Abstract
The aim of the study was to determine the effect of yeast probiotic on body weight, and the activities of anti-oxidant enzymes: superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx), and malondialdehyde (MDA) concentration of broiler chickens. The experiment was carried out on hybrid Hubbard broiler chickens (n = 200). Two-hundred day-old chicks were randomly selected and distributed into four groups of 50 day-old chicks each: Control, C, and treatment groups comprising T1, T2 and T3 administered with 0.25 mL, 0.5 mL and 1.0 mL yeast probiotic, respectively. Chicks were fed a commercial starter diet for the first 28 days of age, followed by pelleted finisher diet from 29 to 42 days. Chickens in T1 had a significantly (p < 0.01) higher body weight at 4th week of age when compared with the control. SOD activity in all treatment groups was not significantly (p > 0.05) different when compared with the control. GPx activity was significantly (p < 0.01) higher in T1, when compared with the control. GPx activity in T2 was higher (p < 0.01) when compared with the control. There was no significant (p > 0.05) difference in MDA level in all the treatment groups. In conclusion, administering yeast probiotic supplement increased body weight and enhanced serum anti-oxidant enzyme activities of broiler chickens.
1. Introduction
Probiotics are viable microbial feed supplements, which stimulate the growth and health as well as modify the ecology of the intestine in a beneficial manner for the host [1,2]. They have been successfully used to improve the health of poultry and to achieve better production [3,4]. Feeding of Saccharomyces cerevisiae to broiler chickens enhances growth performance in a dose-dependent manner [5]. Nutrition plays a huge role in maintaining the pro-oxidant-antioxidant balance [6]. An excessive production of reactive species (RS) causes an imbalance in the pro-oxidants/anti-oxidants system, and any imbalance in favor of the pro-oxidants potentially leading to damage is termed oxidative stress [7]. Recently, an adapted concept of oxidative stress (OxS) was advanced by Jones [8], which states that OxS causes disruption of redox signaling and control, emphasizing the impact of the redox ratio as vital tools for the quantification of oxidative stress. Broiler chickens are exposed to natural or induced stressors, some of which induce reactive oxygen species (ROS) generation, which may promote morphological and/or physiological malfunctions. Presently, much attention has been focused on new approaches of anti-oxidant therapy by providing anti-oxidant enzymes. All aerobic organisms possess antioxidant enzymes capable of preventing membrane cell damage, enzyme inactivation and nucleic acid alterations. Main anti-oxidant enzymes that constitute the first line of anti-oxidant enzymatic defenses include superoxide dismutase (SOD) [EC.1.15.1.1], catalase (CAT) [EC.1.11.1.6] and glutathione peroxidase (GPx) [EC.1.11.1.9]. SOD catalyzes dismutation of superoxide radicals to hydrogen-peroxide and oxygen; CAT catalyzes the breakdown of hydrogen-peroxide to water and molecular oxygen. GPx is a selenium-dependent enzyme, which decomposes peroxides using the peptide glutathione (GSH) as its co-substrate [9]. Enzymatic ROS scavengers are catalase and peroxidases that lower the steady-state concentration of H2O2, a precursor of potent radical species. Thus, the cytotoxic potential of H2O2 is predominantly a function of intracellular catalase and peroxidase activities that scavenge H2O2 and concentration of free ions of transition metals that promote generation of OH from H2O2. ROS have been considered as the major mediators of oxygen cytotoxicity and as important messengers, stimulating cell division and demonstrating cellular signaling effects [10]. A concentration of MDA in tissues and urine are generally used as a biomarker for radical-induced damage and endogenous lipid peroxidation [11,12].
Saccharomyces cerevisiae cells respond to oxidative stress by altering their transcriptional program in a complex manner [13,14,15]. Zhang et al. [5] reported improve oxidative stability in male broiler chickens supplemented with S. cerevisiae. However, there is paucity of information on anti-oxidant enzyme activity of yeast probiotic used as feed supplement in broiler chickens using in vivo assays. Based on this background, the aim of the study was to determine the effect of yeast probiotic on growth and activity of main anti-oxidant enzymes and malondialdehyde concentration in broiler chickens. We hypothesized that different concentrations of yeast probiotic administered orally to broiler chickens enhance growth, anti-oxidant enzyme activities and maintain tissue malondialdehyde concentration.
2. Experimental Section
2.1. Experimental Design and Animal Management
The experiment was carried out on hybrid Hubbard broiler chickens. Two hundred day-old chicks were randomly selected and distributed into four groups of 50 day-old chicks each: Control, C; T1 0.25 mL, T2 0.5 mL and T3 1.0 mL yeast probiotic treatment groups. Birds were housed in an environmentally controlled poultry house with floor covered with wood shavings. The shavings were kept dry throughout the experimental period by routine replacement of the spoiled litter. Ambient temperature and relative humidity were 28.9 °C and 92%, respectively. Chicks were administered with Newcastle disease vaccine (NDV-intra-ocular), Infectious bursal disease vaccine (IBDV), and Newcastle disease vaccine (NDV-La Sota) on day 3, 14, and 21, respectively. The yeast probiotic was orally administered to each chick in the experimental groups through the beak, at the concentration of 0.5 mL/L of drinking water daily, using Unitract® 1 mL Tuberculin (TB) syringes (Clinicare, Mumbai, India). This is a single culture probiotic, containing Saccharomyces cerevisiae at 4.125 × 106 CFU per 100 mL. The quantity of probiotic administered to the first treatment group (T1) 0.25 mL) was ½ the standard concentration and unreconstituted in drinking water. Chicks were fed a commercial broiler starter diet for the first 28 days of age, and pelleted finisher diet from 29 to 42 days of age. Birds had 15 h of light and 9 h of darkness throughout the experimental period. The ingredients and nutrient levels of experimental diets, and the chemical composition of diet carried out by proximate analysis are shown in Table 1 and Table 2, respectively. Feed and water were provided ad libitum. Body weight (BW), feed conversion ratio (FCR), and feed intake (FI) were recorded on a weekly basis for comparative evaluation and interaction effects of all treatment groups. Mortality was observed and recorded on daily basis. After 42 days of feeding, blood was collected from 15 randomly selected broiler chickens from each group through the jugular or brachial vein into polythene tubes after 5 h of fasting. Blood (i.e., serum samples) was used for the analysis of antioxidant enzymes and MDA.

