Systemic and CSF Interleukin-1α Expression in a Rabbit Closed Cranium Subarachnoid Hemorrhage Model: An Exploratory Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Study Design, and Anesthesia
2.2. Digital Subtraction Angiography and SAH Induction
2.3. Blood and CSF Samples Analysis
2.4. Statistical Analysis
2.5. Ethic Approval
3. Results
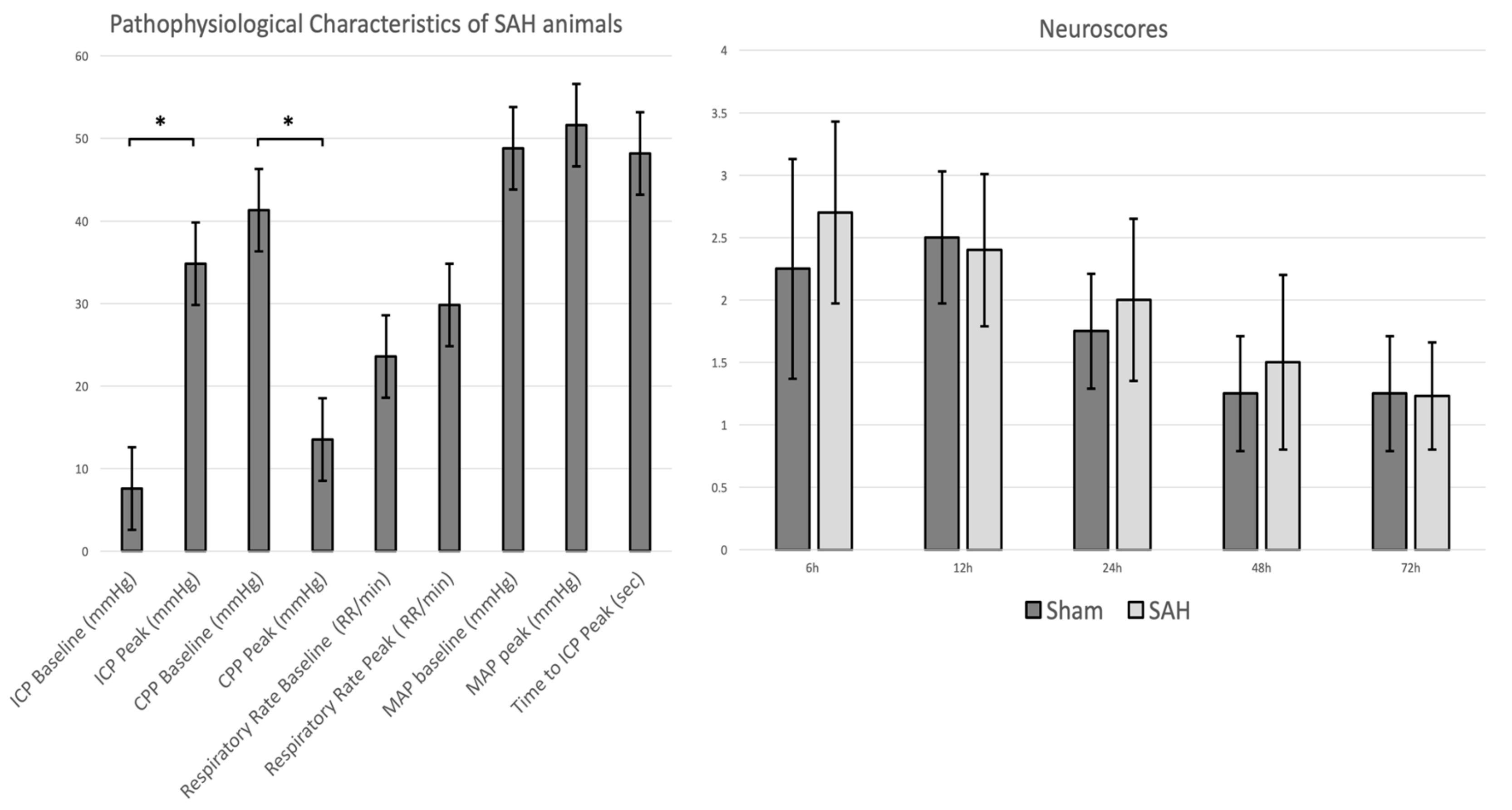
3.1. Physiological Parameters, ICP Time Course, and SAH and Clinical Scores
3.2. Angiographic Delayed Cerebral Vasospasm
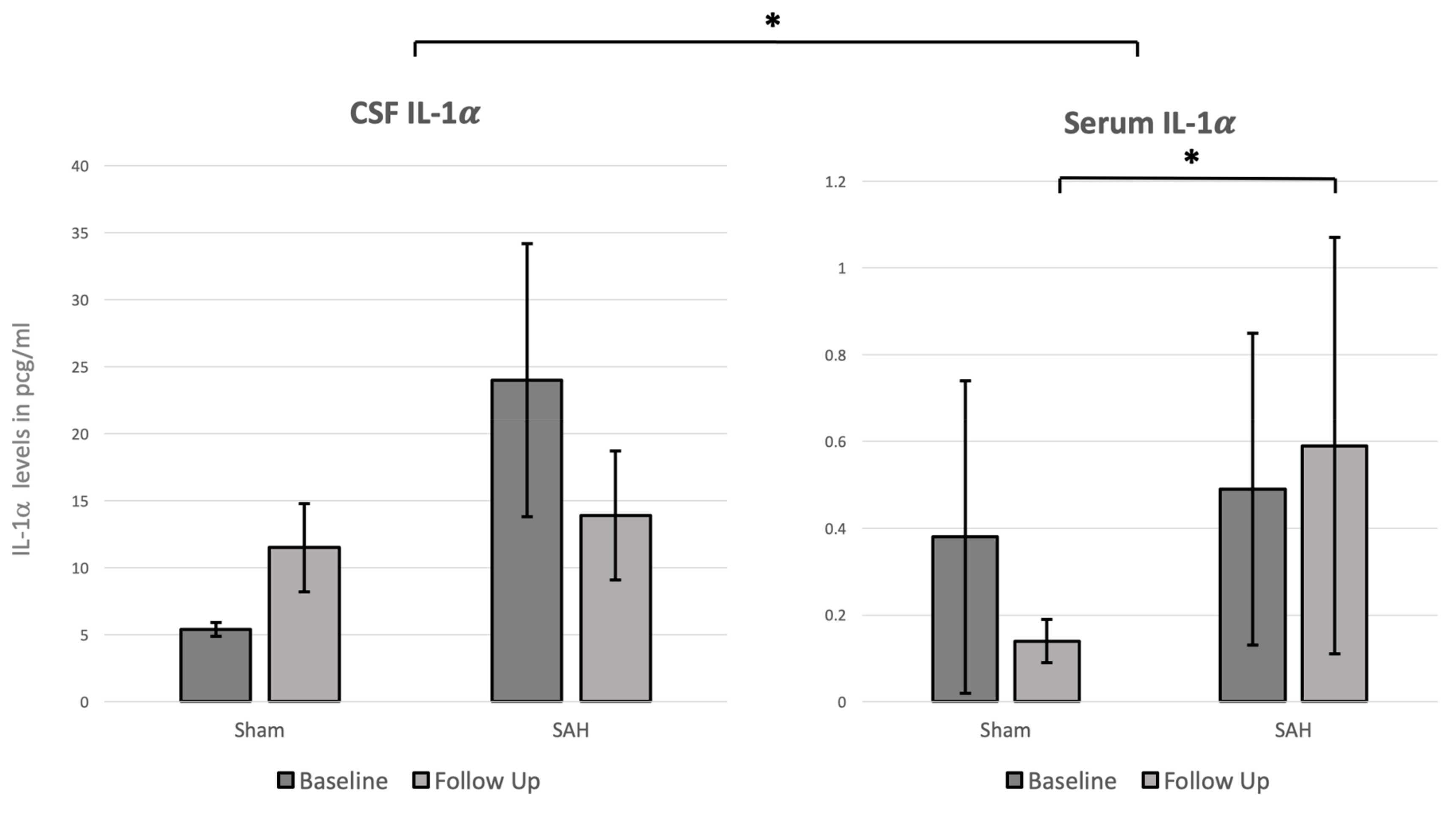
3.3. CSF and Systemic IL-1α Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Croci, D.; Nevzati, E.; Danura, H.; Schöpf, S.; Fandino, J.; Marbacher, S.; Muroi, C. The relationship between IL-6, ET-1 and cerebral vasospasm, in experimental rabbit subarachnoid hemorrhage. J. Neurosurg. Sci. 2016, 63, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Niwa, A.; Osuka, K.; Nakura, T.; Matsuo, N.; Watabe, T.; Takayasu, M. Interleukin-6, MCP-1, IP-10, and MIG are sequentially expressed in cerebrospinal fluid after subarachnoid hemorrhage. J. Neuroinflamm. 2017, 13, 217. [Google Scholar] [CrossRef] [PubMed]
- Fassbender, K.; Hodapp, B.; Rossol, S.; Bertsch, T.; Schmeck, J.; Schütt, S.; Fritzinger, M.; Horn, P.; Vajkoczy, P.; Kreisel, S.; et al. Inflammatory cytokines in subarachnoid haemorrhage: Association with abnormal blood flow velocities in basal cerebral arteries. J. Neurol. Neurosurg. Psychiatry 2001, 70, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Osuka, K.; Suzuki, Y.; Tanazawa, T.; Hattori, K.; Yamamoto, N.; Takayasu, M.; Shibuya, M.; Yoshida, J. Interleukin-6 and development of vasospasm after subarachnoid haemorrhage. Acta Neurochir. (Wien.) 1998, 140, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur. J. Immunol. 