Trial Evaluation of Protection and Immunogenicity of Piscine Bivalent Streptococcal Vaccine: From the Lab to the Farms
Abstract
:1. Introduction
2. Materials and Methods
2.1. S. agalactiae and Experimental Fish
2.2. Inactivated Whole-Cell S. agalactiae Preparation
2.3. Evaluation of Vaccine Efficacy in Laboratory Scale: S. agalactiae Challenge Test
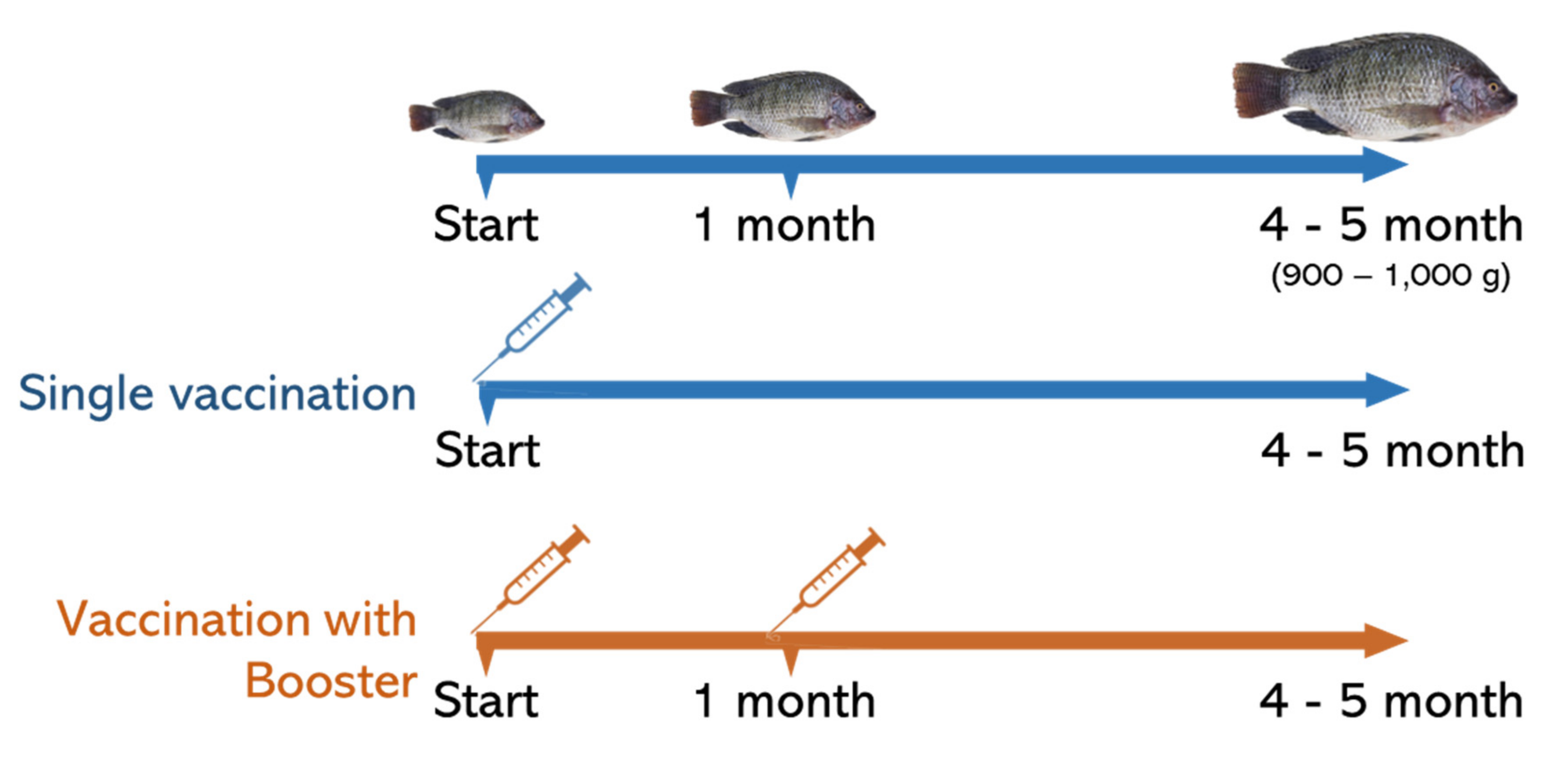
2.3.1. Immunization with Monovalent and Bivalent Streptococcal Vaccine
2.3.2. Challenge Test
2.4. Determination of Serum Antibody Titer
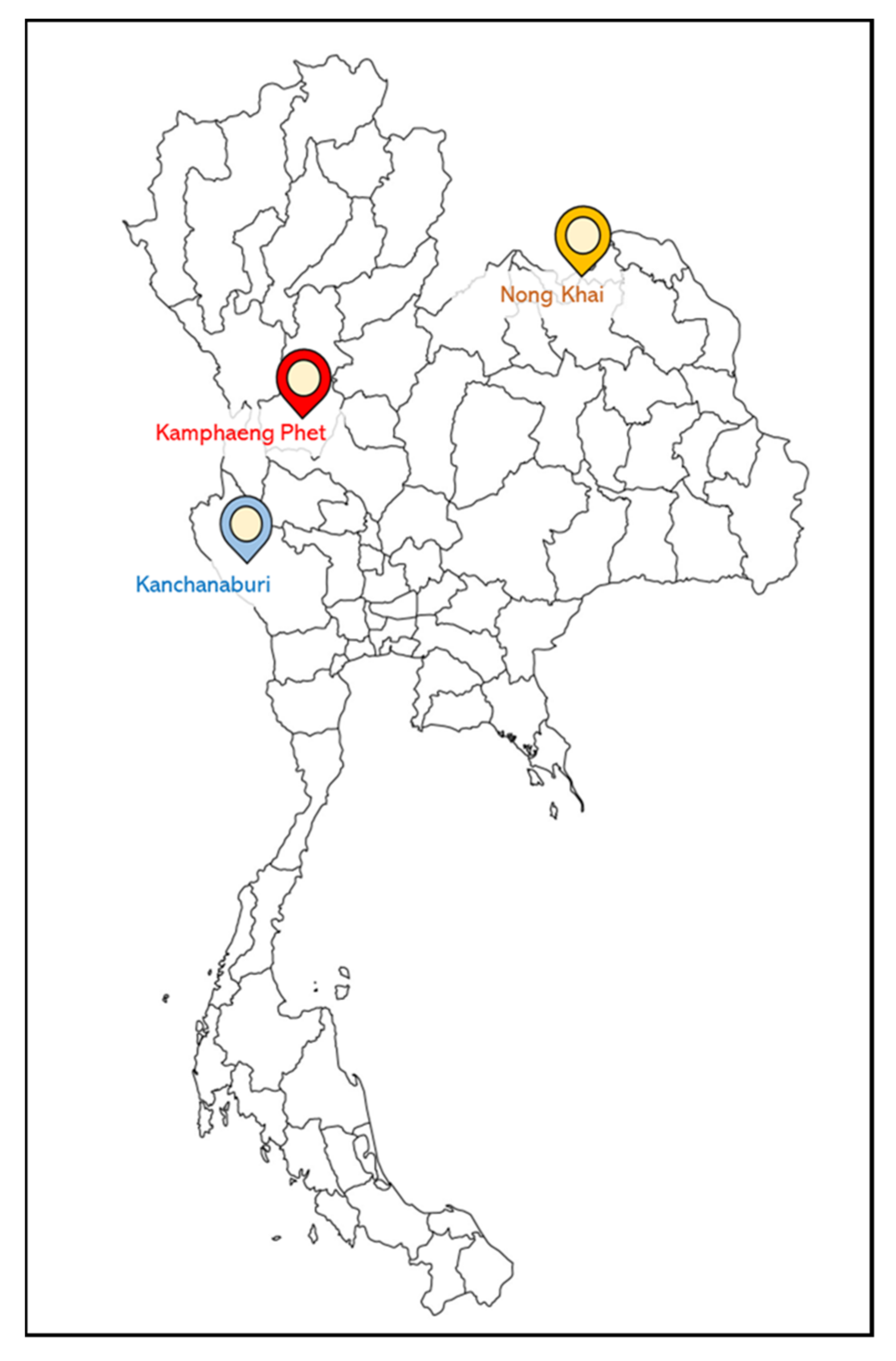
2.5. Evaluation of Vaccine Efficacy in Farm Scale
2.6. Average Weight Gain of Vaccinated Fish
2.7. Statistical Analysis
3. Results
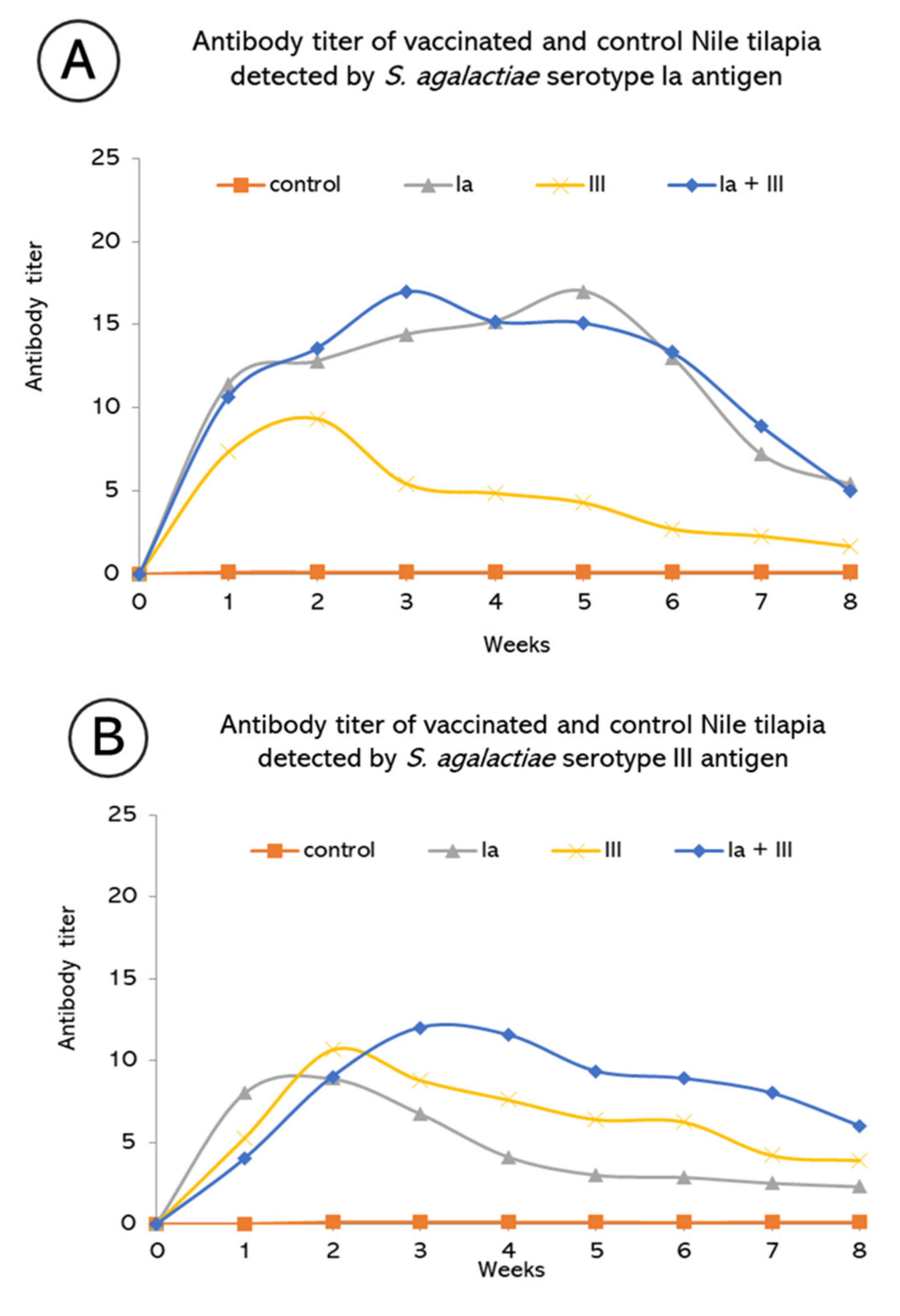
3.1. Specific Serum Antibody Titer of Tilapia Vaccinated with Monovalent and Bivalent Vaccine
3.2. Evaluation of Vaccine Efficacy: Laboratory Test
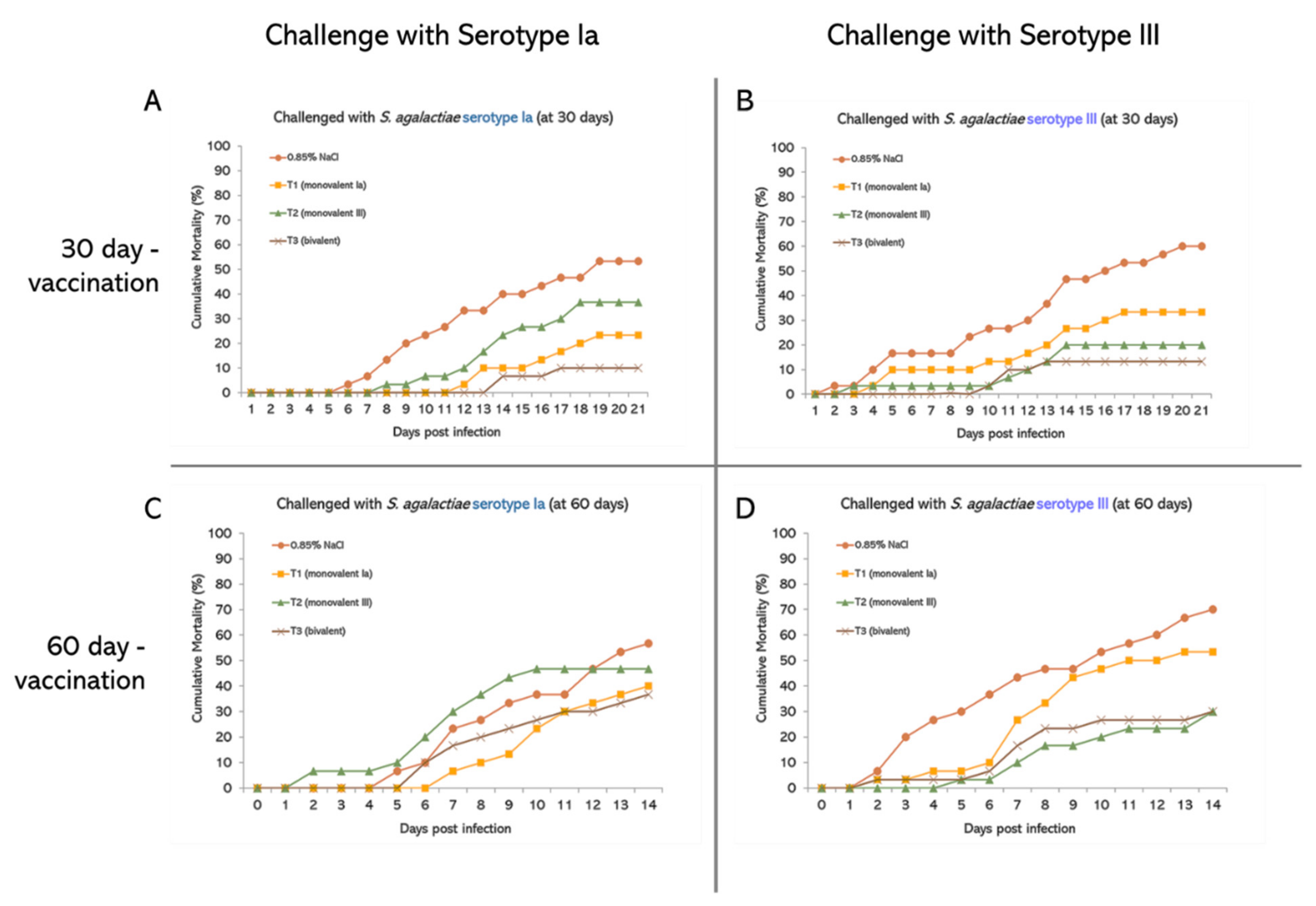
3.2.1. Percent Cumulative Mortality
3.2.2. Percent Survival
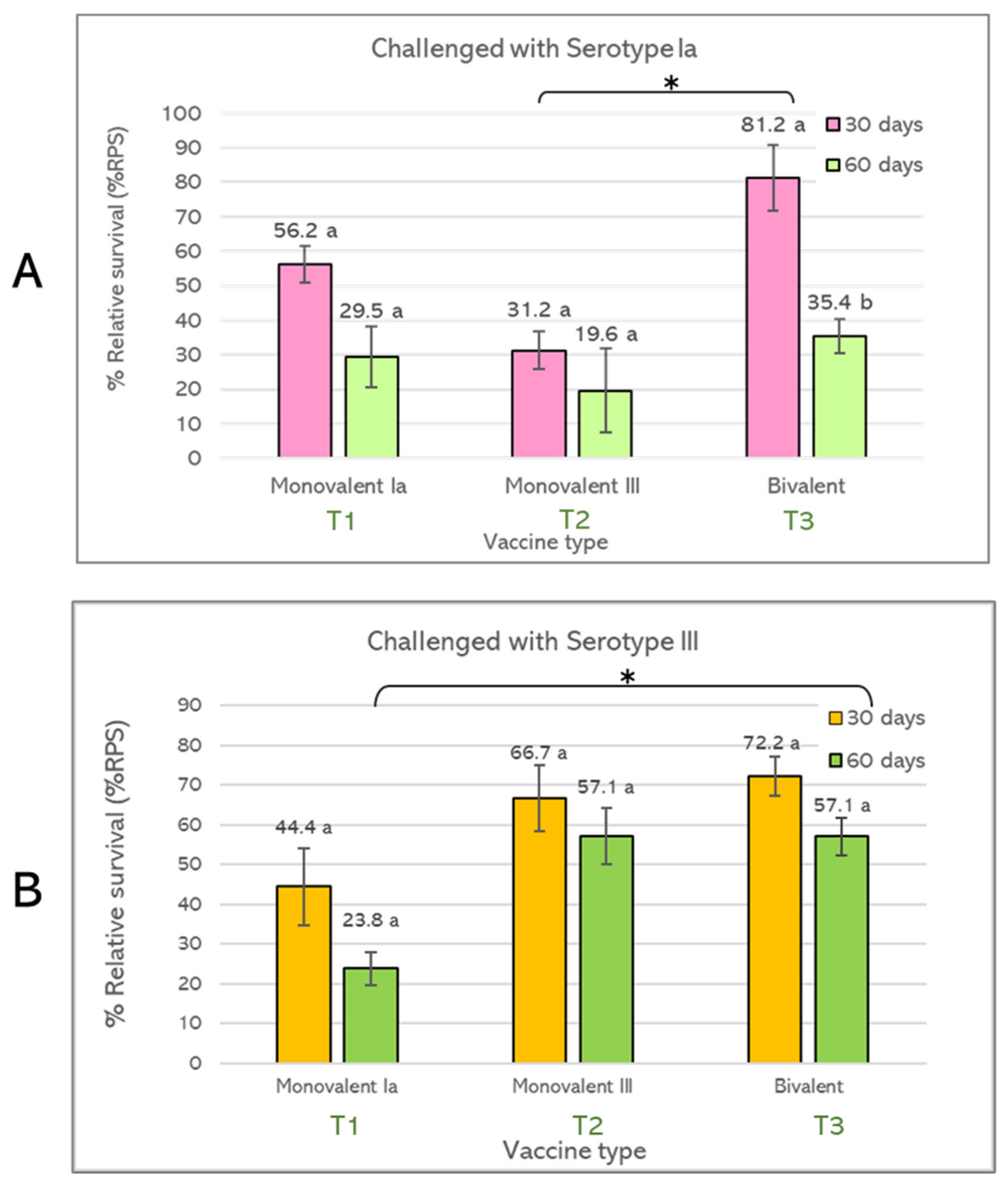
3.2.3. Relative Percent Survival (RPS)
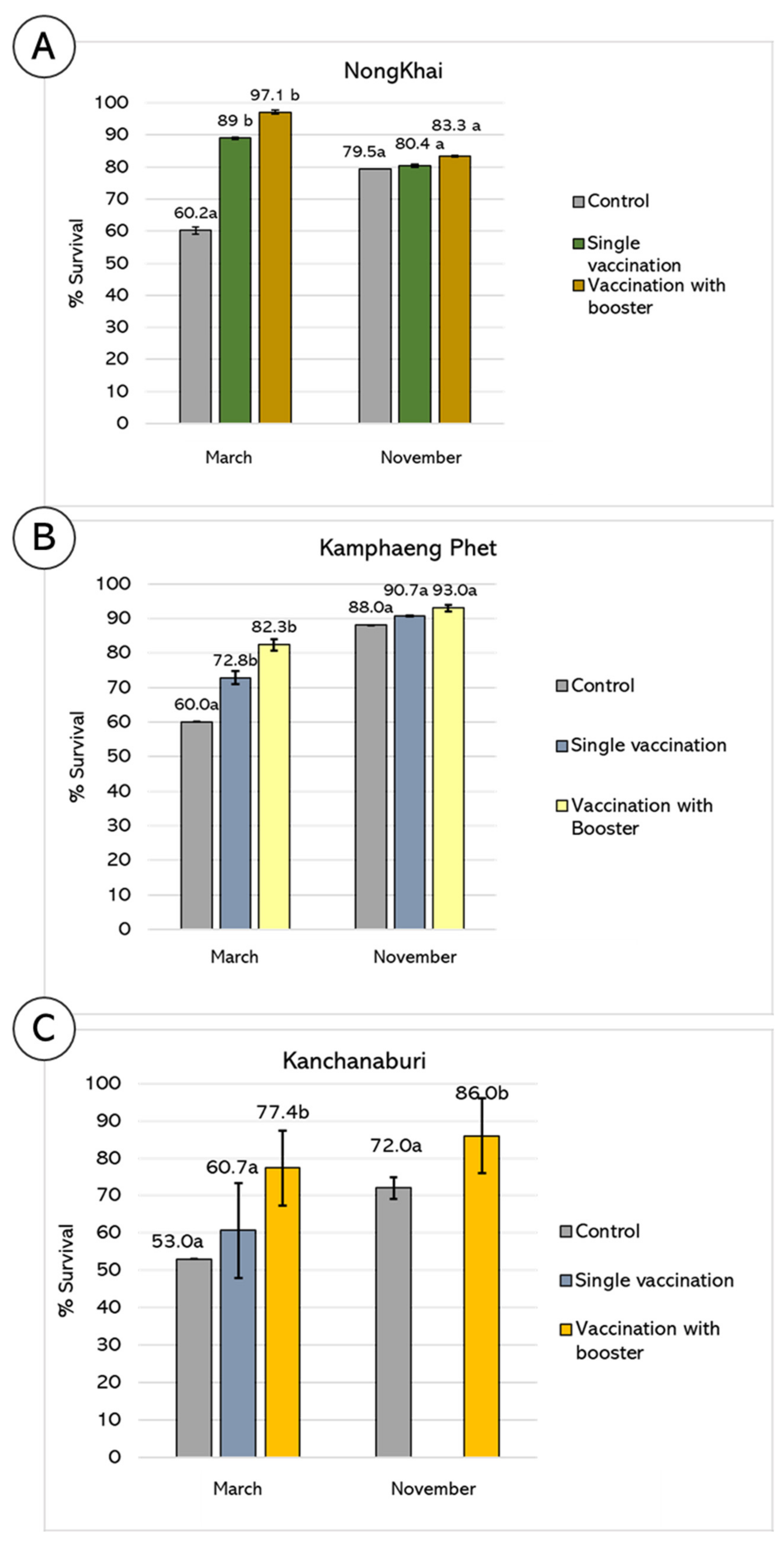
3.3. Evaluation of Vaccine Efficacy: Farm Scale
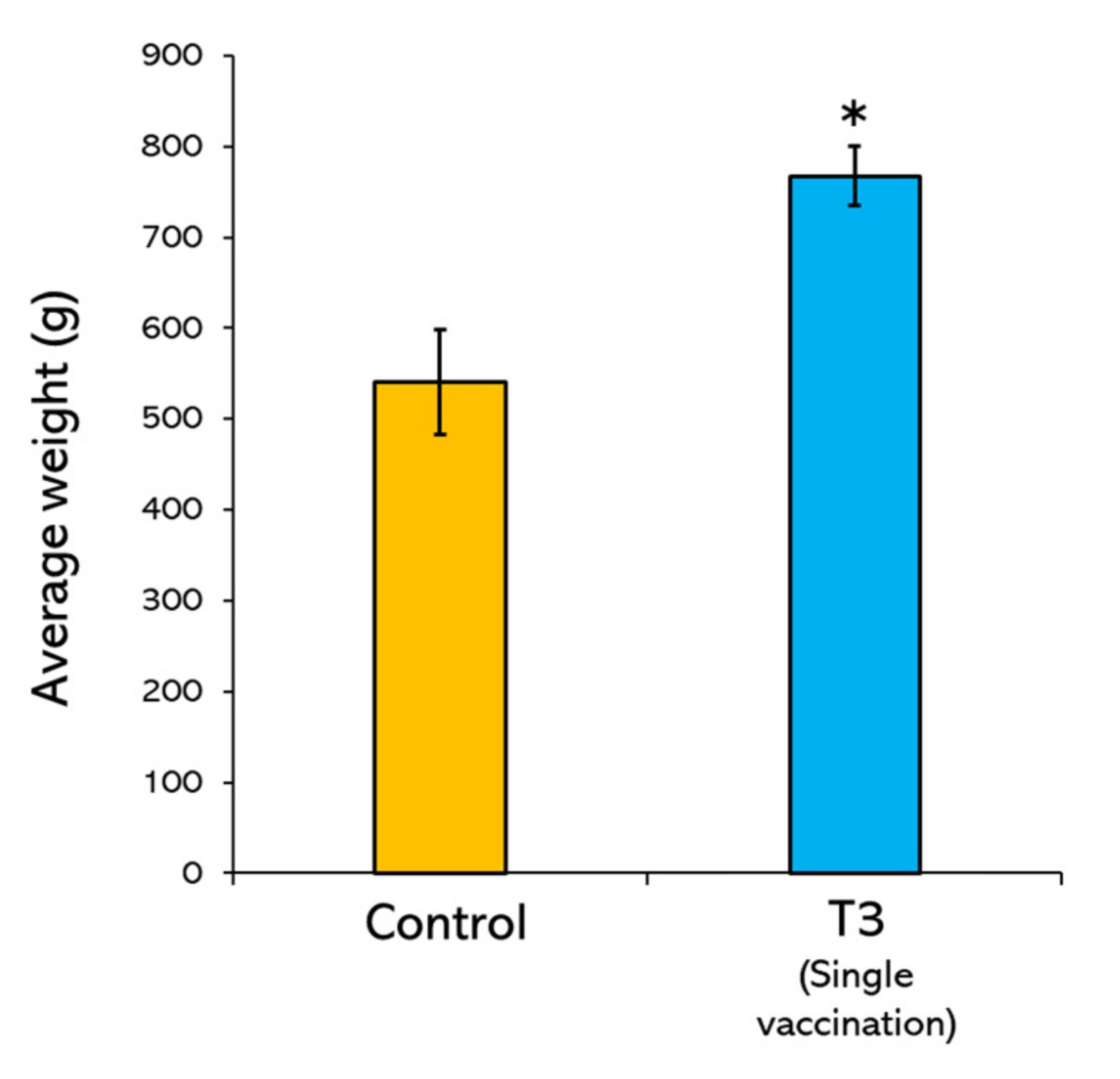
3.4. Average Weight Gain of Vaccinated Fish
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture, Sustainability in Action; the Food and Agriculture Organization of the United Nations: Rome, Italy, 2020; p. 244. [Google Scholar]
- Sommerset, I.; Krossøy, E.; Biering, E.; Frost, P. Vaccines for fish in aquaculture. Expert Rev. Vaccines 2005, 4, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Klesius, P.H.; Shoemaker, C.A. Development and use of modified live Edwardsiella ictaluri vaccine against enteric septicemia of catfish. Adv. Vet. Med. 1999, 41, 523–537. [Google Scholar] [PubMed]
