A Modified Bacillus Calmette-Guérin (BCG) Vaccine with Reduced Activity of Antioxidants and Glutamine Synthetase Exhibits Enhanced Protection of Mice despite Diminished in Vivo Persistence
Abstract
:1. Introduction
2. Results and Discussion
2.1. Construction of 4dBCG
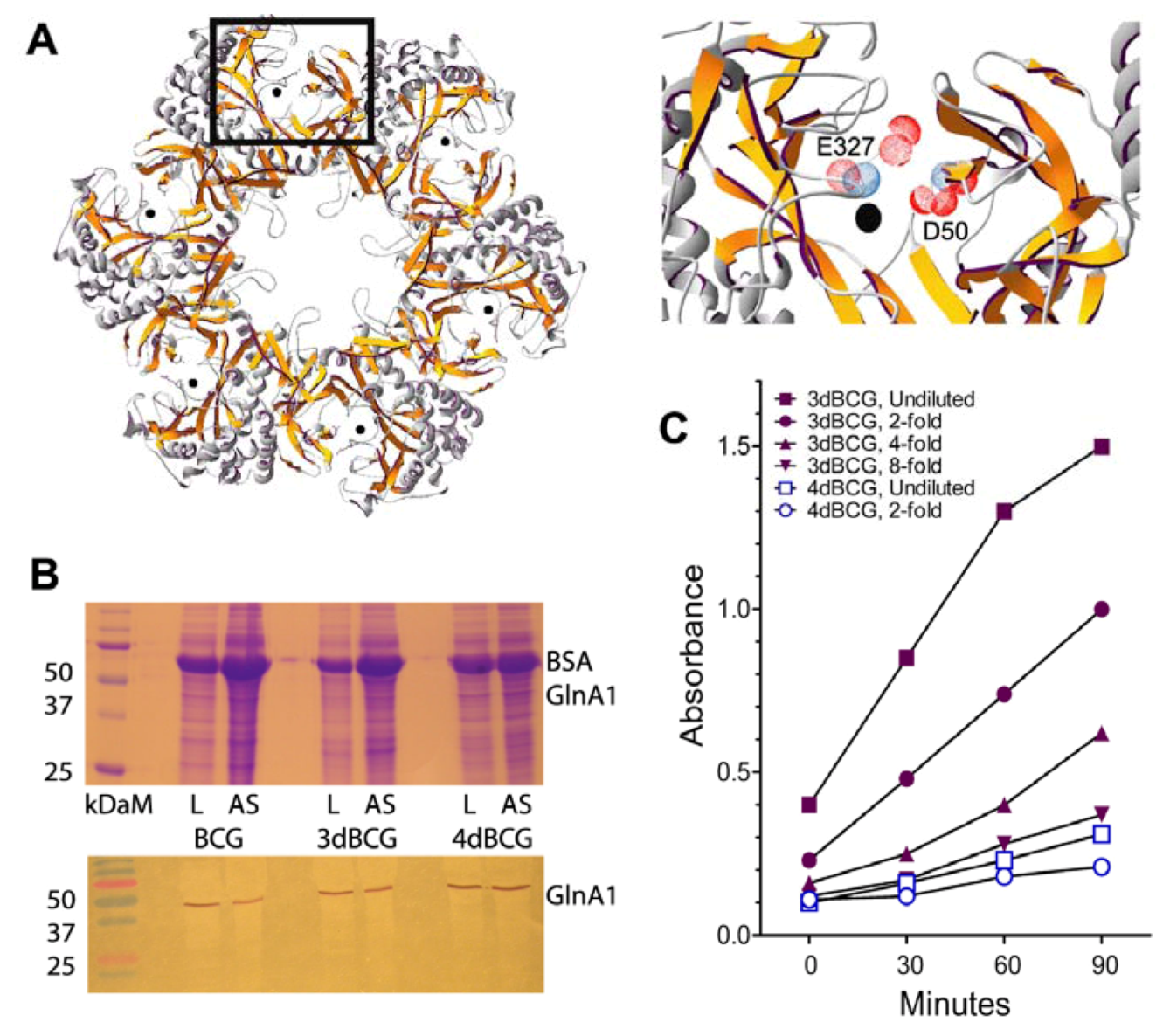
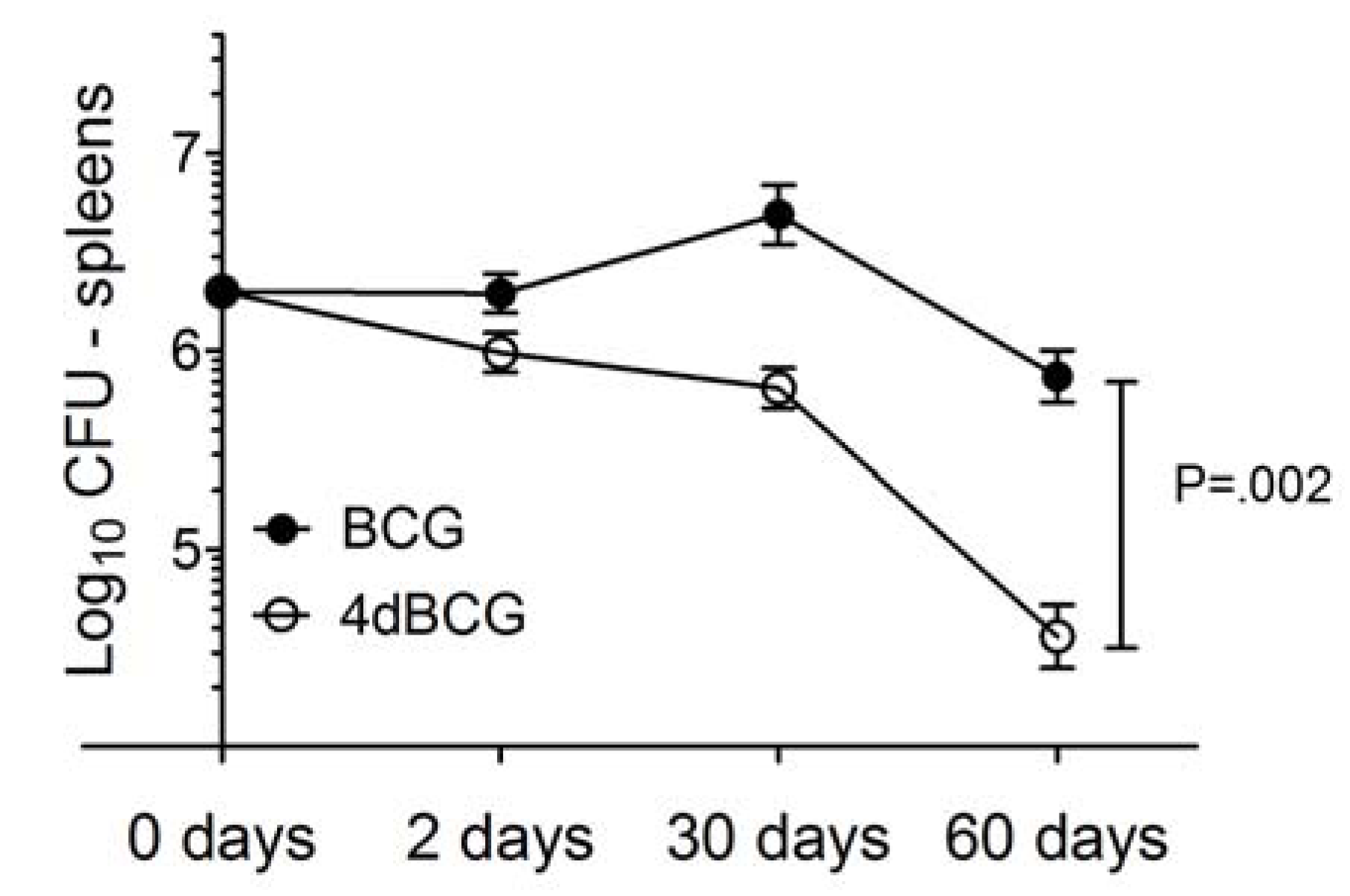
2.2. 4dBCG Is Cleared More Rapidly than BCG from the Spleens of Mice
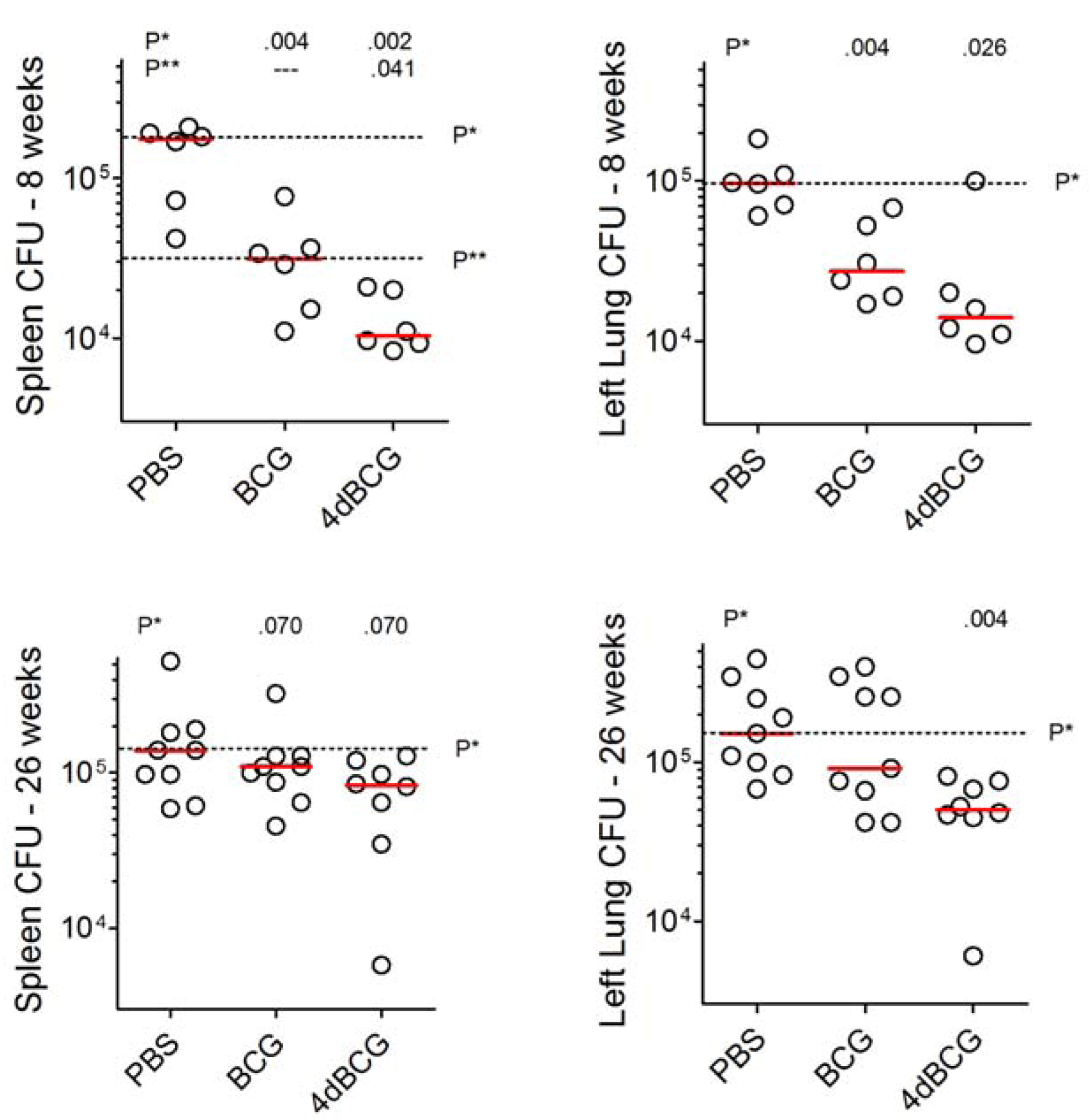
2.3. Vaccination with 4dBCG Exhibits Greater Protection than BCG against Early Dissemination of Aerosolized M. tuberculosis to the Spleen
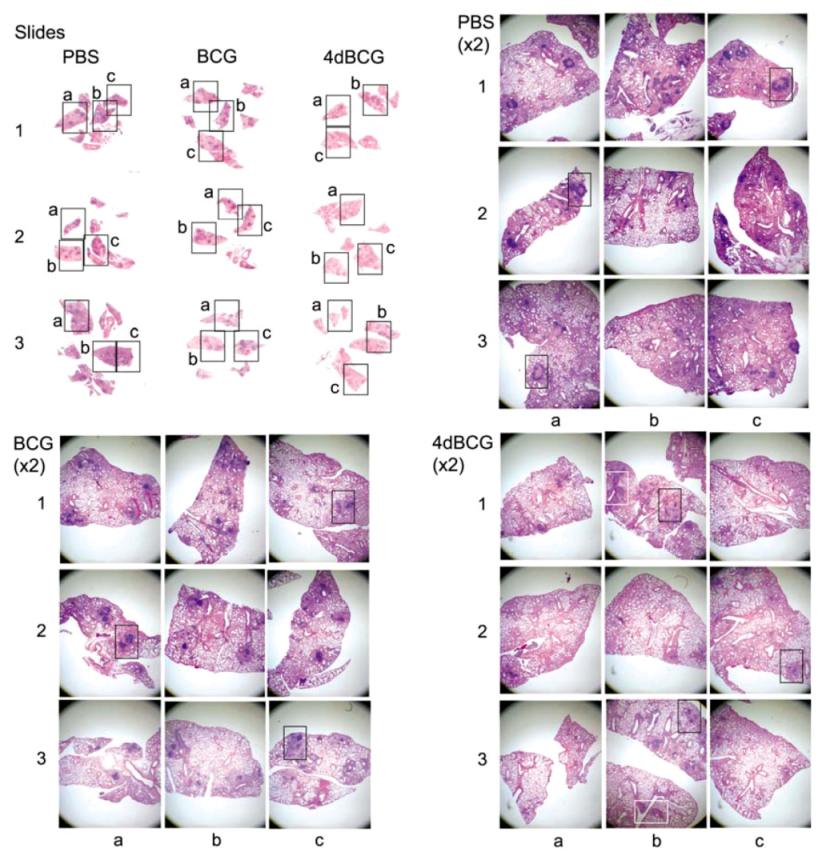
2.4. Vaccination with 4dBCG Alters the Pattern of Lymphohistiocytic Infiltration of Lung Parenchyma at 8 Weeks Post-Challenge
2.5. Vaccination with 4dBCG Reduces Lung Cfu Counts at 26 Weeks Post-Challenge
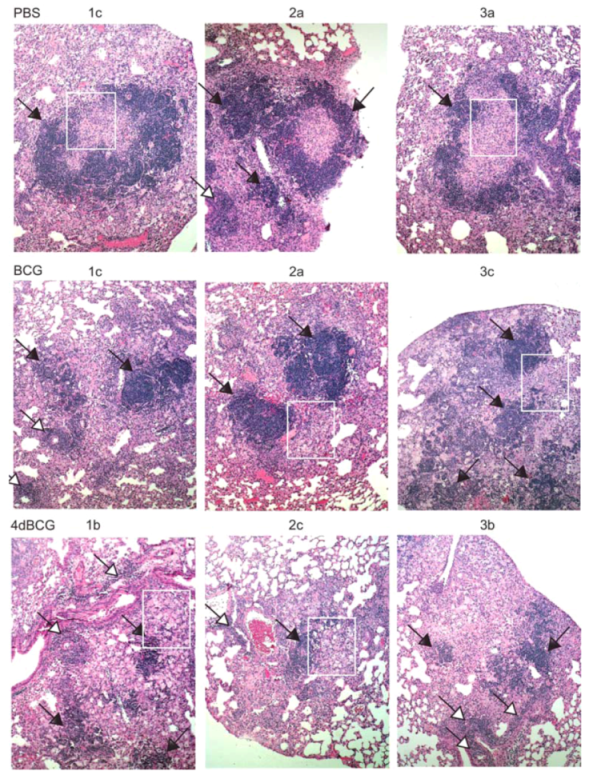
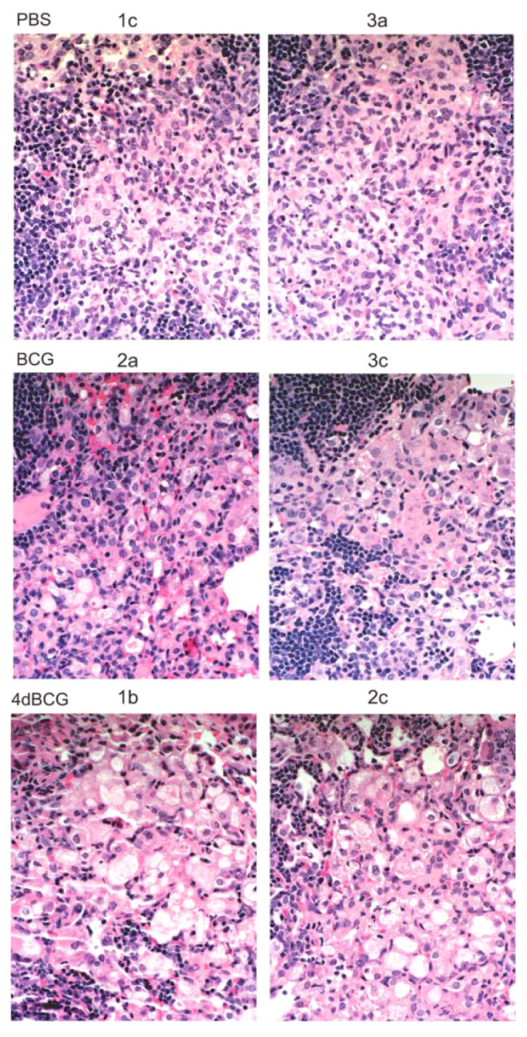
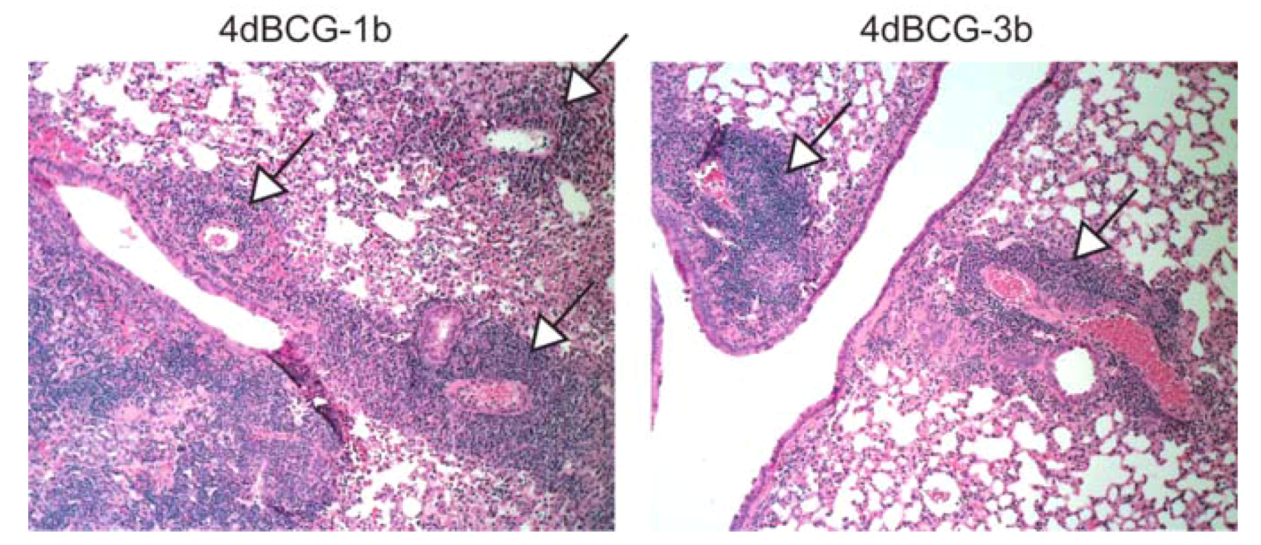
2.6. Advantages and Limitations of This Vaccine Strategy and Its Implications for Human Vaccination
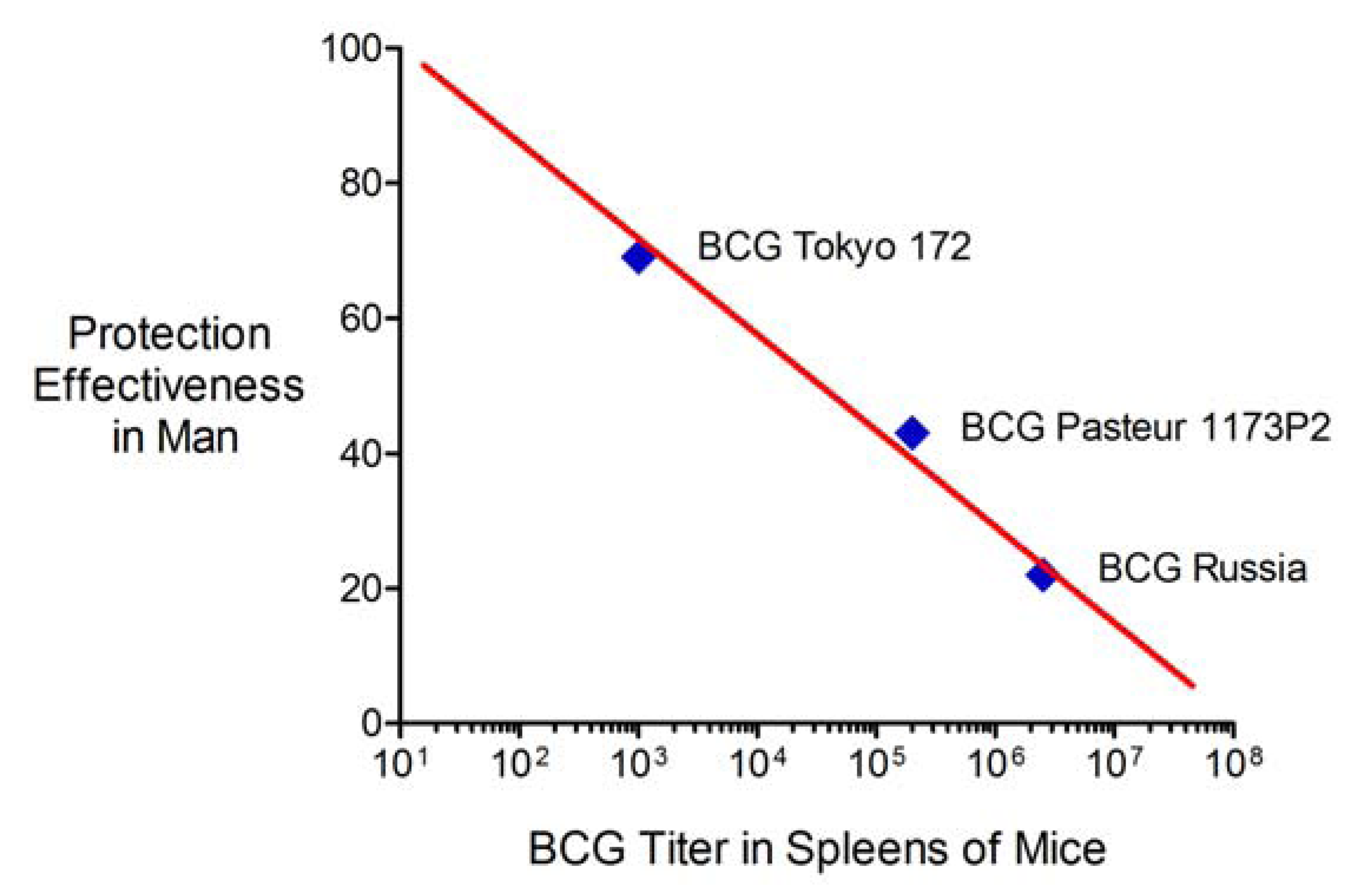
3. Experimental
3.1. Bacterial Isolates, Plasmids, Antibiotics, and Media
| Name | Description | Reference or source |
|---|---|---|
| Plasmids | ||
| pCR2.1-TOPO | Plasmid for cloning PCR products | Invitrogen Corp., Carlsbad, California |
| pBC SK+ | E. coli phagemid vector | Stratagene, La Jolla, CA |
| pHV203 | E. coli-mycobacterial shuttle plasmid with kanamycin resistance gene | [16] |
| pHV203-dnglnA1-ΔD54ΔE335 | pHV203 with ΔD54ΔE335 dn mutant glnA1 gene with its own promoter | This study |
| Strains | ||
| Erdman S-1 | M. tuberculosis WHO Standard lot 1 (Mtb S-1) prepared from virulent Erdman lot K01 from the Trudeau Institute and filled by Mycos Research, Loveland, CO | Amy Yang, FDA, Bethesda, MD [64] |
| TOP 10 | Host strain for cloning PCR products, used in combination with pCR2.1-TOPO | Invitrogen Corp., Carlsbad, California |
| DH5α | E. coli host strain for genetic manipulation, construction of mutant enzyme expression vectors | Life Technologies, Gaithersburg, MD [87] |
| BCG Tice | Bacillus Calmette-Guérin, substrain Tice | Organon Teknika Corp., Durham, NC [82,83] |
| 3rd generation modified BCG vaccine (three modifications) | ||
| 3dBCG | BCG Tice with inactivated sigH and secA2 containing the chromosomal integration vector pMP399-dnsodA-ΔH28ΔH76 | [12] |
| 4th generation modified BCG vaccine (four modifications) | ||
| 4dBCG | BCG Tice with inactivated sigH and secA2 containing pMP399-dnsodA-ΔH28ΔH76 and pHV203-dnglnA1-ΔD54ΔE335 | This study |
3.2. Molecular Genetic Manipulations
3.3. Expression of Mutant Enzyme Alleles in BCG and Assays of Enzyme Activity
3.4. Animal Procedures
3.5. Histopathology
3.6. Statistical Comparisons
4. Conclusions
Conflict of Interests
Acknowledgments
References
- Fine, P.E.M.; Carneiro, I.A.M.; Milstien, J.B.; Clements, J.D. Issues Relating to the Use of BCG in Immunization Programs, a Discussion Document, Document WHO/V&B/99.23 ed; World Health Organization Department of Vaccines and Biologicals: Geneva, Switzerland, 1999. [Google Scholar]