2011, 41, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. The biological properties of interleukin-1. Eur. Cytokine Netw. 1994, 5, 517–531. [Google Scholar]
- Arend, W.P.; Malyak, M.; Guthridge, C.J.; Gabay, C. Interleukin-1 receptor antagonist: Role in biology. Annu. Rev. Immunol. 1998, 16, 27–55. [Google Scholar] [CrossRef]
- Kohno, K.; Kurimoto, M. Interleukin 18, a cytokine which resembles IL-1 structurally and IL-12 functionally but exerts its effect independently of both. Clin. Immunol. Immunopathol. 1998, 86, 11–15. [Google Scholar] [CrossRef]
- Mandrup-Poulsen, T. The role of interleukin-1 in the pathogenesis of IDDM. Diabetologia 1996, 39, 1005–1029. [Google Scholar] [CrossRef]
- Hansen, M.K.; Taishi, P.; Chen, Z.; Krueger, J.M. Vagotomy blocks the induction of interleukin-1beta (IL-1beta) mRNA in the brain of rats in response to systemic IL-1beta. J. Neurosci. 1998, 18, 2247–2253. [Google Scholar] [CrossRef] [PubMed]
- Goehler, L.E.; Relton, J.K.; Dripps, D.; Kiechle, R.; Tartaglia, N.; Maier, S.F.; Watkins, L.R. Vagal paraganglia bind biotinylated interleukin-1 receptor antagonist: A possible mechanism for immune-to-brain communication. Brain Res. Bull. 1997, 43, 357–364. [Google Scholar] [CrossRef]
- Zheng, H.; Fletcher, D.; Kozak, W.; Jiang, M.; Hofmann, K.J.; Conn, C.A.; Soszynski, D.; Grabiec, C.; Trumbauer, M.E.; Shaw, A. Resistance to fever induction and impaired acute-phase response in interleukin-1 beta-deficient mice. Immunity 1995, 3, 9–19. [Google Scholar] [CrossRef]