- Arias, C.R.; Shoemaker, C.A.; Evans, J.J.; Klesius, P.H. A comparative study of Edwardsiella ictaluri parent (EILO) and E. ictaluri rifampicinmutant (RE-33) isolates using lipopolysaccharides, outer membrane proteins, fatty acids, Biolog, API 20E and genomic analyses. J. Fish Dis. 2003, 26, 415–421. [Google Scholar] [CrossRef]
- Klesius, P.H.; Shoemaker, C.A.; Evans, J.J. Efficacy of a killed Streptococcus iniae vaccine in tilapia (Oreochromis niloticus). Bull. Eur. Assoc. Fish Pathol. 1999, 19, 38–41. [Google Scholar]
- Klesius, P.H.; Shoemaker, C.A.; Evans, J.J. Efficacy of single and combined Streptococcus iniae isolate vaccine administered by intraperitoneal and intramuscular routes in tilapia (Oreochromis niloticus). Aquaculture 2000, 188, 237–246. [Google Scholar] [CrossRef]
- Klesius, P.H.; Evans, J.J.; Shoemaker, C.A.; Pasnik, D.J. Vaccines to prevent S. iniae and S. agalactiae disease in tilapia, O. niloticus. In Proceedings of the 7th International Symposium on Tilapia in Aquaculture, Veracuz, Mexico, 6–8 September 2006. [Google Scholar]
- Locke, J.B.; Aziz, R.K.; Vicknair, M.R.; Nizet, V.; Buchanan, J.T. Streptococcus iniae M-like protein contributes to virulence in fish and is a target for live attenuated vaccine development. PLoS ONE 2008, 3, e2824. [Google Scholar] [CrossRef] [PubMed]
- Locke, J.B.; Vicknair, M.R.; Ostland, V.E.; Nizet, V.; Buchanan, J.T. Evaluation of Streptococcus iniae killed bacterin and live attenuated vaccines in hybrid striped bass through injection and bath immersion. Dis. Aquat. Org. 2010, 89, 117–123. [Google Scholar] [CrossRef]
- Ra, C.-H.; Park, S.-J.; Kim, K.-H.; Kim, S.-K. Production of recombinant ghost bacterial vaccine against streptococcal disease of olive flounder. Process Biochem. 2010, 45, 317–322. [Google Scholar] [CrossRef]
- Evans, J.J.; Klesius, P.H.; Gilbert, P.M.; Shoemaker, C.A.; Al-Sarawi, M.A.; Lansberg, J.; Durendez, R.; Al-Marzouk, A.; Al-Zenki, S. Characterization of β-hemolytic group B Streptococcus agalactiae in cultured seabream, Sparus auratus and and mullet, Liza klunzingeri, in Kuwait. J. Fish Dis. 2002, 25, 505–513. [Google Scholar] [CrossRef]