Table 1.
Ingredients and nutrient levels of experimental diets.
| Ingredients | Starter | Finisher | Starter Nutrient levels | Finisher Nutrient levels | ||
|---|---|---|---|---|---|---|
| Maize | N/S | N/S | ME, Kcal/Kg | 2800 | ME, Kcal/kg | 2900 |
| Soybean | N/S | N/S | CP, % | 20 | CP, % | 19 |
| Palm kernel cake | N/S | N/S | Ca, % | 1.0 | Ca, % | 1.0 |
| Wheat offal | N/S | N/S | Available P, % | 0.45 | Available P, % | 0.40 |
| Fish meal | N/S | N/S | CF, % | 9 | CF, % | 10 |
| Blood meal | N/S | N/S | Fat, % | 10 | Fat, % | 10 |
| Bone meal | N/S | N/S | ||||
| Oyster shell | N/S | N/S | ||||
| l-Lysine | N/S | N/S | ||||
| l-Methionine | N/S | N/S | ||||
| Vit/Min premix | N/S | N/S | ||||
| Salt | N/S | N/S | ||||
| Total | N/S | N/S | ||||
N.B. Nutrient levels are values declared by manufacturer on the feed labels. N/S = Not stated.

Table 2.
Proximate analysis of starter and finisher feeds.
| Ingredient (%) | Starter | Finisher |
|---|---|---|
| Dry matter | 93.87 | 94.86 |
| Crude protein | 23.69 | 21.20 |
| Crude fibre | 4.87 | 6.14 |
| Oil | 6.04 | 5.31 |
| Ash | 7.86 | 7.88 |
| NFE | 57.54 | 59.47 |
2.2. Body Weight Measurement
Birds were weighed individually at weekly intervals using Mettler Toledo® digital precision weighing balance with a sensitivity of 0.01 g (Model MT-500D).
2.3. Carcass and Organ Weight Measurement
Ten fasted broiler chickens from a total of 50 birds per treatment group were randomly selected, and each was reweighed just before slaughtering. Immediately after stunning in an electrobath, the birds were slaughtered by severing the jugular vein. After evisceration, the abdominal fat was collected and weighed. Eviscerated carcasses and organs were individually weighed using a Mettler Toledo® digital precision weighing balance. The main meat parts (i.e., thighs and drumsticks), wings, shanks and other organs were weighed for each carcass to determine the individual part to carcass ratio. That is, organ yield was calculated using the formula: organ weight/carcass weight × 10.
2.4. Enzymatic Measurements
The activity of GPx [EC.1.11.1.9] was measured using the Paglia and Valentine [16] spectrophotometry method based on the Northwest Life Science Specialties (NWLSS™) Glutathione peroxidase assay kits protocol NWK-GPX01. The activity of SOD [EC.1.15.1.1] was assessed using the NWLSS™ Superoxide dismutase activity assay, which provided a simple, rate method for determining SOD activity. This method is based on monitoring the auto-oxidation rate of haematoxylin as originally described by Martin Jr., et al. [17], with modifications to increase robustness and reliability. Briefly, in the presence of SOD enzyme at specific assay pH (pH 7.4), the rate of auto-oxidation was inhibited and the percentage of inhibition is linearly proportional to the amount of SOD present within a specific range. Sample SOD activity was determined by measuring ratios of auto-oxidation rates in the presence and absence of the sample and expressed as traditional McCord-Fridovich “cytochrome c” units. The activity of CAT [EC.1.11.1.6] was determined spectrophotomerically according to the method of Beers and Sizer [18], with modifications to increase robustness and convenience using the NWLSS™ Catalase activity assay kits protocol NWK-CAT01. MDA level was analyzed using the standard method of NWLSS™ MDA assay kits NWK-MDA01. This was based on the reaction of MDA with thiobarbituric acid (TBA); forming an MDA-TBA2 adduct that absorbed strongly at 532 nm [19]. Enzyme activity was expressed as units per millilitre (U/mL) of the sample.
2.5. Statistical Analysis
GraphPad Windows 4.03 (GraphPad Software, San Diego, CA, USA) was used for statistical analyses and data obtained were expressed as the mean ± standard error of the mean (Mean ± SEM). Data were analyzed using repeated measures ANOVA. Turkey’s post hoc test was used to compare all treatment groups with the control and between treated groups. Values of p < 0.05 were considered significant.
3. Results and Discussion
3.1. Body Weight, Feed Intake and Feed Conversion Ratio of Broiler Chickens
Results are shown in Table 3, Table 4 and Table 5. Body weights from week 1 and 2 were highly significant (p < 0.001) in T2 group but significantly (p < 0.05) different in the same T2 group in week 3 when compared with the control group, respectively. In addition, there was a significant (p < 0.001) difference in body weight in T2 and T3 in week 2 when T2 and T3 were compared with T1 and T2, respectively. In week 3, significance differences (p < 0.01) and (p < 0.05) in body weights were observed between T2 and T1, and between T3 and T2, respectively. There were significant (p < 0.01) differences in body weight between T2 and T1 and between T3 and T1, respectively in week 4. However, in week 5, a significant (p < 0.05) difference in body weight only exists between T3 and T1. There were highly significant (p < 0.001) differences between T1 and T2; T1 and T3. In the sixth week of the experiment, there was no significant difference (p > 0.05) in body weight between the treated groups and the control. Overall, the mean body weight obtained in T1 (1269 ± 31.17 g) in the sixth week was higher when compared with other experimental groups and the control. Feed intake in week 3 decreased (p < 0.05) in T2 and T3 when compared with the control group. However, there was a significant reduction (p < 0.01) in FI between T1 and T2, and between T1 and T3, respectively. In the fourth week, FI decreased (p < 0.001) significantly in T2 and T3 when compared between treated groups, and with the control. A similar trend of decreased (p < 0.001) in FI was observed in week 5 and 6, respectively. A significant improvement in FCR was recorded in the first (T1) treatment group. In the sixth week of age, there was significant (p < 0.05) decrease in feed conversion ratio of broiler chickens in T1 when compared with the control. The feed conversion ratio of broiler chickens in the sixth week of the experiment for control, T1, T2, and T3 were 0.97, 0.49, 0.64, and 0.53, respectively. Feed conversion efficiency did not differ (p > 0.05) in all the treatment groups in the 1st week of the experiment. During the six weeks of the experimental period, the percentage of mortality in each group was 24, 12, 20 and 16% for control, T1, T2, and T3, respectively.