- Shadiack, A.M.; Hart, R.P.; Carlson, C.D.; Jonakait, G.M. Interleukin-1 induces substance P in sympathetic ganglia through the induction of leukemia inhibitory factor (LIF). J. Neurosci. 1993, 13, 2601–2609. [Google Scholar] [CrossRef] [PubMed]
- Allan, S.M.; Tyrrell, P.J.; Rothwell, N.J. Interleukin-1 and neuronal injury. Nat. Rev. Immunol. 2005, 5, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Allan, S.M. Pragmatic target discovery from novel gene to functionally defined drug target: The interleukin-1 story. Methods Mol. Med. 2005, 104, 333–346. [Google Scholar]
- Kandere-Grzybowska, K.; Letourneau, R.; Kempuraj, D.; Donelan, J.; Poplawski, S.; Boucher, W.; Athanassiou, A.; Theoharides, T.C. IL-1 induces vesicular secretion of IL-6 without degranulation from human mast cells. J. Immunol. 2003, 171, 4830–4836. [Google Scholar] [CrossRef]
- Pluta, R.M.; Hansen-Schwartz, J.; Dreier, J.; Vajkoczy, P.; Macdonald, R.L.; Nishizawa, S.; Kasuya, H.; Wellman, G.; Keller, E.; Zauner, A.; et al. Cerebral vasospasm following subarachnoid hemorrhage: Time for a new world of thought. Neurol. Res. 2009, 31, 151–158. [Google Scholar] [CrossRef]
- Hasan, D.; Chalouhi, N.; Jabbour, P.; Hashimoto, T. Macrophage imbalance (M1 vs. M2) and upregulation of mast cells in wall of ruptured human cerebral aneurysms: Preliminary results. J. Neuroinflamm. 2012, 9, 222–227. [Google Scholar] [CrossRef]
- Korherr, C.; Hofmeister, R.; Wesche, H.; Falk, W. A critical role for interleukin-1 receptor accessory protein in interleukin-1 signaling. Eur. J. Immunol. 1997, 27, 262–267. [Google Scholar] [CrossRef]
- Mosley, B.; Urdal, D.L.; Prickett, K.S.; Larsen, A.; Cosman, D.; Conlon, P.J.; Gillis, S.; Dower, S.K. The interleukin-1 receptor binds the human interleukin-1 alpha precursor but not the interleukin-1 beta precursor. J. Biol. Chem. 1987, 262, 2941–2944. [Google Scholar]