- Evans, J.J.; Bohnsack, J.F.; Klesius, P.H.; Whiting, A.A.; Garcia, J.C.; Shoemaker, C.A.; Takahashi, S. Phylogenetic relationships among Streptococcus agalactiae isolated from piscine, dolphin, bovine and human sources: A dolphin and piscine lineage associated with a fish epidemic in Kuwait is also associated with human neonatal infections in Japan. J. Med. Microbiol. 2008, 57, 1369–1376. [Google Scholar] [CrossRef]
- Baya, A.M.; Lupiani, B.; Hetrick, F.M.; Roberson, B.S.; Lukacovic, R.; May, E.; Poukish, C. Association of Streptococcus sp. with fish mortalities in the Chesapeake Bay and its tributaries. J. Fish Dis. 1990, 13, 251–253. [Google Scholar] [CrossRef]
- Plumb, J.A. Tilapia bacterial diseases. In Health Maintenance and Principal Microbial Diseases of Cultured Fishes; Iowa State University: Ames, IA, USA, 1999; pp. 297–305. [Google Scholar]
- Eldar, A.; Bejerano, Y.; Livoff, A.; Hurvitz, A.; Bercovier, H. Experimental streptococcal meningo-encephalitis in cultured fish. Vet. Microbiol. 1995, 43, 33–40. [Google Scholar] [CrossRef]
- Salvador, R.; Muller, E.E.; Freitas, J.C.; Leonhardt, J.H.; Pretto-Giordano, L.G.; Dias, J.A. Isolation and characterization of Streptococcus spp. group B in Nile tilapia (Oreochromis niloticus) reared in hapas nets and earth nurseries in the northern region of Parana State, Brazil. Cienc. Rural 2005, 35, 1374–1378. [Google Scholar] [CrossRef]
- Kayansamruaj, P.; Pirarat, N.; Katagiri, T.; Hirono, I.; Rodkhum, C. Molecular characterization and virulence gene profiling of pathogenic Streptococcus agalactiae populations from tilapia (Oreochromis sp.) farms in Thailand. J. Vet. Diagn. Investig. 2014, 26, 488–495. [Google Scholar] [CrossRef]
- Kayansamruaj, P.; Pirarat, N.; Kondo, H.; Hirono, I.; Rodkhum, C. Genomic comparison between pathogenic Streptococcus agalactiae isolated from Nile tilapia in Thailand and fish-derived ST7 strains. Infect. Genet. Evol. 2015, 36, 301–314. [Google Scholar] [CrossRef]