- Andersen, P.; Doherty, T.M. The success and failure of BCG—Implications for a novel tuberculosis vaccine. Nat. Rev. Microbiol. 2005, 3, 656–662. [Google Scholar] [CrossRef]
- Behr, M.A.; Wilson, M.A.; Gill, W.P.; Salamon, H.; Schoolnik, G.K.; Rane, S.; Small, P.M. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 1999, 284, 1520–1523. [Google Scholar] [CrossRef]
- Brosch, R.; Gordon, S.V.; Garnier, T.; Eiglmeier, K.; Frigui, W.; Valenti, P.; Dos, S.S.; Duthoy, S.; Lacroix, C.; Garcia-Pelayo, C.; et al. Genome plasticity of BCG and impact on vaccine efficacy. Proc. Natl. Acad. Sci. USA 2007, 104, 5596–5601. [Google Scholar]
- Trunz, B.B.; Fine, P.; Dye, C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: A meta-analysis and assessment of cost-effectiveness. Lancet 2006, 367, 1173–1180. [Google Scholar]
- Bonifachich, E.; Chort, M.; Astigarraga, A.; Diaz, N.; Brunet, B.; Pezzotto, S.M.; Bottasso, O. Protective effect of Bacillus Calmette-Guérin (BCG) vaccination in children with extra-pulmonary tuberculosis, but not the pulmonary disease. A case-control study in Rosario, Argentina. Vaccine 2006, 24, 2894–2899. [Google Scholar] [CrossRef]
- Bjartveit, K. Olaf Scheel and Johannes Heimbeck: Their contribution to understanding the pathogenesis and prevention of tuberculosis. Int. J. Tuberc. Lung Dis. 2003, 7, 306–311. [Google Scholar]
- Aronson, J.D.; Aronson, C.F.; Taylor, H.C. A twenty-year appraisal of BCG vaccination in the control of tuberculosis. Arch. Intern. Med. 1958, 101, 881–893. [Google Scholar] [CrossRef]
- Aronson, N.E.; Santosham, M.; Comstock, G.W.; Howard, R.S.; Moulton, L.H.; Rhoades, E.R.; Harrison, L.H. Long-term efficacy of BCG vaccine in American Indians and Alaska Natives: A 60-year follow-up study. JAMA 2004, 291, 2086–2091. [Google Scholar] [CrossRef]
- Behr, M.A.; Small, P.M. Has BCG attenuated to impotence? Nature 1997, 389, 133–134. [Google Scholar]
- Kernodle, D.S. Decrease in the effectiveness of Bacille Calmette-Guérin vaccine against pulmonary tuberculosis: A consequence of increased immune suppression by microbial antioxidants, not overattenuation. Clin. Infect. Dis. 2010, 51, 177–184. [Google Scholar] [CrossRef]
- Sadagopal, S.; Braunstein, M.; Hager, C.C.; Wei, J.; Daniel, A.K.; Bochan, M.R.; Crozier, I.; Smith, N.E.; Gates, H.O.; Barnett, L.; et al. Reducing the activity and secretion of microbial antioxidants enhances the immunogenicity of BCG. PLoS One 2009, 4, e5531. [Google Scholar]
- Horwitz, M.A.; Harth, G.; Dillon, B.J.; Maslesa-Galic’, S. Recombinant bacillus Calmette-Guérin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc. Natl. Acad. Sci. USA 2000, 97, 13853–13858. [Google Scholar]
- Grode, L.; Seiler, P.; Baumann, S.; Hess, J.; Brinkmann, V.; Eddine, A.N.; Mann, P.; Goosmann, C.; Bandermann, S.; Smith, D.; et al. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guérin mutants that secrete listeriolysin. J. Clin. Invest. 2005, 115, 2472–2479. [Google Scholar] [CrossRef]