The present study revealed that probiotic supplementation had an impact on the body weight of broiler chickens from the fourth week of life. This may be explained as one of the yeast probiotic beneficial effects of promoting a healthy gastrointestinal tract environment by nourishing the enterocytes, improving ileal mucosal development and reinforcing mucosal barrier function through maintaining epithelial integrity. This finding is similar with the result obtained by Zhang et al. [5], who reported improve growth rate of male broiler chickens supplemented with S. cerevisiae. In addition, this finding agrees with the results obtained by several authors [20,21] in studies with broiler chickens. The highest body weight was recorded in the experimental group administered with the lowest probiotic concentration (T1 0.25 mL). This very concentration was directly administered to the birds daily without reconstitution in drinking water. However, T2 could not cause an increase in body weight as seen in T3. This probably may be associated with the dose effect in T3, which is higher than the T1 dose. This result demonstrated that single strain Saccharomyces cerevisiae (Antox® probiotic) supplementation has a more positive effect on body weight when administered in lower concentrations, without being reconstituted in drinking water as prescribed by the manufacturer (Montajat Veterinary Pharmaceutical Co. Ltd., Dammam, Saudi Arabia). Santin et al. [22] showed that the cell walls of Saccharomyces cerevisiae improve nutrient absorption from the intestinal mucosa and suggested that this factor may be responsible for the improvement in performance of broiler chickens supplemented with S. cerevisiae. A probiotic acts by reducing the feed conversion ratio, resulting in an increase in daily live weight gain [23], which is achieved through a natural physiological way and improvement of digestion by balancing the resident gut microflora. The differences in final body weight in the sixth week may be associated with differences in feed intake.

Table 3.
Effect of supplemental yeast probiotic on body weight of broilers. Significance difference is indicated by single and double asterisk as: * p < 0.05 vs. control, ** p < 0.01 vs. control, *** p < 0.001 vs. control; C, control group (without probiotic); T1, first treatment group; T2, second treatment group; T3, third treatment group. The data are presented as Mean ± SEM, (n = 30). Significance difference at p < 0.05.
| Week | C | T1 0.25 mL | T2 0.5 mL | T3 1.0 mL | p-values |
|---|---|---|---|---|---|
| 1 | 90.97 ± 1.98 | 91.63 ± 1.14 | 67.63 ± 2.45 *** | 89.77 ± 1.61 *** | p < 0.0001 |
| 2 | 185.4 ± 5.25 | 199 ± 3.84 | 147.9 ± 6.34 *** | 185.4 ± 5.29 *** | p < 0.0001 |
| 3 | 402.7 ± 12.55 | 425.3 ± 10.13 | 355.4 ± 16.57 * | 407.9 ± 12.48 * | 0.0015 |
| 4 | 647.4 ± 17.29 | 733.6 ± 13.93 ** | 611.6 ± 23.92 *** | 544.7 ± 18.15 ** | p < 0.0001 |
| 5 | 886.0 ± 30.77 | 926.5 ± 18.59 | 862.1 ± 38.19 | 804 ± 32.50 * | 0.0322 |
| 6 | 1157 ± 49.55 | 1269 ± 31.17 | 1141 ± 43.29 | 1143 ± 46.48 | 0.1307 |

Table 4.
Effects of dietary yeast probiotic supplement on feed intake of broilers. Significance difference is indicated by single and double asterisk as: * p < 0.05 vs. control, ** p < 0.01 vs. 0.5, *** p < 0.001 vs. control. C, control group (without probiotic); T1, first treatment group; T2, second treatment group; T3, third treatment group. The data are presented as Mean ± SEM, (n = 30). Significance difference at p < 0.05.
| Week | C | T1 0.25 mL | T2 0.5 mL | T3 1.0 mL | p-values |
|---|---|---|---|---|---|
| 1 | 424.3 ± 68 | 574.3 ± 116 | 365.7 ± 33 | 557.4 ± 108 | 0.0395 |
| 2 | 1161.0 ± 91 | 1175.0 ± 103 | 1097.0 ± 97 | 1089.0 ± 91 | p < 0.0001 |
| 3 | 2149.0 ± 222 | 2206.0 ± 205 | 1771.0 ± 139 * | 1805.0 ± 137 * | p < 0.0001 |
| 4 | 2958.0 ± 133 | 3007.0 ± 132 | 2559.0 ± 107 *** | 2579.0 ± 105 *** | p < 0.0001 |
| 5 | 5379.0 ± 424 | 5521.0 ± 343 | 4342.0 ± 273 *** | 4377.0 ± 271 *** | p < 0.0001 |
| 6 | 7283.0 ± 116 | 7570.0 ± 173 | 5856.0 ± 65 *** | 6159.0 ± 107 *** | p < 0.0001 |

Table 5.