- Hannum, C.H.; Wilcox, C.J.; Arend, W.P.; Joslin, F.G.; Dripps, D.J.; Heimdal, P.L.; Armes, L.G.; Sommer, A.; Eisenberg, S.P.; Thompson, R.C. Interleukin-1 receptor antagonist activity of a human interleukin-1 inhibitor. Nature 1990, 343, 336–340. [Google Scholar] [CrossRef]
- Greenhalgh, A.D.; Brough, D.; Robinson, E.M.; Girard, S.; Rothwell, N.J.; Allan, S.M. Interleukin-1 receptor antagonist is beneficial after subarachnoid haemorrhage in rat by blocking haem-driven inflammatory pathology. Dis. Model. Mech. 2012, 5, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Brough, D.; Dénes, Á. Interleukin-1α and brain inflammation. IUBMB Life 2015, 67, 323–330. [Google Scholar] [CrossRef]
- Orsini, F.; Fumagalli, S.; Császár, E.; Tóth, K.; De Blasio, D.; Zangari, R.; Lénárt, N.; Dénes, Á.; De Simoni, M.-G. Mannose-Binding Lectin Drives Platelet Inflammatory Phenotype and Vascular Damage After Cerebral Ischemia in Mice via IL (Interleukin)-1α. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2678–2690. [Google Scholar] [CrossRef]
- Luheshi, N.M.; Kovács, K.J.; Lopez-Castejon, G.; Brough, D.; Dénes, Á. Interleukin-1α expression precedes IL-1β after ischemic brain injury and is localised to areas of focal neuronal loss and penumbral tissues. J. Neuroinflamm. 2011, 8, 186. [Google Scholar] [CrossRef]
- Eisenhut, M. Vasospasm in cerebral inflammation. Int. J. Inflam. 2014, 2014, 509707–509714. [Google Scholar] [CrossRef]
- Randomisation and online databases for clinical trials. Available online: https://www.sealedenvelope.com/ (accessed on 5 March 2018).