- Sun, R.; Skeiky, Y.A.; Izzo, A.; Dheenadhayalan, V.; Imam, Z.; Penn, E.; Stagliano, K.; Haddock, S.; Mueller, S.; Fulkerson, J.; et al. Novel recombinant BCG expressing perfringolysin O and the over-expression of key immunodominant antigens; pre-clinical characterization, safety and protection against challenge with Mycobacterium tuberculosis. Vaccine 2009, 27, 4412–4423. [Google Scholar]
- Edwards, K.M.; Cynamon, M.H.; Voladri, R.K.; Hager, C.C.; DeStefano, M.S.; Tham, K.T.; Lakey, D.L.; Bochan, M.R.; Kernodle, D.S. Iron-cofactored superoxide dismutase inhibits host responses to Mycobacterium tuberculosis. Am. J. Respir. Crit. Care Med. 2001, 164, 2213–2219. [Google Scholar]
- Braunstein, M.; Espinosa, B.J.; Chan, J.; Belisle, J.T.; Jacobs, W.R., Jr. SecA2 functions in the secretion of superoxide dismutase A and in the virulence of Mycobacterium tuberculosis. Mol. Microbiol. 2003, 48, 453–464. [Google Scholar] [CrossRef]
- Hinchey, J.; Lee, S.; Jeon, B.Y.; Venkataswamy, M.M.; Chen, B.; Chan, J.; Braunstein, M.; Orme, I.M.; Derrick, S.C.; Morris, S.L.; et al. Enhanced priming of adaptive immunity by a proapoptotic mutant of Mycobacterium tuberculosis. J. Clin. Investig. 2007, 117, 2279–2288. [Google Scholar] [CrossRef]
- Jain, R.; Dey, B.; Khera, A.; Srivastav, P.; Gupta, U.D.; Katoch, V.M.; Ramanathan, V.D.; Tyagi, A.K. Over-expression of superoxide dismutase obliterates the protective effect of BCG against tuberculosis by modulating innate and adaptive immune responses. Vaccine 2011, 29, 8118–8125. [Google Scholar]
- Kaushal, D.; Schroeder, B.G.; Tyagi, S.; Yoshimatsu, T.; Scott, C.; Ko, C.; Carpenter, L.; Mehrotra, J.; Manabe, Y.C.; Fleischmann, R.D.; et al. Reduced immunopathology and mortality despite tissue persistence in a Mycobacterium tuberculosis mutant lacking alternative σ factor, SigH. Proc. Natl. Acad. Sci. USA 2002, 99, 8330–8335. [Google Scholar]
- Mehra, S.; Golden, N.A.; Stuckey, K.; Didier, P.J.; Doyle, L.A.; Russell-Lodrigue, K.E.; Sugimoto, C.; Hasegawa, A.; Sivasubramani, S.K.; Roy, C.J.; et al. The Mycobacterium tuberculosis stress response factor SigH is required for bacterial burden as well as immunopathology in primate lungs. J. Infect. Dis. 2012, 205, 1203–1213. [Google Scholar] [CrossRef]
- Kernodle, D.S. SigH, antioxidants, and the pathogenesis of pulmonary tuberculosis. J. Infect. Dis. 2012, 205, 1186–1188. [Google Scholar] [CrossRef]
- Raynaud, C.; Etienne, G.; Peyron, P.; Laneelle, M.A.; Daffe, M. Extracellular enzyme activities potentially involved in the pathogenicity of Mycobacterium tuberculosis. Microbiology 1998, 144, 577–587. [Google Scholar] [CrossRef]
- Miller, B.H.; Shinnick, T.M. Evaluation of Mycobacterium tuberculosis genes involved in resistance to killing by human macrophages. Infect. Immun. 2000, 68, 387–390. [Google Scholar] [CrossRef]