Effect of supplemental yeast probiotic on feed conversion ratio of broilers. C, control group (without probiotic); T1, first treatment group; T2, second treatment group; T3, third treatment group.
| Week | C | T1 0.25 mL | T2 0.5 mL | T3 1.0 mL |
|---|---|---|---|---|
| 1 | 0.16 | 0.21 | 0.24 | 0.22 |
| 2 | 0.33 | 0.24 | 0.39 | 0.31 |
| 3 | 0.27 | 0.21 | 0.26 | 0.22 |
| 4 | 0.33 | 0.22 | 0.30 | 0.52 |
| 5 | 0.66 | 0.64 | 0.53 | 0.48 |
| 6 | 0.79 | 0.49 | 0.64 | 0.53 |
In the present study, there were significant differences in feed intake between treated birds and control birds. This may be attributed to improved digestion and absorption of nutrient in the digestive tract due to the presence of live yeast cells of Saccharomyces cerevisiae. These findings are consistent with a series of experimental studies, which revealed that dietary prebiotics [24] and probiotics [25] increase feed intake of broiler chickens. However, it is in contrast with the findings by [26,27] who reported that dietary additions of probiotics and organic acid preparations, respectively, did not affect feed intake of broiler chickens. Improvement in feed intake by dietary probiotic and prebiotic supplementation often resulted in improved growth performance. Furthermore, significant differences in feed intake existing between treated birds and the control group may be partly responsible for the variation in growth performance. Nevertheless, the birds supplemented with the lowest concentration of the probiotic were heavier than the birds in other treatment groups with the control (C) inclusive. A significant improvement in feed conversion efficiency was recorded in the first experimental group (T1) treated with the probiotic. FCR values for broiler chickens treated with 0.25 mL of dietary Saccharomyces cerevisiae probiotic possessed the highest body weight at the 6th week. These results are in agreement with the findings of Jin et al. [28] who reported that although a significant improvement in feed conversion ratio was observed in probiotic-supplemented broilers chickens, the results were inconsistent. Probiotics act by reducing the feed conversion, thereby resulting in an increase in daily live weight gain. This may be attributable to efficient ileal digestibility of nutrients. Szymczyk et al. [29] reported a marked reduction in feed conversion efficiency in animals fed 1.5% conjugated linoleic acid-supplemented diet relative to control. In addition, Bansal et al. [23] reported significant and better weekly feed conversion efficiency on probiotic supplementation in the diet of commercial broiler chicks.
3.2. Carcass and Organ Weights of Broiler Chickens
Weights and yields for carcasses and some organs of broiler chickens are presented in Table 3. Birds in T1 experimental group tended to be heavier than birds in T2 and T3 experimental groups. However, they were much heavier (p < 0.01) when compared with the control group. Weights of the thighs and drum sticks in T1 were higher (p < 0.05) when compared with the control group, but the yield percentages were not. Abdominal fat weight was significantly low (p < 0.001) in all probiotic-supplemented groups when compared with control group. This was consistent with the decreased yield percentages in all the probiotic supplemented groups. There was no significant difference (p > 0.05) in the weights of the heart and intestine in all the treated groups. In addition, there was no significant difference (p > 0.05) in the weights of the gizzards, liver, spleen, gall bladder and lungs. The differences in carcass weights of broiler chickens may originate from differences in feed conversion ratio and feed intake. In the present study, the feed conversion ratio was low in T1 probiotic group (results not included) and this same group recorded the highest body weight in the xith week of the experimental period. These results further corroborated the recent findings that probiotic act by reducing the feed conversion ratio, thereby resulting in an increase in daily live weight gain [23]. The yeast probiotic in T1 was more effective in elevating the live weight of broiler chickens followed by T3 group. This may be explained as the improved digestion and absorption of nutrients in the digestive tract of broiler chickens by the live yeast cells of Saccharomyces cerevisiae. Abdominal fat represents the main fat deposition in broiler chickens and it seems to be directly related to total carcass fat [30,31]. Excess accumulation of abdominal fat means both processing and waste problems, but also indicates inefficient energy use [32]. In the present study, significant differences were observed in abdominal fat in probiotic-supplemented groups. There was overt decreased in weight of abdominal fat in all the probiotic-supplemented groups, indicating the fact that probiotics enhance efficient energy usage. This study is similar with several studies that reported lowering of abdominal fat by probiotic supplementation [24,30,32,33]. On the contrary, Bozkurt et al. [34] showed that dietary supplementation of prebiotics, organic acids and probiotics had no significant effect on abdominal fat pad accumulation in broiler chickens. The entire mass of the intestine in T1 group was heavier but not significant than the weights of intestine from other experimental groups. This result disagrees with the findings by Alcicek et al. [35], who reported that dietary supplementation of probiotics lowered the weight of the small intestine. In addition, there was no significant effect of the probiotic on the weights of organs like the liver, gizzard and lungs. In some previous findings, dietary prebiotic and probiotic supplementation, respectively, did not increase the liver weights of broiler chickens [28,36]. Results of probiotic effect on the carcass and organ weights of broilers are shown below (Table 6).

Table 6.
Effect of supplemental yeast probiotic on carcass and organ weights of broilers. Significance difference is indicated by single, double and triple asterisk as: * p < 0.05 vs. control, ** p < 0.01 vs. control, *** p < 0.001 vs. control; C, control group (without probiotic); T1, first treatment group; T2, second treatment group; T3, third treatment group. The data are presented as Mean ± SEM, (n = 10). Significance difference at p < 0.05.