- Areechon, N.; Kannika, K.; Hirono, I.; Kondo, H.; Unajak, S. Draft genome sequences of Streptococcus agalactiae serotype Ia and III isolates from tilapia farms in Thailand. Genome Announc. 2016, 4, e00122-16. [Google Scholar] [CrossRef]
- Chitmanat, C.; Lebel, P.; Whangchai, N.; Promya, J.; Lebel, L. Tilapia diseases and management in river-based cage aquaculture in Northern Thailand. J. Appl. Aquac. 2016, 28, 1–8. [Google Scholar] [CrossRef]
- Kannika, K.; Pisuttharachai, D.; Srisapoome, P.; Wongtavatchai, J.; Kondo, H.; Hirono, I.; Unajak, S.; Areechon, N. Molecular serotyping, virulence gene profiling and pathogenicity of Streptococcus agalactiae isolated from tilapia farms in Thailand by multiplex PCR. J. Appl. Microbiol. 2017, 122, 1497–1507. [Google Scholar] [CrossRef]
- Niu, G.; Khattiya, R.; Zhang, T.; Boonyayatra, S.; Wongsathein, D. Phenotypic and genotypic characterization of Streptococcus spp. isolated from tilapia (Oreochromis spp.) cultured in river-based cage and earthen ponds in northern Thailand. J. Fish Dis. 2020, 43, 391–398. [Google Scholar] [CrossRef]
- Amal, M.N.A.; Zamri-Saad, M.; Siti-Zahrah, A.; Zulkafli, A.R.; Nur-Nazifah, M. Molecular characterization of S. agalactiae strains isolated from fishes in Malaysia. J. Appl. Microbiol. 2013, 115, 20–29. [Google Scholar] [CrossRef]
- Zamri-Saad, M.; Azmai, M.N.A.; Siti-Zahrah, A.; Zulkafli, A.R. Control and prevention of streptococcosis in cultured tilapia in Malaysia: A review. Pertanika J. Trop. Agric. Sci. 2014, 37, 389–410. [Google Scholar]
- Firdaus-Nawi, M.; Mohd Yusoff, S.; Yusof, H.; Abdullah, S.-Z.; Zamri-Saad, M. Efficacy of feed-based adjuvant vaccine against Streptococcus agalactiae in Oreochromis spp. in Malaysia. Aquac. Res. 2014, 45, 87–96. [Google Scholar] [CrossRef]