- Tullius, M.V.; Harth, G.; Horwitz, M.A. Glutamine synthetase GlnA1 is essential for growth of Mycobacterium tuberculosis in human THP-1 macrophages and guinea pigs. Infect. Immun. 2003, 71, 3927–3936. [Google Scholar]
- Manganelli, R.; Voskuil, M.I.; Schoolnik, G.K.; Dubnau, E.; Gomez, M.; Smith, I. Role of the extracytoplasmic-function σ factor σH in Mycobacterium tuberculosis global gene expression. Mol. Microbiol. 2002, 45, 365–374. [Google Scholar] [CrossRef]
- Raman, S.; Song, T.; Puyang, X.; Bardarov, S.; Jacobs, W.R., Jr.; Husson, R.N. The alternative sigma factor SigH regulates major components of oxidative and heat stress responses in Mycobacterium tuberculosis. J. Bacteriol. 2001, 183, 6119–6125. [Google Scholar] [CrossRef]
- Harth, G.; Maslesa-Galic, S.; Tullius, M.V.; Horwitz, M.A. All four Mycobacterium tuberculosis glnA genes encode glutamine synthetase activities but only GlnA1 is abundantly expressed and essential for bacterial homeostasis. Mol. Microbiol. 2005, 58, 1157–1172. [Google Scholar] [CrossRef]
- Dong, Y.; Demaria, S.; Sun, X.; Santori, F.R.; Jesdale, B.M.; de Groot, A.S.; Rom, W.N.; Bushkin, Y. HLA-A2-restricted CD8+-cytotoxic-T-cell responses to novel epitopes in Mycobacterium tuberculosis superoxide dismutase, alanine dehydrogenase, and glutamine synthetase. Infect. Immun. 2004, 72, 2412–2415. [Google Scholar] [CrossRef]
- Gill, H.S.; Pfluegl, G.M.; Eisenberg, D. Multicopy crystallographic refinement of a relaxed glutamine synthetase from Mycobacterium tuberculosis highlights flexible loops in the enzymatic mechanism and its regulation. Biochemistry 2002, 41, 9863–9872. [Google Scholar]
- Milstien, J.B.; Gibson, J.J. Quality control of BCG vaccine by WHO: A review of factors that may influence vaccine effectiveness and safety. Bull. World Health Organ. 1990, 68, 93–108. [Google Scholar]
- Ladefoged, A.; Bunch-Christensen, K.; Guld, J. Tuberculin sensitivity in guinea-pigs after vaccination with varying doses of BCG of 12 different strains. Bull. World Health Organ. 1976, 53, 435–443. [Google Scholar]
- Kupferschmidt, K. Infectious disease. Taking a new shot at a TB vaccine. Science 2011, 334, 1488–1490. [Google Scholar] [CrossRef]
- Ottenhoff, T.H.; Kaufmann, S.H. Vaccines against tuberculosis: Where are we and where do we need to go? PLoS Pathog. 2012, 8, e1002607. [Google Scholar] [CrossRef]
- Dubos, R.J.; Pierce, C.H. Differential characteristics in vitro and in vivo of several substrains of BCG. IV. Immunizing effectiveness. Am. Rev. Tuberc. 1956, 74, 699–717. [Google Scholar]
- Dubos, R.J.; Pierce, C.H.; Schaefer, W.B. Differential characteristics in vitro and in vivo of several substrains of BCG. III. Multiplication and survival in vivo. Am. Rev. Tuberc. 1956, 74, 683–698. [Google Scholar]
- Lagranderie, M.R.; Balazuc, A.M.; Deriaud, E.; Leclerc, C.D.; Gheorghiu, M. Comparison of immune responses of mice immunized with five different Mycobacterium bovis BCG vaccine strains. Infect. Immun. 1996, 64, 1–9. [Google Scholar]