| Parameters | C | T1 0.25 mL | T2 0.5 mL | T3 1.0 mL | p-values |
|---|---|---|---|---|---|
| Live weight (g) | 1382.0 ± 37.95 | 1678.0 ± 64.34 ** | 1482.0 ± 34.69 | 1515.0 ± 56.08 | 0.0034 |
| Carcass weight | 903.5 ± 35.47 | 1094.0 ± 46.68 ** | 987.5 ± 26.82 | 996.7 ± 31.77 | 0.0096 |
| % | 65.38 | 65.20 | 66.63 | 65.79 | |
| Thigh (g) | 270.0 ± 8.65 | 318.6 ± 14.49 * | 298.9 ± 8.11 | 303.8 ± 10.91 | 0.0424 |
| % | 29.88 | 29.12 | 30.27 | 30.48 | |
| Drum stick (g) | 153.9 ± 4.68 | 178.5 ± 7.73 * | 171.5 ± 5.55 | 170.7 ± 3.56 | 0.0368 |
| % | 17.03 | 16.32 | 17.37 | 17.13 | |
| Abdominal fat | 9.10 ± 0.18 | 6.60 ± 0.16 *** | 7.60 ± 0.16 *** | 7.10 ± 0.23 *** | p < 0.0001 |
| % | 1.01 | 0.60 | 0.77 | 0.71 | |
| Gizzard (g) | 45.00 ± 4.79 | 57.50 ± 2.32 | 54.80 ± 2.34 | 56.30 ± 3.89 | 0.1050 |
| % | 4.98 | 5.26 | 5.55 | 5.65 | |
| Liver (g) | 44.50 ± 1.92 | 45.80 ± 1.98 | 47.10 ± 1.96 | 50.00 ± 1.76 | 0.1455 |
| % | 4.93 | 4.19 | 4.77 | 5.02 | |
| Heart (g) | 9.40 ± 0.45 | 10.40 ± 0.52 | 9.40 ± 0.31 | 10.90 ± 0.32 | 0.0376 |
| % | 1.04 | 0.95 | 0.95 | 1.09 | |
| Lungs (g) | 10.70 ± 0.42 | 11.80 ± 0.49 | 10.80 ± 0.51 | 11.60 ± 0.43 | 0.2633 |
| % | 1.18 | 1.08 | 1.09 | 1.16 | |
| Intestine (g) | 151.7 ± 8.23 | 177.6 ± 7.64 | 157.3 ± 4.64 | 168.0 ± 7.11 | 0.0590 |
| % | 16.79 | 16.23 | 15.93 | 16.86 |
3.3. Anti-Oxidant Enzyme Activities and Malondialdehyde Concentration of Broiler Chickens
Results of serum anti-oxidant enzyme activities are shown in Figure 1. CAT activity was significantly higher (45.53 ± 1.60 U/mL; p < 0.01) in T1 when compared with T2. In addition, there was significant (p < 0.05) CAT activity in T3 when compared with T2. SOD activity in all the treatment groups was not significantly (p > 0.05) different when compared with the control and treatment groups. GPx activity was greater (43.20 ± 1.02 U/mL; p < 0.001) in T1 when compared with T3 and control group.
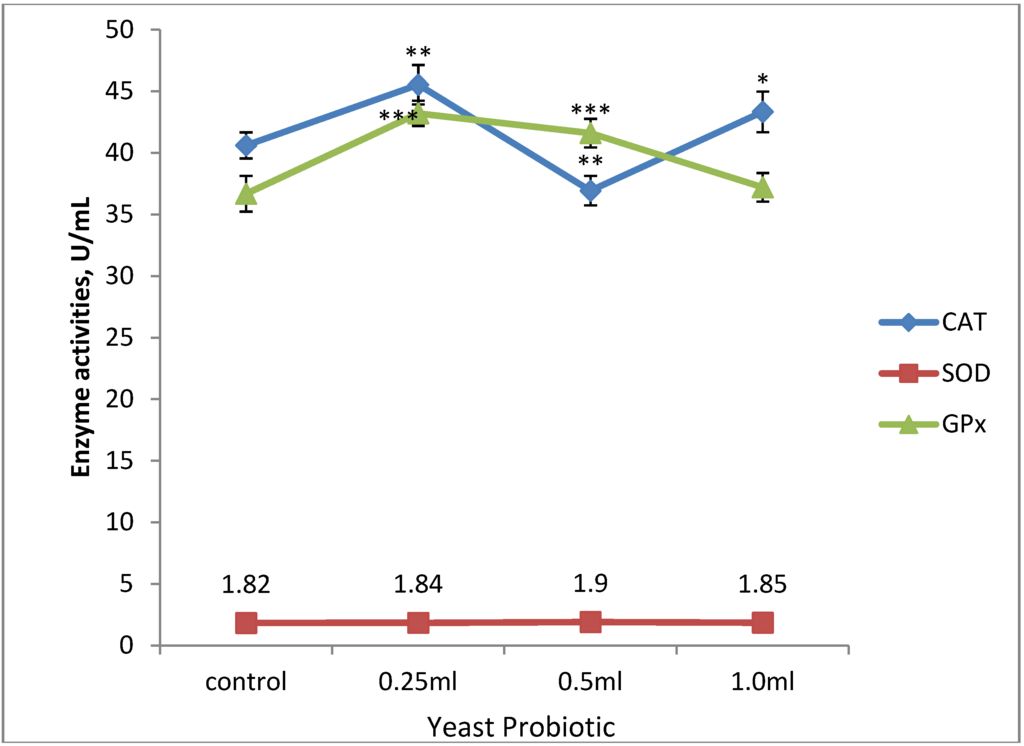
Figure 1.
Effect of supplemental yeast probiotic on serum antioxidant enzyme activities of broiler chickens. Significance difference is indicated by single, double and triple asterisk as: * p < 0.05 vs. 1.0, ** p < 0.01 vs. 0.5, *** p < 0.001 vs. control. Data are presented as Mean ± SEM, (n = 15).
In addition, a more significant (p < 0.01) difference in GPx activity exists between T2 and the control. When T1 was compared with T3 and T2 with T3, a significant (p < 0.05) difference was observed between these treatment groups. There was significant (p < 0.05) difference in MDA concentration in T1 when compared with T3.
