epidemiology of equine influenza in the australian outbreak. Aust. Vet. J. 2011, 89, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, A.J. Equine Influenza in South Africa 2003 Outbreak. In Proceedings of the 9th International Congress of World Equine Veterinary Association, Marrakech, Morocco, 22–26 January 2006; World Equine Veterinary Association: Marrakech, Morocco, 2006. [Google Scholar]
- Garner, M.G.; Cowled, B.; East, I.J.; Moloney, B.J.; Kung, N. Evaluating the effectiveness of the response to equine influenza in the australian outbreak and the potential role of early vaccination. Aust. Vet. J. 2011, 89, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Garner, M.G.; Cowled, B.; East, I.J.; Moloney, B.J.; Kung, N.Y. Evaluating the effectiveness of early vaccination in the control and eradication of equine influenza—A modelling approach. Prev. Vet. Med. 2011, 99, 15–27. [Google Scholar] [CrossRef] [PubMed]
- May, T. Vaccines as community-focused therapy. Expert Rev. Vaccines 2003, 2, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Major, D.A.; Jones, B. Behaviour of equine influenza virus in a naive population: A practitioner’s perspective. Aust. Vet. J. 2011, 89, 13–14. [Google Scholar] [CrossRef] [PubMed]
- Kannegieter, N.J.; Frogley, A.; Crispe, E.; Kirkland, P.D. Clinical outcomes and virology of equine influenza in a naive population and in horses infected soon after receiving one dose of vaccine. Aust. Vet. J. 2011, 89, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Galvin, P.; Gildea, S.; Arkins, S.; Walsh, C.; Cullinane, A. The evaluation of a nucleoprotein elisa for the detection of equine influenza antibodies and the differentiation of infected from vaccinated horses (diva). Influenza Other Respir Viruses 2013, 7, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R. Vaccination contre l’herpèsvirus équin 1. Pratique Vétérinaire Equine 2012, 44, 15–23. [Google Scholar]
- Paillot, R.; Case, R.; Ross, J.; Newton, J.R.; Nugent, J. Equine herpes virus-1: Virus, immunity and vaccines. Open Vet. Sci. J. 2008, 2, 68–91. [Google Scholar] [CrossRef]
- HBLB Horserace Betting Levy Board (HBLB) Codes of Practice 2017, Equine Herpesvirus-EHV. Available online: http://codes.hblb.org.uk/index.php/page/32 (accessed on 3 October 2017).
- Lunn, D.P.; Davis-Poynter, N.; Flaminio, M.J.; Horohov, D.W.; Osterrieder, K.; Pusterla, N.; Townsend, H.G. Equine herpesvirus-1 consensus statement. J. Vet. Intern. Med. 2009, 23, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Ivens, P. EHM—What every equine practitioner needs to know. Livestock 2014, 19, 180–185. [Google Scholar] [CrossRef]
- Henninger, R.W.; Reed, S.M.; Saville, W.J.; Allen, G.P.; Hass, G.F.; Kohn, C.W.; Sofaly, C. Outbreak of neurologic disease caused by equine herpesvirus-1 at a university equestrian center. J. Vet. Intern. Med. 2007, 21, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Kydd, J.H.; Slater, J.; Osterrieder, N.; Lunn, D.P.; Antczak, D.F.; Azab, W.; Balasuriya, U.; Barnett, C.; Brosnahan, M.; Cook, C.; et al. Third international havemeyer workshop on equine herpesvirus type 1. Equine Vet. J. 2012, 44, 513–517. [Google Scholar] [CrossRef] [PubMed]
- AAEP American Association of Equine Practitioners (AAEP) Equine Herpes (EHV) Control Guidelines. Available online: https://aaep.org/guidelines/infectious-disease-control/equine-herpesvirus-resources (accessed on 3 October 2017).
- Rash, A.; Morton, R.; Woodward, A.; Maes, O.; McCauley, J.; Bryant, N.; Elton, D. Evolution and divergence of H3N8 equine influenza viruses circulating in the united kingdom from 2013 to 2015. Pathogens 2017, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Fougerolle, S.; Legrand, L.; Lecouturier, F.; Sailleau, C.; Paillot, R.; Hans, A.; Pronost, S. Genetic evolution of equne influenza virus strains (H3N8) isolated in france from 1967 to 2015 and the implications of several potential pathogenic factors. Virology 2017, 505, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R.; Hannant, D.; Kydd, J.H.; Daly, J.M. Vaccination against equine influenza: Quid novi? Vaccine 2006, 24, 4047–4061. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R. A systematic review of recent advances in equine influenza vaccination. Vaccines 2014, 2, 797–831. [Google Scholar] [CrossRef] [PubMed]
- Van de Zande, S. Intervet International, Prime-Boost Vaccine for the Protection of Equine Viral Infection; World Intellectual Property Organisation: Geneva, Switzerland, 2007; Available online: http://patentscope.wipo.int/search/en/detail.jsf?docId=WO2007051763&recNum=1&maxRec=&office=&prevFilter=&sortOption=&queryString=&tab=PCT+Biblio (accessed on 18 September 2014).