- Freudenstein, H.; Pranter, W.; Schweinsberg, H. Assessment of several BCG vaccines in different animal test systems (additional studies to an IABS collaborative assay). J. Biol. Stand. 1979, 7, 203–212. [Google Scholar]
- Smith, D.; Harding, G.; Chan, J.; Edwards, M.; Hank, J.; Muller, D.; Sobhi, F. Potency of 10 BCG vaccines as evaluated by their influence on the bacillemic phase of experimental airborne tuberculosis in guinea-pigs. J. Biol. Stand. 1979, 7, 179–197. [Google Scholar] [CrossRef]
- Hank, J.A.; Chan, J.K.; Edwards, M.L.; Muller, D.; Smith, D.W. Influence of the virulence of Mycobacterium tuberculosis on protection induced by bacille Calmette-Guérin in guinea pigs. J. Infect. Dis. 1981, 143, 734–738. [Google Scholar] [CrossRef]
- Sekhuis, V.M.; Freudenstein, H.; Sirks, J.L. Report on results of a collaborative assay of BCG vaccines organized by the International Associaton of Biological Standardization. J. Biol. Stand. 1977, 5, 85–109. [Google Scholar]
- Festjens, N.; Bogaert, P.; Batni, A.; Houthuys, E.; Plets, E.; Vanderschaeghe, D.; Laukens, B.; Asselbergh, B.; Parthoens, E.; de Rycke, R.; et al. Disruption of the SapM locus in Mycobacterium bovis BCG improves its protective efficacy as a vaccine against M. tuberculosis. EMBO Mol. Med. 2011, 3, 222–234. [Google Scholar] [CrossRef]
- Saleh, M.T.; Belisle, J.T. Secretion of an acid phosphatase (SapM) by Mycobacterium tuberculosis that is similar to eukaryotic acid phosphatases. J. Bacteriol. 2000, 182, 6850–6853. [Google Scholar] [CrossRef]
- Vergne, I.; Chua, J.; Lee, H.H.; Lucas, M.; Belisle, J.; Deretic, V. Mechanism of phagolysosome biogenesis block by viable Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2005, 102, 4033–4038. [Google Scholar]
- Johansen, P.; Fettelschoss, A.; Amstutz, B.; Selchow, P.; Waeckerle-Men, Y.; Keller, P.; Deretic, V.; Held, L.; Kundig, T.M.; Bottger, E.C.; et al. Relief from Zmp1-mediated arrest of phagosome maturation is associated with facilitated presentation and enhanced immunogenicity of mycobacterial antigens. Clin. Vaccine Immunol. 2011, 18, 907–913. [Google Scholar] [CrossRef]
- Rutault, K.; Alderman, C.; Chain, B.M.; Katz, D.R. Reactive oxygen species activate human peripheral blood dendritic cells. Free Radic. Biol. Med. 1999, 26, 232–238. [Google Scholar] [CrossRef]
- Van der Veen, R.C.; Dietlin, T.A.; Karapetian, A.; Holland, S.M.; Hofman, F.M. Extra-cellular superoxide promotes T cell expansion through inactivation of nitric oxide. J. Neuroimmunol. 2004, 153, 183–189. [Google Scholar]
- Pacheco, R.; Oliva, H.; Martinez-Navio, J.M.; Climent, N.; Ciruela, F.; Gatell, J.M.; Gallart, T.; Mallol, J.; Lluis, C.; Franco, R. Glutamate released by dendritic cells as a novel modulator of T cell activation. J. Immunol. 2006, 177, 6695–6704. [Google Scholar]
- Gras, G.; Porcheray, F.; Samah, B.; Leone, C. The glutamate-glutamine cycle as an inducible, protective face of macrophage activation. J. Leukoc. Biol. 2006, 80, 1067–1075. [Google Scholar] [CrossRef]