Anti-oxidant enzymes are most effective when acting synergistically with one another or with other components of the anti-oxidant barrier of the organism when their activity remains balanced. It has been shown that nutrition plays a vital role in maintaining the pro-oxidant-antioxidant balance [6]. In the present study, there was increased in both catalase and glutathione peroxidase activities. The increased in activity of these antioxidant enzymes may be attributed to the age, colonization resistance, and susceptibility to environmental pathogens of the birds. This is in agreement with the work of numerous scholars [37,38] who reported that an increased in the activity of catalase in the blood is caused by environmental burdens to which birds are exposed to during their growth. In addition, most importantly growth processes in early life is characterized with the generation of ROS through cellular division and apoptosis. This is because ROS are considered as the major mediators of oxygen cytotoxicity and as important messengers stimulating cell division and manifesting cellular signaling effects [10]. A similar study in turkey reported that mannanoligosaccharides a component of S. cerevisiae used as dietary additive stimulate the mechanisms of oxidative defense and improve the growth performance of the birds [39]. Krizkova et al. [40] showed that mannans from S. cerevisiae have antioxidative property in vitro. This suggests that dietary yeast can protect the gastrointestinal tract in ways other than just removing undesirable bacteria. In addition, Kogan et al. [41] suggested that yeast cell wall β-glucans may have antioxidant activity. The steady state of SOD activity in the probiotic-supplemented groups may reflect a significant improvement in health and oxidative status of the birds. These findings are contrary to results obtained by Milinkovic-Tur et al. [42], who reported increased activities of SOD and CAT in the heart muscles of broiler chickens. Catalase activity, as well as the activity of other anti-oxidant enzymes, depends on the presence of anti-oxidants in the diet. Oxidative damage develops when anti-oxidant potential is reduced and/or when factors contributing to oxidative stress increase [43,44]. The increase in GPx activity in T1 suggests greater oxidative stress. Also, GPx increased activity in T2 may be attributed to the sodium selenite fraction of the yeast (S. cerevisiae) probiotic, which partly enhances anti-oxidative activity. The present results further corroborate the existing knowledge on the positive effect of selenium on GPx activity in chicken erythrocytes, blood, muscles and the liver [45,46]. GPx displays its activity mainly in the cellular cytoplasm and only about ten percent of activity is displayed in the mitochondria [47]. In this manner, the safe removal of hydrogen peroxide is attained through the joint action of GPx and CAT. Malondialdehyde level endogenously reflects lipid peroxidation, which is the sequela of diminished anti-oxidant protection as ROS levels increase. MDA level was higher in T1 (Figure 2), and this is a direct reflection of GPx activity in T1. This may be attributed to the probiotic inability to confer adequate anti-oxidant protection against lipid peroxidation during the growth phase of the birds. We are unaware of any research work on S. cerevisiae as anti-oxidant and used to study oxidative stress during growth in broiler chickens.
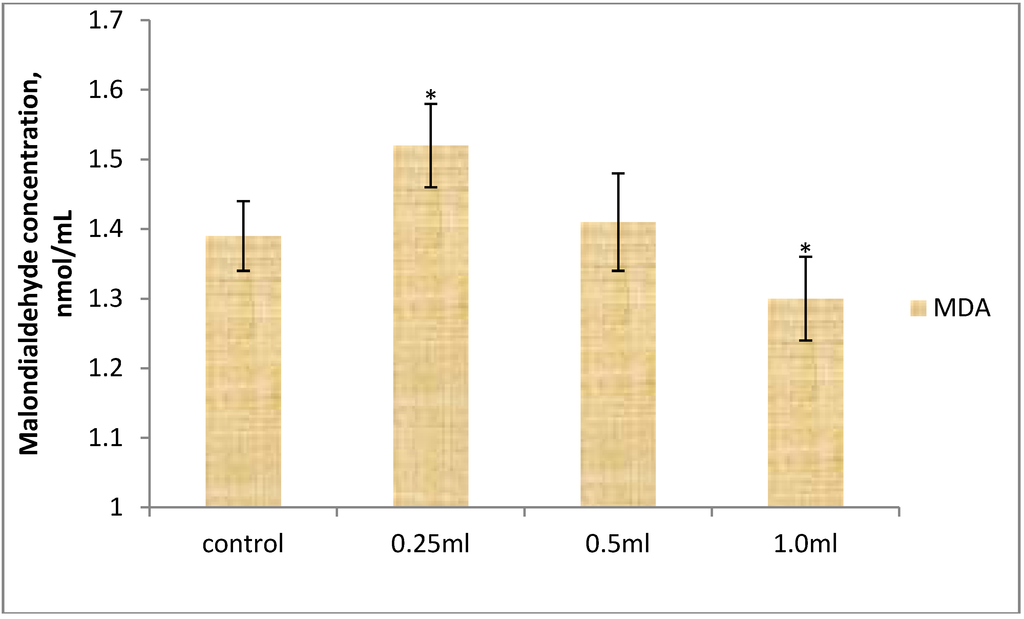
Figure 2.
Effect of supplemental yeast probiotic on serum malondialdehyde concentration of broiler chickens. Significance difference is indicated by single asterisk as: * p < 0.05 vs. 1.0. Data are presented as Mean ± SEM, (n = 15).
4. Conclusions
In conclusion, to maintain the physiological grade of oxidative stress needed for a number of bio-functions like growth, broiler chickens have an integrated antioxidant defense system that is enhanced by the yeast probiotic. However, it has an inability to confer adequate anti-oxidant protection against lipid peroxidation during growth. This therefore, confirms that S. cerevisiae is an optimal eukaryotic model to study cellular events related to oxidative stress.