- Endo, S.; Branson, P.J.; Alksne, J.F. Experimental model of symptomatic vasospasm in rabbits. Stroke 1988, 19, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Marbacher, S.; Nevzati, E.; Croci, D.; Erhardt, S.; Muroi, C.; Jakob, S.M.; Fandino, J. The rabbit shunt model of subarachnoid haemorrhage. Transl. Stroke Res. 2014, 5, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Andereggen, L.; Neuschmelting, V.; von Gunten, M.; Widmer, H.R.; Takala, J.; Jakob, S.M.; Fandino, J.; Marbacher, S. The rabbit blood-shunt model for the study of acute and late sequelae of subarachnoid hemorrhage: Technical aspects. J. Vis. Exp. 2014, 92, e52132. [Google Scholar] [CrossRef]
- Marbacher, S.; Fathi, A.-R.; Muroi, C.; Coluccia, D.; Andereggen, L.; Neuschmelting, V.; Widmer, H.R.; Jakob, S.M.; Fandino, J. The rabbit blood shunt subarachnoid haemorrhage model. Acta Neurochir. Suppl. 2015, 120, 337–342. [Google Scholar] [PubMed]
- Zhang, Z.-W.; Yanamoto, H.; Nagata, I.; Miyamoto, S.; Nakajo, Y.; Xue, J.-H.; Iihara, K.; Kikuchi, H. Platelet-derived growth factor-induced severe and chronic vasoconstriction of cerebral arteries: Proposed growth factor explanation of cerebral vasospasm. Neurosurgery 2010, 66, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Marbacher, S.; Neuschmelting, V.; Andereggen, L.; Widmer, H.R.; von Gunten, M.; Takala, J.; Jakob, S.M.; Fandino, J. Early brain injury linearly correlates with reduction in cerebral perfusion pressure during the hyperacute phase of subarachnoid hemorrhage. Intensive Care Med. Exp. 2014, 2, 30. [Google Scholar] [CrossRef] [PubMed]
- Marbacher, S.; Sherif, C.; Neuschmelting, V.; Schläppi, J.-A.; Takala, J.; Jakob, S.M.; Fandino, J. Extra-intracranial blood shunt mimicking aneurysm rupture: Intracranial-pressure-controlled rabbit subarachnoid hemorrhage model. J. Neurosci. Methods 2010, 191, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Marbacher, S.; Andereggen, L.; Neuschmelting, V.; Widmer, H.R.; von Gunten, M.; Takala, J.; Jakob, S.M.; Fandino, J. A new rabbit model for the study of early brain injury after subarachnoid hemorrhage. J. Neurosci. Methods 2012, 208, 138–145. [Google Scholar] [CrossRef]
- Marbacher, S.; Milavec, H.; Neuschmelting, V.; Andereggen, L.; Erhardt, S.; Fandino, J. Outer skull landmark-based coordinates for measurement of cerebral blood flow and intracranial pressure in rabbits. J. Neurosci. Methods 2011, 201, 322–326. [Google Scholar] [CrossRef] [PubMed]
- NC3Rs Reporting Guidelines Working Group. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. J. Physiol. (Lond.) 2010, 588, 2519–2521. [Google Scholar] [CrossRef]
- Zheng, Y.; Humphry, M.; Maguire, J.J.; Bennett, M.R.; Clarke, M.C.H. Intracellular interleukin-1 receptor 2 binding prevents cleavage and activity of interleukin-1α, controlling necrosis-induced sterile inflammation. Immunity 2013, 38, 285–295. [Google Scholar] [CrossRef]
- Emsley, H.C.A.; Smith, C.J.; Georgiou, R.F.; Vail, A.; Hopkins, S.J.; Rothwell, N.J.; Tyrrell, P.J. Acute Stroke Investigators A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1366–1372. [Google Scholar] [CrossRef]
- Galea, J.; Ogungbenro, K.; Hulme, S.; Patel, H.; Scarth, S.; Hoadley, M.; Illingworth, K.; McMahon, C.J.; Tzerakis, N.; King, A.T.; et al. Reduction of inflammation after administration of interleukin-1 receptor antagonist following aneurysmal subarachnoid hemorrhage: Results of the Subcutaneous Interleukin-1Ra in SAH (SCIL-SAH) study. J. Neurosurg. 2018, 128, 515–523. [Google Scholar] [CrossRef]