- Auzureen, M.Z.A.; Hamdan, R.H.; Jamaludin, M.H. Identification and antibiotic resistance of Streptococcus agalactiae from red hybrid tilapia (Oreochromis niloticus) in local wet markets. In Proceedings of the International Seminar on Livestock Production and Veterinary Technology, Bali, Indonesia, 10–12 August 2016; pp. 561–566. [Google Scholar]
- Laith, A.A.; Ambak, M.A.; Hassan, M.; Sheriff, S.; Nadirah, M.; Draman, A.S.; Wahab, W.; Ibrahim, W.N.W.; Aznan, A.S.; Jabar, A.; et al. Molecular identification and histopathological study of natural Streptococcus agalactiae infection in hybrid tilapia (Oreochromis niloticus). Vet. World 2017, 10, 101–111. [Google Scholar] [PubMed]
- Lusiastuti, A.M.; Textor, M.; Seeger, H.; Akineden, Ö.; Zschöck, M. The occurrence of Streptococcus agalactiae sequence type 261 from fish disease outbreaks of tilapia Oreochromis niloticus in Indonesia. Aquac. Res. 2014, 45, 1260–1263. [Google Scholar] [CrossRef]
- Anshary, H.; Kurniawan, R.A.; Sriwulan, S.; Ramli, R.; Baxa, D.V. Isolation and molecular identification of the etiological agents of streptococcosis in Nile tilapia (Oreochromis niloticus) cultured in net cages in Lake Sentani, Papua, Indonesia. SpringerPlus 2014, 3, 627. [Google Scholar] [CrossRef]
- Legario, F.S.; Choresca, C.H., Jr.; Turnbull, J.F.; Crumlish, M. Isolation and molecular characterization of streptococcal species recovered from clinical infections in farmed Nile tilapia (Oreochromis niloticus) in the Philippines. J. Fish Dis. 2020, 43, 1431–1442. [Google Scholar] [CrossRef]
- Wang, B.; Jian, J.; Lu, Y.; Cai, S.; Huang, Y.; Tang, J.; Wu, Z. Complete genome sequence of Streptococcus agalactiae ZQ0910, a pathogen causing meningo-encephalitis in the GIFT strain of Nile tilapia (Oreochromis niloticus). J. Bacteriol. 2012, 194, 5132–5133. [Google Scholar] [CrossRef]
- Li, Y.W.; Liu, L.; Huang, P.R.; Fang, W.; Luo, Z.P.; Peng, H.L.; Wang, Y.X.; Li, A.X. Chronic streptococcosis in Nile tilapia, Oreochromis niloticus (L.), caused by Streptococcus agalactiae. J. Fish Dis. 2014, 37, 757–763. [Google Scholar] [CrossRef]