Acknowledgments
The work was supported by the Academic Staff of the Department of Veterinary Physiology, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria, Nigeria that constitute the Nutritional physiology research group. The authors acknowledge the excellent technical assistance of all staff working in the Poultry Unit of the Veterinary Teaching Hospital, Ahmadu Bello University, Zaria, Nigeria.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Breves, G.; Walter, C.; Burmeister, M.; Shroder, B. In vitro studies on the effects of Saccharomyces boulardii and Bacillus cereus var. toyoi on nutrient transport in pig jejunum. J. Anim. Physiol. Anim. Nutr. 2000, 84, 9–20. [Google Scholar] [CrossRef]
- Simon, O.; Vahjen, W.; Scharek, L. Microorganisms as Feed Additive-Probiotics. In Proceedings of the 9th International Symposium on Digestive Physiology in Pigs, Banff, Alberta, Canada, 14–17 May 2003; Volume 1, pp. 295–318.
- O’dea, E.E.; Fasenko, G.M.; Allison, G.E.; Korver, D.R.; Tannock, G.W.; Guan, L.L. Investigating the effects of commercial probiotics on broiler chick quality and production efficiency. Poult. Sci. 2006, 85, 1855–1863. [Google Scholar] [CrossRef] [PubMed]
- Peric, L.; Milosevic, N.; Zikic, D.; Bjedov, S.; Cvetkovic, D.; Markov, S.; Mohnl, M.; Steiner, T. Effect of probiotic and phytobiotic products on performance, gut morphology and cecal microflora of broiler chicks. Arch. Tierz. 2010, 53, 350–359. [Google Scholar]
- Zhang, A.W.; Lee, B.D.; Lee, S.K.; Lee, K.W.; An, G.H.; Song, K.B.; Lee, C.H. Effects of yeast (Saccharomyces cerevisiae) cell components on growth performance, meat quality and ileal mucosa development of broiler chicks. Poult. Sci. 2005, 84, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Cowey, C.B. The role of nutritional factors in the prevention of peroxidative damage to tissues. Fish Physiol. Biochem. 1986, 2, 171–178. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: Oxidants and Antioxidants; Academic Press: New York, NY, USA, 1991. [Google Scholar]
- Jones, D.P. Redefining oxidative stress. Antioxid. Redox Signal. 2006, 8, 1865–1879. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive species and antioxidants redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef]
- Buetler, T.M.; Krauskopf, A.; Ruegg, U.T. Role of superoxide as a signalling molecule. News Physiol. Sci. 2004, 19, 120–123. [Google Scholar] [PubMed]
- Day, C.P. Is necro-inflammation a prerequisite for fibrosis? Hepatogastroenterology 1996, 43, 104–120. [Google Scholar] [PubMed]
- Wang, Y.Z.; Xu, C.L.; An, Z.H.; Liu, J.-X.; Feng, J. Effect of dietary bovine lactoferin on performance and antioxidant status of piglets. Anim. Feed Sci. Technol. 2008, 140, 326–336. [Google Scholar] [CrossRef]
- Gasch, A.P.; Spellman, P.T.; Kao, C.M.; Carmel-Harel, O.; Eisen, M.B.; Storz, G.; Botstein, D.; Brown, P.O. Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell. 2000, 11, 4241–4257. [Google Scholar] [CrossRef]
- Ikner, A.; Shiozaki, K. Yeast signalling pathways in the oxidative stress response. Mutat. Res. 2005, 569, 13–27. [Google Scholar] [CrossRef]
- Temple, M.D.; Perrone, G.G.; Dawes, I.W. Complex cellular responses to reactive species. Trends Cell Biol. 2005, 15, 319–326. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- Martin, J.P., Jr.; Dailey, M.; Sugarman, E. Negative and positive assays of superoxide dismutase based on haematoxylin auto-oxidation. Arch. Biochem. Biophys. 1987, 255, 329–336. [Google Scholar] [CrossRef]
- Beers, R.F., Jr.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by Catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [PubMed]
- Placer, Z.A.; Cushman, L.L.; Johnson, B.C. Estimation of lipid peroxidation, malondialdehyde in biochemical system. Ann. Biochem. 1996, 16, 359–367. [Google Scholar]
- Gohain, A.K.; Sapcota, D. Effect of probiotics feeding on the performance of broilers. Indian J. Poult. Sci. 1998, 33, 101–105. [Google Scholar]
- Morales-Lopez, R.; Auclair, E.; van Immerseel, F.; Ducatelle, R.; Garcia, F.; Brufau, J. Effects of different yeast cell wall supplements added to maize- or wheat-based diets for broiler chickens. Br. Poult. Sci. 2010, 51, 399–408. [Google Scholar] [CrossRef]
- Santin, E.; Maiorka, A.; Macari, M.; Grecco, M.; Sanchez, J.C.; Okada, T.M.; Myasaka, A.M. Performance and intestinal mucosa development of broiler chickens fed diets containing Saccharomyces cerevisiae cell wall. J. Appl. Poult. Res. 2001, 10, 236–244. [Google Scholar] [CrossRef]
- Bansal, G.R.; Singh, V.P.; Sachan, N. Effect of probiotic supplementation on the performance of broilers. Asian J. Anim. Sci. 2011, 5, 277–284. [Google Scholar] [CrossRef]
- Safalaoh, A.C.L. Body weight gain, dressing percentage, abdominal fat and serum cholesterol of broilers supplemented with a microbial preparation. Afr. J. Food Agric. Nutr. Dev. 2006, 6, 1–10. [Google Scholar]
- Sanchez, R.; Ayaya, J.A. Effect of MOS on Broiler Performance under Field Conditions; Alltech’s INC.: Selden, NY, USA, 1998. [Google Scholar]
- Yeo, J.; Kyu-il, K. Effect of feeding diets containing an antibiotic, a probiotic, or yucca extract on growth and intestinal urease activity in broiler chicks. Poult. Sci. 1997, 76, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Sims, M.D.; Sefton, A.E. Comparative Effects of Mannan-Oligosaccharide and an Antibiotic Growth Promoter on Performance of Commercial Broilers. In Presented at the 50th North Central Avian Disease Conference, Vancouver, BC, Canada, 1999.