- Singh, N.; Hopkins, S.J.; Hulme, S.; Galea, J.P.; Hoadley, M.; Vail, A.; Hutchinson, P.J.; Grainger, S.; Rothwell, N.J.; King, A.T.; et al. The effect of intravenous interleukin-1 receptor antagonist on inflammatory mediators in cerebrospinal fluid after subarachnoid haemorrhage: A phase II randomised controlled trial. J. Neuroinflamm. 2014, 11, 1. [Google Scholar] [CrossRef] [PubMed]

| Parameters | SAH Group (n = 10) | Sham Group (n = 4) | p-Value |
|---|---|---|---|
| Baseline | |||
| pH | 7.3 (±0.1) | 7.3 (±0.1) | 0.310 |
| pCO2 (mmHg) | 77.1 (±12.5) | 70.5 (±14.5) | 0.215 |
| pO2 (mmHg) | 340.5 (±95.2) | 308 (±150.6) | 0.330 |
| HCO3-mmol/L | 28.9 (±7.1) | 30.0 (±0.6) | 0.325 |
| BE mmol/L | 10.8 (±5.9) | 9.1 (±2.9) | 0.301 |
| SO2% | 99.8 (±0.5) | 99.5 (±0.7) | 0.268 |
| ctHb (g/dL) | 12.3 (±5.3) | 12.5 (±0.1) | 0.323 |
| Na+ mmol/L | 143.3 (±2.2) | 142 (±2.2) | 0.164 |
| K+ mmol/L | 3.5 (±0.3) | 3.47 (±2.2) | 0.401 |
| Ca2+ mmol/L | 1.6 (±0.1) | 1.5 (±0.1) | 0.187 |
| Glu mmol/L | 15.8 (±3.4) | 15.7 (±1.1) | 0.487 |
| Lac mmol/L | 0.5 (±0.3) | 0.5 (±0.1) | 0.371 |
| Heart rate/min | 187.6 (±18.6) | 175.7 (±3.8) | 0.223 |
| Middle Arterial Pressure | 54.6 (±4.6) | 63.7 (±0.3) | 0.082 |
| Weight Kg | 3.73 (±0.3) | 3.53 (±0.2) | 0.183 |
| Follow up | SAH Group (n = 9) | Sham Group (n = 3) | p-Value |
| pH | 7.3 (±0.1) | 7.3 (±0.1) | 0.349 |
| pCO2 (mmHg) | 70 (±14.3) | 68.9 (±14.7) | 0.457 |
| pO2 (mmHg) | 351 (±27.8) | 431.3 (±29) | 0.078 |
| HCO3-mmol/L | 13.2 (±6.2) | 22.5 (±7.2) | 0.138 |
| BE mmol/L | 7.3 (±7.2) | 14 (±7.3) | 0.186 |
| SO2% | 99.2 (±0.8) | 99.5 (±0.7) | 0.338 |
| ctHb (g/dL) | 10.8 (±0.8) | 10.8 (±0.4) | 0.50 |
| Na+ mmol/L | 142.7 (±3.8) | 143 (±3.5) | 0.435 |
| K+ mmol/L | 3.5 (±1.7) | 3.9 (±1.5) | 0.006 |
| Ca2+ mmol/L | 1.5 (±0.1) | 1.5 (±0.1) | 0.449 |
| Glu mmol/L | 18.8 (±4.1) | 18.6 (±0.4) | 0.428 |
| Lac mmol/L | 0.6 (±0.1) | 0.6 (±0.2) | 0.415 |
| Heart rate/min | 177.5 (±20.2) | 184.5 (±7.5) | 0.371 |
| Middle Arterial Pressure | 53 (±12.7) | 57 (±12.7) | 0.399 |
| Weight Kg | 3.52 (±0.4) | 3.35 (±0.1) | 0.07 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Croci, D.M.; Wanderer, S.; Strange, F.; Grüter, B.E.; Casoni, D.; Sivanrupan, S.; Widmer, H.R.; Di Santo, S.; Fandino, J.; Mariani, L.; et al. Systemic and CSF Interleukin-1α Expression in a Rabbit Closed Cranium Subarachnoid Hemorrhage Model: An Exploratory Study. Brain Sci. 2019, 9, 249. https://doi.org/10.3390/brainsci9100249
Croci DM, Wanderer S, Strange F, Grüter BE, Casoni D, Sivanrupan S, Widmer HR, Di Santo S, Fandino J, Mariani L, et al. Systemic and CSF Interleukin-1α Expression in a Rabbit Closed Cranium Subarachnoid Hemorrhage Model: An Exploratory Study. Brain Sciences. 2019; 9(10):249. https://doi.org/10.3390/brainsci9100249
Chicago/Turabian StyleCroci, Davide Marco, Stefan Wanderer, Fabio Strange, Basil E. Grüter, Daniela Casoni, Sivani Sivanrupan, Hans Rudolf Widmer, Stefano Di Santo, Javier Fandino, Luigi Mariani, and et al. 2019. "Systemic and CSF Interleukin-1α Expression in a Rabbit Closed Cranium Subarachnoid Hemorrhage Model: An Exploratory Study" Brain Sciences 9, no. 10: 249. https://doi.org/10.3390/brainsci9100249
APA StyleCroci, D. M., Wanderer, S., Strange, F., Grüter, B. E., Casoni, D., Sivanrupan, S., Widmer, H. R., Di Santo, S., Fandino, J., Mariani, L., & Marbacher, S. (2019). Systemic and CSF Interleukin-1α Expression in a Rabbit Closed Cranium Subarachnoid Hemorrhage Model: An Exploratory Study. Brain Sciences, 9(10), 249. https://doi.org/10.3390/brainsci9100249