- Zhang, D.; Li, A.; Guo, Y.; Zhang, Q.; Chen, X.; Gong, X. Molecular characterization of Streptococcus agalactiae in diseased farmed tilapia in China. Aquaculture 2013, 412–413, 64–69. [Google Scholar] [CrossRef]
- Sun, J.; Fang, W.; Ke, B.; He, D.; Liang, Y.; Ning, D.; Tan, H.; Peng, H.; Wang, Y.; Ma, Y.; et al. Inapparent Streptococcus agalactiae infection in adult/commercial tilapia. Sci. Rep. 2016, 6, 26319. [Google Scholar] [CrossRef]
- Amend, D.F. Potency Testing of Fish Vaccines. Fish Biologics: Serodiagnostics and Vaccines; Development in Biological Standardization; Karger: Basel, Switzerland, 1981; pp. 447–454. [Google Scholar]
- Duncan, D.B. Multiple range and multiple F tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Dangwetngam, M.; Suanyuk, N.; Kong, F.; Phromkunthong, W. Serotype distribution and antimicrobial susceptibilities of Streptococcus agalactiae isolated from infected cultured tilapia (Oreochromis niloticus) in Thailand: Nine-year perspective. J. Med. Microbiol. 2016, 65, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Syuhada, R.; Zamri-Saad, M.; Ina-Salwany, M.Y.; Mustafa, M.; Nasruddin, N.N.; Desa, M.N.M.; Nordin, S.A.; Barkham, T.; Amal, M.N.A. Molecular characterization and pathogenicity of Streptococcus agalactiae serotypes IaST7 and IIIST283 isolated from cultured red hybrid tilapia in Malaysia. Aquaculture 2020, 515, 734543. [Google Scholar] [CrossRef]
- Zhang, D.; Ke, X.; Liu, Z.; Cao, J.; Su, Y.; Lu, M.; Gao, F.; Wang, M.; Yi, M.; Qin, F. Capsular polysaccharide of Streptococcus agalactiae is an essential virulence factor for infection in Nile tilapia (Oreochromis niloticus Linn.). J. Fish Dis. 2019, 42, 293–302. [Google Scholar]
- Liu, G.; Zhu, J.; Chen, K.; Gao, T.; Yao, H.; Liu, Y.; Zhang, W.; Lu, C. Development of Streptococcus agalactiae vaccines for tilapia. Dis. Aquat. Org. 2016, 122, 163–170. [Google Scholar] [CrossRef]