- Barquero, N.; Daly, J.M.; Newton, J.R. Risk factors for influenza infection in vaccinated racehorses: Lessons from an outbreak in newmarket, UK in 2003. Vaccine 2007, 25, 7520–7529. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R.; Parizot, V.; Garrett, D.; Birand, I.; Lopez-Alvarez, M.R.; Horspool, L.; Hurley, M. Large scale sero-epidemiological investigation of equine influenza vaccination in Hong Kong. Equine Vet. J. 2016, 39. [Google Scholar] [CrossRef]
- Nugent, J.; Birch-Machin, I.; Smith, K.C.; Mumford, J.A.; Swann, Z.; Newton, J.R.; Bowden, R.J.; Allen, G.P.; Davis-Poynter, N. Analysis of equid herpesvirus 1 strain variation reveals a point mutation of the DNA polymerase strongly associated with neuropathogenic versus nonneuropathogenic disease outbreaks. J. Virol. 2006, 80, 4047–4060. [Google Scholar] [CrossRef] [PubMed]
- Cullinane, A.; Gildea, S.; Weldon, E. Comparison of primary vaccination regimes for equine influenza—Working towards an evidence based regime. Equine Vet. J. 2013, 46, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Gildea, S.; Arkins, S.; Walsh, C.; Cullinane, A. A comparison of antibody responses to commercial equine influenza vaccines following annual booster vaccination of national hunt horses—A randomised blind study. Vaccine 2011, 29, 3917–3922. [Google Scholar] [CrossRef] [PubMed]
- Gildea, S.; Arkins, S.; Walsh, C.; Cullinane, A. A comparison of antibody responses to commercial equine influenza vaccines following primary vaccination of thoroughbred weanlings—A randomised blind study. Vaccine 2011, 29, 9214–9223. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R.; Fraser, S.; Prowse-Davis, L.; Rash, N.; Montesso, F.; Slootmans, N.; Thomas, A.; Besognet, B.; Meinert, T.; Ons, E.; et al. Iscom-based equine influenza vaccine: Duration of immunity and randomised clinical trials to assess an accelerated schedule of immunisation and efficacy. Trials Vaccinol. 2015, 4, 61–70. [Google Scholar] [CrossRef]
- Paillot, R.; Prowse, L.; Donald, C.; Medcalf, E.; Montesso, F.; Bryant, N.; Watson, J.; Jeggo, M.; Elton, D.; Newton, R.; et al. Efficacy of a whole inactivated EI vaccine against a recent EIV outbreak isolate and comparative detection of virus shedding. Vet. Immunol. Immunopathol. 2010, 136, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R.; Prowse, L. Iscom-matrix-based equine influenza (EIV) vaccine stimulates cell-mediated immunity in the horse. Vet. Immunol. Immunopathol. 2012, 145, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R.; Prowse, L.; Montesso, F.; Huang, C.M.; Barnes, H.; Escala, J. Whole inactivated equine influenza vaccine: Efficacy against a representative clade 2 equine influenza virus, ifngamma synthesis and duration of humoral immunity. Vet. Microbiol. 2013, 162, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Heldens, J.G.; van Loon, A.A.; van de Zande, S. Is there a benefit from an early booster vaccination in the control of equine influenza? Vet. J. 2007, 174, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Fougerolle, S.; Legrand, L.; Garrett, D.; Birand, I.; Foursin, M.; D’Ablon, X.; Bayssat, P.; Newton, R.J.; Pronost, S.; Paillot, R. Influential factors inducing suboptimal humoral response to vector-based influenza immunisation in thoroughbred foals. Vaccine 2016, 34, 3787–3795. [Google Scholar] [CrossRef] [PubMed]


| Variable | Category | EI Cases | Controls | Odds Ratio | p-Value |
|---|---|---|---|---|---|
| Number of EI vaccines administered | 1 type | 89 (76.7%) | 27 (23.3%) | Reference | na |
| 2 types | 110 (68.3%) | 51 (31.7%) | 0.65 | 0.13 | |
| 3 types | 57 (55.3%) | 46 (44.7%) | 0.38 | 0.001 | |
| ≥4 types | 15 (71.4%) | 6 (28.6%) | 0.76 | 0.6 | |
| Last EI vaccine administered | Whole inactivated, hydory-aluminium adjuvanted vaccine | 193 (79.1%) | 51 (20.9%) | Reference | na |
| Other type | 78 (49.7%) | 79 (50.3%) | 0.26 | <0.001 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paillot, R.; Marcillaud Pitel, C.; D’Ablon, X.; Pronost, S. Equine Vaccines: How, When and Why? Report of the Vaccinology Session, French Equine Veterinarians Association, 2016, Reims. Vaccines 2017, 5, 46. https://doi.org/10.3390/vaccines5040046
Paillot R, Marcillaud Pitel C, D’Ablon X, Pronost S. Equine Vaccines: How, When and Why? Report of the Vaccinology Session, French Equine Veterinarians Association, 2016, Reims. Vaccines. 2017; 5(4):46. https://doi.org/10.3390/vaccines5040046
Chicago/Turabian StylePaillot, Romain, Christel Marcillaud Pitel, Xavier D’Ablon, and Stéphane Pronost. 2017. "Equine Vaccines: How, When and Why? Report of the Vaccinology Session, French Equine Veterinarians Association, 2016, Reims" Vaccines 5, no. 4: 46. https://doi.org/10.3390/vaccines5040046
APA StylePaillot, R., Marcillaud Pitel, C., D’Ablon, X., & Pronost, S. (2017). Equine Vaccines: How, When and Why? Report of the Vaccinology Session, French Equine Veterinarians Association, 2016, Reims. Vaccines, 5(4), 46. https://doi.org/10.3390/vaccines5040046