- Xue, H.; Field, C.J. New role of glutamate as an immunoregulator via glutamate receptors and transporters. Front. Biosci. (Schol. Ed.) 2011, 3, 1007–1020. [Google Scholar]
- Jabs, T. Reactive oxygen intermediates as mediators of programmed cell death in plants and animals. Biochem. Pharmacol. 1999, 57, 231–245. [Google Scholar] [CrossRef]
- Winau, F.; Kaufmann, S.H.; Schaible, U.E. Apoptosis paves the detour path for CD8 T cell activation against intracellular bacteria. Cell. Microbiol. 2004, 6, 599–607. [Google Scholar]
- Romani, L.; Fallarino, F.; de Luca, A.; Montagnoli, C.; D’Angelo, C.; Zelante, T.; Vacca, C.; Bistoni, F.; Fioretti, M.C.; Grohmann, U.; et al. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature 2008, 451, 211–215. [Google Scholar]
- Tse, H.M.; Thayer, T.C.; Steele, C.; Cuda, C.M.; Morel, L.; Piganelli, J.D.; Mathews, C.E. NADPH oxidase deficiency regulates Th lineage commitment and modulates autoimmunity. J. Immunol. 2010, 185, 5247–5258. [Google Scholar]
- Jespersen, A. Use of red mice (Clethrionomys g. glareolus Schreb.) for estimation of the ability of vaccines to immunize against tuberculosis. Acta Pathol. Microbiol. Scand. 1954, 34, 157–160. [Google Scholar] [CrossRef]
- Jespersen, A. Studies on tuberculin sensitivity and immunity in guinea-pigs induced by vaccination with varying doses of BCG vaccine. Acta Pathol. Microbiol. Scand. 1956, 38, 203–210. [Google Scholar] [CrossRef]
- Jespersen, A.; Bentzon, M.W.; Magnusson, M. Development of tuberculin sensitivity and acquired resistance to tuberculosis in guinea pigs vaccinated with a small dose of BCG vaccine. Acta Pathol. Microbiol. Scand. 1962, 54, 291–304. [Google Scholar]
- Ladefoged, A.; Bunch-Christensen, K.; Guld, J. The protective effect in bank voles of some strains of BCG. Bull. World Health Organ. 1970, 43, 71–90. [Google Scholar]
- World Health Organization Department of Vaccines and Biologicals, Adverse Events Following BCG Vaccination, Chapter 2 in Clements, C. J., Supplementary Information on Vaccine Safety, Document WHO/V&B/00.36 ed; Geneva, Switzerland, 2000.
- Gheorghiu, M.; Carnus, H.; Lagrange, P.; Chambon, L. Potency and suppurative adenitis in BCG vaccination. Dev. Biol. Stand 1978, 41, 79–84. [Google Scholar]
- Bunch-Christensen, K.; Ladefoged, A.; Guld, J. The virulence of some strains of BCG for golden hamsters. Bull. World Health Organ. 1968, 39, 821–828. [Google Scholar]
- Bunch-Christensen, K.; Ladefoged, A.; Guld, J. The virulence of some strains of BCG for golden hamsters. Further studies. Bull. World Health Organ. 1970, 43, 65–70. [Google Scholar]
- Smith, D.W.; Wiegeshaus, E.; Navalkar, R.; Grover, A.A. Host-parasite relationships in experimental airborne tuberculosis. I. Preliminary studies in BCG-vaccinated and nonvaccinated animals. J. Bacteriol. 1965, 91, 718–724. [Google Scholar]