- Munang’andu, H.M.; Paul, J.; Evensen, Ø. An overview of vaccination strategies and antigen delivery systems for Streptococcus agalactiae vaccines in Nile tilapia (Oreochromis niloticus). Vaccines 2016, 4, 48. [Google Scholar] [CrossRef]
- Kayansamruaj, P.; Areechon, N.; Unajak, S. Development of fish vaccine in Southeast Asia: A challenge for the sustainability of SE Asia aquaculture. Fish Shellfish Immunol. 2020, 103, 73–87. [Google Scholar] [CrossRef]
- Miccoli, A.; Manni, M.; Picchietti, S.; Scapigliati, G. State-of-the-art vaccine research for aquaculture use: The case of three economically relevant fish species. Vaccines 2021, 9, 140. [Google Scholar] [CrossRef]
- Zhang, Z. Research Advances on Tilapia Streptococcosis. Pathogens 2021, 10, 558. [Google Scholar] [CrossRef]
- Ismail, M.S.; Syafiq, M.R.; Siti-Zahrah, A.; Fahmi, S.; Shahidan, H.; Hanan, Y.; Amal, M.N.A.; Zamri Saad, M. The effect of feed-based vaccination on tilapia farm endemic for streptococcosis. Fish Shellfish Immunol. 2017, 60, 21–24. [Google Scholar] [CrossRef]
- Ramos-Espinoza, F.C.; Cueva-Quiroz, V.A.; Yunis-Aguinaga, J.; de Moraes, J.R.E. A comparison of novel inactivation methods for production of a vaccine against Streptococcus agalactiae in Nile tilapia Oreochromis niloticus. Aquaculture 2020, 528, 735484. [Google Scholar] [CrossRef]
- Li, L.P.; Wang, R.; Liang, W.W.; Huang, T.; Huang, Y.; Luo, F.G.; Lei, A.Y.; Chen, M.; Gan, X. Development of live attenuated Streptococcus agalactiae vaccine for tilapia via continuous passage in vitro. Fish Shellfish Immunol. 2015, 45, 955–963. [Google Scholar] [CrossRef]
- Liu, L.; Lu, D.-Q.; Xu, J.; Luo, H.-L.; Li, A.-X. Development of attenuated erythromycin-resistant Streptococcus agalactiae vaccine for tilapia (Oreochromis niloticus) culture. J. Fish Dis. 2019, 42, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Pumchan, A.; Krobthong, S.; Roytrakul, S.; Sawatdichaikul, O.; Kondo, H.; Hirono, I.; Areechon, N.; Unajak, S. Novel chimeric multiepitope vaccine for streptococcosis disease in Nile tilapia (Oreochromis niloticus Linn.). Sci. Rep. 2020, 10, 603. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.Y.; Wang, K.Y.; Xiao, D.; Chen, D.F.; Geng, Y.; Wang, J.; He, Y.; Wang, E.L.; Huang, J.L.; Xiao, G.Y. Safety and immunogenicity of an oral DNA vaccine encoding Sip of Streptococcus agalactiae from Nile tilapia Oreochromis niloticus delivered by live attenuated Salmonella typhimurium. Fish Shellfish Immunol. 2014, 38, 34–41. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, Q.; Huang, L.; Wang, K.; Wang, X.; Chen, D.; Geng, Y.; Huang, X.; Ouyang, P.; Lai, W. Effectivity of oral recombinant DNA vaccine against Streptococcus agalactiae in Nile tilapia. Dev. Comp. Immunol. 2017, 77, 77–87. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kannika, K.; Sirisuay, S.; Kondo, H.; Hirono, I.; Areechon, N.; Unajak, S. Trial Evaluation of Protection and Immunogenicity of Piscine Bivalent Streptococcal Vaccine: From the Lab to the Farms. Vaccines 2022, 10, 1625. https://doi.org/10.3390/vaccines10101625
Kannika K, Sirisuay S, Kondo H, Hirono I, Areechon N, Unajak S. Trial Evaluation of Protection and Immunogenicity of Piscine Bivalent Streptococcal Vaccine: From the Lab to the Farms. Vaccines. 2022; 10(10):1625. https://doi.org/10.3390/vaccines10101625
Chicago/Turabian StyleKannika, Korntip, Soranut Sirisuay, Hidehiro Kondo, Ikuo Hirono, Nontawith Areechon, and Sasimanas Unajak. 2022. "Trial Evaluation of Protection and Immunogenicity of Piscine Bivalent Streptococcal Vaccine: From the Lab to the Farms" Vaccines 10, no. 10: 1625. https://doi.org/10.3390/vaccines10101625
APA StyleKannika, K., Sirisuay, S., Kondo, H., Hirono, I., Areechon, N., & Unajak, S. (2022). Trial Evaluation of Protection and Immunogenicity of Piscine Bivalent Streptococcal Vaccine: From the Lab to the Farms. Vaccines, 10(10), 1625. https://doi.org/10.3390/vaccines10101625