- Jin, L.Z.; Ho, Y.W.; Abdullahi, N.; Ali, M.A.; Jalaludin, S. Effects of adherent Lactobacillus cultures on growth, weight of organs and intestinal microflora and volatile fatty acids in broilers. Anim. Feed Sci. 1998, 70, 197–209. [Google Scholar] [CrossRef]
- Szymczyk, B.; Pisulewski, M.P.; Szczurek, S. Effects of conjugated linoleic acid on growth performance, feed conversion efficiency, and subsequent carcass quality in broiler chickens. Br. J. Nutr. 2001, 85, 465–473. [Google Scholar] [CrossRef]
- Santoso, U.; Tanaka, K.; Ohtani, S. Effect of dried Bacillus subtilis culture on growth, body composition and hepatic lipogenic enzyme activity in female broiler chicks. Br. J. Nutr. 1995, 74, 523–529. [Google Scholar] [CrossRef]
- Gaya, L.G.; Mourao, G.B.; Rzende, F.M.; Chicaronide Matos, E.; Filho, T.M.; Figueiredo, L.G.G.; Ferraz, J.B.S.; Eler, J.P. Genetic trends of abdominal fat content in a male broiler chicken line. Genet. Mol. Res. 2005, 4, 760–764. [Google Scholar] [PubMed]
- Homma, H.; Shinohara, T. Effects of probiotic Bacillus cereus toyoi on abdominal fat accumulation in Japanese quail (Coturnix japonica). Anim. Sci. J. 2004, 75, 37–41. [Google Scholar] [CrossRef]
- Anjum, M.L.; Khan, A.G.; Azim, A.; Afzal, M. Effect of dietary supplementation of multi-strain probiotic on broiler growth performance. Pak. Vet. J. 2005, 25, 25–29. [Google Scholar]
- Bozkurt, M.; Kucukyilmaz, K.; Cath, A.U.; Cinar, M. Growth Performance and Carcass Yield of Broiler Chickens Given Antibiotic, Mannan Oligosaccharide and Dextran Oligosaccharide Supplemented Diets. In Nutritional Biotechnology in the Feed and Food Industries, Proceedings of Alltech’s 21st Annual Symposium, Lexington, KY, USA, 22–25 May 2005; pp. 5–13.
- Alcicek, A.; Bozkurt, M.; Cabuk, M. The effects of a mixture of herbal essential oil, an organic acid or a probiotic on broiler performance. S. Afr. J. Anim. Sci. 2004, 34, 217–222. [Google Scholar]
- Bozkurt, M.; Kucukyilmaz, K.; Cath, A.U.; Cinar, M. The effect of single or combined dietary supplementation of prebiotics, organic acid and probiotics on performance and slaughter characteristics of broilers. S. Afr. J. Anim. Sci. 2009, 39, 197–205. [Google Scholar]
- Gutowicz, M.; Cholojczyk, M.; Pyrzanowska, R.M.; Cholojczyk, M.; Pyrzanowska, J.; Widy-Tyszkiewicz, E.; Baranczyk-Kuzma, A. Effect of curcumin on antioxidant and detoxification mechanisms in the livers of aging rats. Med. Weter. 2008, 64, 955–957. (in Polish). [Google Scholar]
- Lecewicz, A.; Jankowski, J.; Zdunczyk, Z.; Juskiewicz, J. Selected factors stimulating the development of some gastrointestinal parts in turkeys. Med. Weter. 2008, 64, 1184–1186. [Google Scholar]
- Ognik, K.; Krauze, M. Dietary supplementation of mannanoligosaccharides to turkey hens on their growth performance and antioxidant status in blood. S. Afr. J. Anim. Sci. 2012, 42, 379–388. [Google Scholar]
- Krizkova, L.; Durackova, Z.; Sandula, J.; Sasinkova, V.; Krajcovic, J. Antioxidative and antimutagenic activity of yeast cell wall mannans in vitro. Mutat. Res. 2001, 497, 213–222. [Google Scholar] [CrossRef]
- Kogan, G.; Pajtinka, M.; Babincova, M.; Miadokova, E.; Rauko, P.; Slamenova, D.; Korolenko, T.A. Yesat cell wall polysaccharides as antioxidants and antimutagens: Can they fight cancer? Neoplasma 2008, 55, 387–393. [Google Scholar] [PubMed]
- Milinkovic-Tur, S.; Aladrovic, J.; Ljubic, B.B.; Poljicak-Milas, N. Age-related antioxidant enzyme activities and lipid peroxidation in heart muscles of broiler chickens fed with supplementary organic selenium. Vet. Arhiv. 2009, 79, 481–489. [Google Scholar]
- Ibrahim, W.; Lee, U.S.; Yen, H.C.; Siclair, D.K.; Chow, C.K. Antioxidant and oxidative stress status in tissues of manganese superoxide dismutase transgenic mice. Free Radic. Biol. Med. 2000, 3, 397–402. [Google Scholar]
- Milinkovic-Tur, S.; Stojevic, Z.; Pirsljin, J.; Zdelar-Tuk, M.; Poljicak-Milas, N.; Beer Ljubic, B.; Gradinski-Vrbanac, B. Effect of fasting and refeeding on the antioxidant system in cockerels and pullets. Acta Vet. Hung. 2007, 55, 181–189. [Google Scholar] [CrossRef]
- Kuricova, S.; Boldizarova, K.; Geresakova, L.; Bobcek, R.; Levkut, M.; Leng, L. Chicken selenium status when fed a diet supplemented with Se-yeast. Acta Vet. Brno 2003, 72, 339–346. [Google Scholar] [CrossRef]
- Pirsljin, J.; Milinkovic-Tur, S.; Beer Ljubic, B.; Zdelar-Tuk, M. The effect of organic selenium supplementation on the antioxidative characteristics and lipid peroxidation of chicken blood during fattening and after fasting. Vet. Arhiv. 2008, 78, 187–196. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C. Free Radical Biology and Medicine, 3rd ed.; Oxford University Press: Oxford, UK, 1999; pp. 23–27. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).