- Jeon, B.Y.; Derrick, S.C.; Lim, J.; Kolibab, K.; Dheenadhayalan, V.; Yang, A.L.; Kreiswirth, B.; Morris, S.L. Mycobacterium bovis BCG immunization induces protective immunity against nine different Mycobacterium tuberculosis strains in mice. Infect. Immun. 2008, 76, 5173–5180. [Google Scholar] [CrossRef]
- Rhoades, E.R.; Frank, A.A.; Orme, I.M. Progression of chronic pulmonary tuberculosis in mice aerogenically infected with virulent Mycobacterium tuberculosis. Tuber. Lung Dis. 1997, 78, 57–66. [Google Scholar] [CrossRef]
- Albina, J.E. On the expression of nitric oxide synthase by human macrophages. Why no NO? J. Leukoc. Biol. 1995, 58, 643–649. [Google Scholar]
- Dutta, N.K.; Mehra, S.; Didier, P.J.; Roy, C.J.; Doyle, L.A.; Alvarez, X.; Ratterree, M.; Be, N.A.; Lamichhane, G.; Jain, S.K.; et al. Genetic requirements for the survival of tubercle bacilli in primates. J. Infect. Dis. 2010, 201, 1743–1752. [Google Scholar] [CrossRef]
- Tsai, M.C.; Chakravarty, S.; Zhu, G.; Xu, J.; Tanaka, K.; Koch, C.; Tufariello, J.; Flynn, J.; Chan, J. Characterization of the tuberculous granuloma in murine and human lungs: Cellular composition and relative tissue oxygen tension. Cell. Microbiol. 2006, 8, 218–232. [Google Scholar]
- Gonzalez-Juarrero, M.; Turner, O.C.; Turner, J.; Marietta, P.; Brooks, J.V.; Orme, I.M. Temporal and spatial arrangement of lymphocytes within lung granulomas induced by aerosol infection with Mycobacterium tuberculosis. Infect. Immun. 2001, 69, 1722–1728. [Google Scholar]
- Khader, S.A.; Guglani, L.; Rangel-Moreno, J.; Gopal, R.; Junecko, B.A.; Fountain, J.J.; Martino, C.; Pearl, J.E.; Tighe, M.; Lin, Y.Y.; et al. IL-23 is required for long-term control of Mycobacterium tuberculosis and B cell follicle formation in the infected lung. J. Immunol. 2011, 187, 5402–5407. [Google Scholar]
- Ober, W.B. Ghon but not forgotten: Anton Ghon and his complex. Pathol. Annu. 1983, 18, 79–85. [Google Scholar]
- Pathan, A.A.; Wilkinson, K.A.; Wilkinson, R.J.; Latif, M.; McShane, H.; Pasvol, G.; Hill, A.V.; Lalvani, A. High frequencies of circulating IFN-γ-secreting CD8 cytotoxic T cells specific for a novel MHC class I-restricted Mycobacterium tuberculosis epitope in M. tuberculosis-infected subjects without disease. Eur. J. Immunol. 2002, 30, 2713–2721. [Google Scholar]
- Tully, G.; Kortsik, C.; Hohn, H.; Zehbe, I.; Hitzler, W.E.; Neukirch, C.; Freitag, K.; Kayser, K.; Maeurer, M.J. Highly focused T cell responses in latent human pulmonary Mycobacterium tuberculosis infection. J. Immunol. 2005, 174, 2174–2184. [Google Scholar]
- Carranza, C.; Juarez, E.; Torres, M.; Ellner, J.J.; Sada, E.; Schwander, S.K. Mycobacterium tuberculosis growth control by lung macrophages and CD8 cells from patient contacts. Am. J. Respir. Crit. Care Med. 2006, 173, 238–245. [Google Scholar]
- Irvine, K.N. B.C.G. Vaccination in Theory and Practice; Blackwell Scientific Publications, Ltd.: London and Oxford, UK, 1949. [Google Scholar]
- Tuberculosis Prevention Trial. Trial of BCG vaccines in south India for tuberculosis prevention: First report. Bull. World Health Organ. 1979, 57, 819–827.
- Comstock, G.W. Identification of an effective vaccine against tuberculosis. Am. Rev. Respir. Dis. Epidemiol. Rev. 1988, 138, 479–480. [Google Scholar]
- Comstock, G.W. Evaluating vaccination effectiveness and vaccine efficacy by means of case-control studies. Epidemiol. Rev. 1994, 16, 77–89. [Google Scholar]
- Comstock, G.W. Simple, practical ways to assess the protective efficacy of a new tuberculosis vaccine. Clin. Infect. Dis. 2000, 30, S250–S253. [Google Scholar] [CrossRef]
- Favorov, M.; Ali, M.; Tursunbayeva, A.; Aitmagambetova, I.; Kilgore, P.; Ismailov, S.; Chorba, T. Comparative tuberculosis (TB) prevention effectiveness in children of Bacillus Calmette-Guérin (BCG) vaccines from different sources, Kazakhstan. PLoS One 2012, 7, e32567. [Google Scholar]
- Dubos, R.J.; Pierce, C.H. Differential characteristics in vitro and in vivo of several substrains of BCG. I. Multiplication and survival in vitro. Am. Rev. Tuberc. 1956, 74, 655–666. [Google Scholar]
- Oettinger, T.; Jorgensen, M.; Ladefoged, A.; Haslov, K.; Andersen, P. Development of the Mycobacterium bovis BCG vaccine: Review of the historical and biochemical evidence for a genealogical tree. Tuber. Lung Dis. 1999, 79, 243–250. [Google Scholar] [CrossRef]
- Dubos, R.J.; Pierce, C.H. Tice strain of BCG. Am. Rev. Tuberc. 1957, 75, 692–693. [Google Scholar]
- Kernodle, D.S.; Bochan, M.R. Pro-apoptotic bacterial vaccines to enhance cellular immune responses (United States Patent no. 8,021,671; World Intellectual Property Organization publication No. WO/2002/062298. Available online: http://patentscope.wipo.int/search/en/WO2002062298/ (accessed on 18 December 2012).
- Kernodle, D.S. Warning: Differences in the copy number of duplication unit 2 (DU2) within BCG Danish 1331 may influence findings involving genetically modified BCG Danish strains. Vaccine 2012, 30, 6013–6014. [Google Scholar] [CrossRef]
- Palanisamy, G.S.; Smith, E.E.; Shanley, C.A.; Ordway, D.J.; Orme, I.M.; Basaraba, R.J. Disseminated disease severity as a measure of virulence of Mycobacterium tuberculosis in the guinea pig model. Tuberculosis (Edinb.) 2008, 88, 295–306. [Google Scholar] [CrossRef]
- Hanahan, D. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 1983, 166, 557–580. [Google Scholar] [CrossRef]
- Braunstein, M.; Bardarov, S.S.; Jacobs, W.R., Jr. Genetic methods for deciphering virulence determinants of Mycobacterium tuberculosis. Meth. Enzymol. 2002, 358, 67–99. [Google Scholar] [CrossRef]
- Ho, S.N.; Hunt, H.D.; Horton, R.M.; Pullen, J.K.; Pease, L.R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 1989, 77, 51–59. [Google Scholar] [CrossRef]
- Woolfolk, C.A.; Shapiro, B.; Stadtman, E.R. Regulation of glutamine synthetase. I. Purification and properties of glutamine synthetase from Escherichia coli. Arch. Biochem. Biophys. 1966, 116, 177–192. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shoen, C.M.; DeStefano, M.S.; Hager, C.C.; Tham, K.-T.; Braunstein, M.; Allen, A.D.; Gates, H.O.; Cynamon, M.H.; Kernodle, D.S. A Modified Bacillus Calmette-Guérin (BCG) Vaccine with Reduced Activity of Antioxidants and Glutamine Synthetase Exhibits Enhanced Protection of Mice despite Diminished in Vivo Persistence. Vaccines 2013, 1, 34-57. https://doi.org/10.3390/vaccines1010034
Shoen CM, DeStefano MS, Hager CC, Tham K-T, Braunstein M, Allen AD, Gates HO, Cynamon MH, Kernodle DS. A Modified Bacillus Calmette-Guérin (BCG) Vaccine with Reduced Activity of Antioxidants and Glutamine Synthetase Exhibits Enhanced Protection of Mice despite Diminished in Vivo Persistence. Vaccines. 2013; 1(1):34-57. https://doi.org/10.3390/vaccines1010034
Chicago/Turabian StyleShoen, Carolyn M., Michelle S. DeStefano, Cynthia C. Hager, Kyi-Toe Tham, Miriam Braunstein, Alexandria D. Allen, Hiriam O. Gates, Michael H. Cynamon, and Douglas S. Kernodle. 2013. "A Modified Bacillus Calmette-Guérin (BCG) Vaccine with Reduced Activity of Antioxidants and Glutamine Synthetase Exhibits Enhanced Protection of Mice despite Diminished in Vivo Persistence" Vaccines 1, no. 1: 34-57. https://doi.org/10.3390/vaccines1010034




