Lead Excretion in Spanish Children with Autism Spectrum Disorder
Abstract
:1. Introduction
- (1)
- To measured urinary Pb concentration in children with ASD and typically developing (TD) children.
- (2)
- Next, we evaluated the relationship between urine Pb levels and the severity of core symptoms of ASD (deficit in communication, socialization and restricted/stereotyped activities) according to the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DMS-IV).
- (3)
- Finally, we studied the influence of several factors such as age, sex, medication, diet, or other neurological comorbidities (sleep or gastrointestinal problems, epilepsy, food allergies/intolerances, level of verbality, etc.) in these associations in order to find any relationship with urinary Pb concentration.
2. Experimental Section
2.1. Participants
2.2. Evaluation of Core Symptoms
2.3. Medical Interview and Evaluation
2.4. Pb Analysis
2.5. Statistical Analysis
3. Results
3.1. Description of the Sample, Evaluation of Core Symptoms and Other Clinical Features
| Age (years) | ASD children: 7.4 ± 0.5 |
| range (4–13 years) | |
| TD children: 7.7 ± 0.9 | |
| Range (4–13 years) | |
| Gender | ASD children: 25 boys 10 girls |
| TD children: 24 boys 10 girls | |
| Body mass index | ASD children: 18.4 ± 0.5 |
| range (14–25) | |
| TD children 18.7 ± 0.8 | |
| (range 15–26) | |
| Head circumference (percentile rank) | 61 ± 5 |
| range (2–100) | |
| ASD type | 30 Autism, 5 NOS-PPD |
| Score of socialization deficit (ADI-R subscale) | 20.9 ± 2.1 |
| Score of communication deficit (ADI-R subscale) | 22.4 ± 2.9 |
| Score of repetitive/restricted behaviours (ADI-R subscale) | 7.2 ± 1.1 |
| Total ADI-R score | 50.4 ± 4.4 |
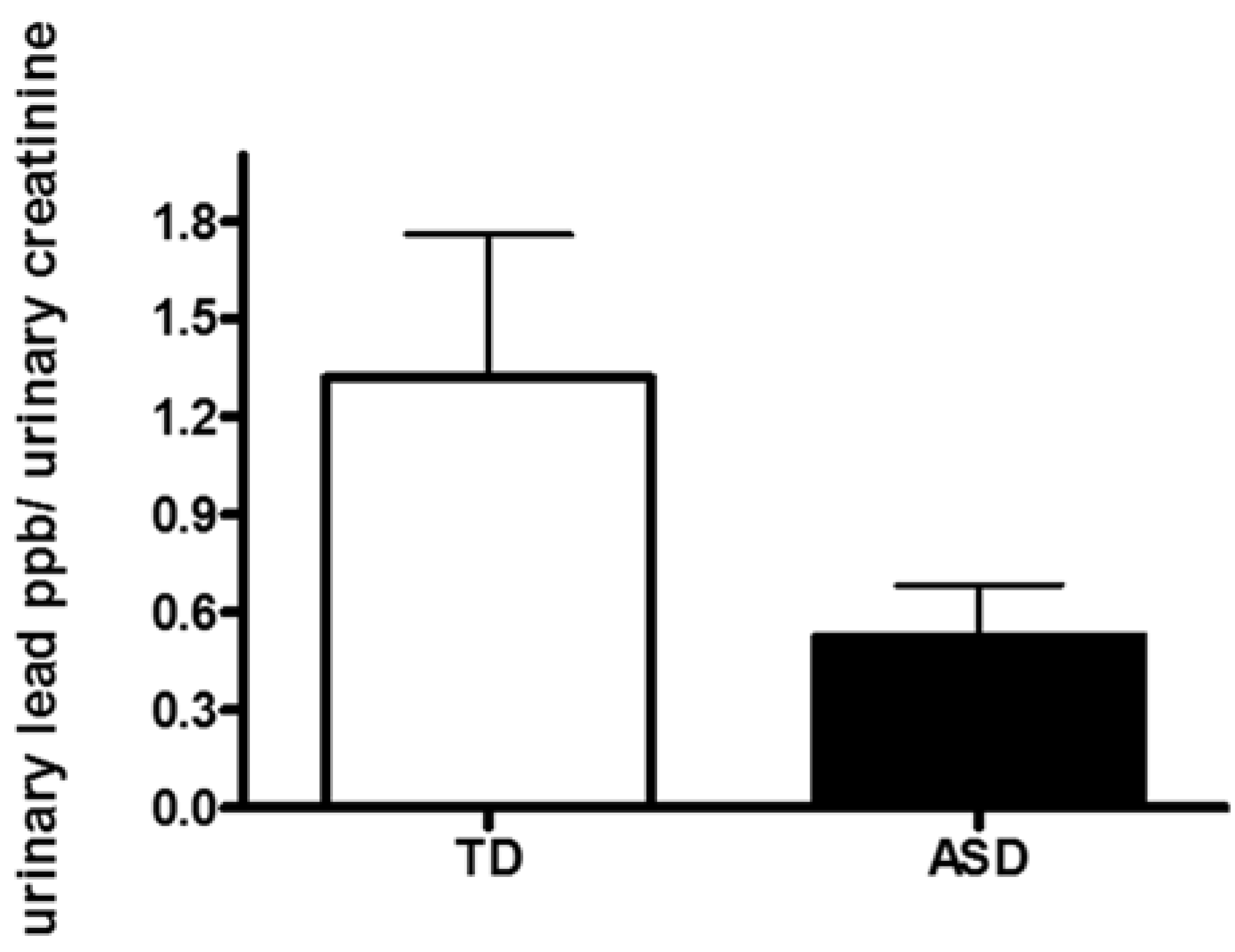
3.2. Evaluation of Urinary Pb Concentration
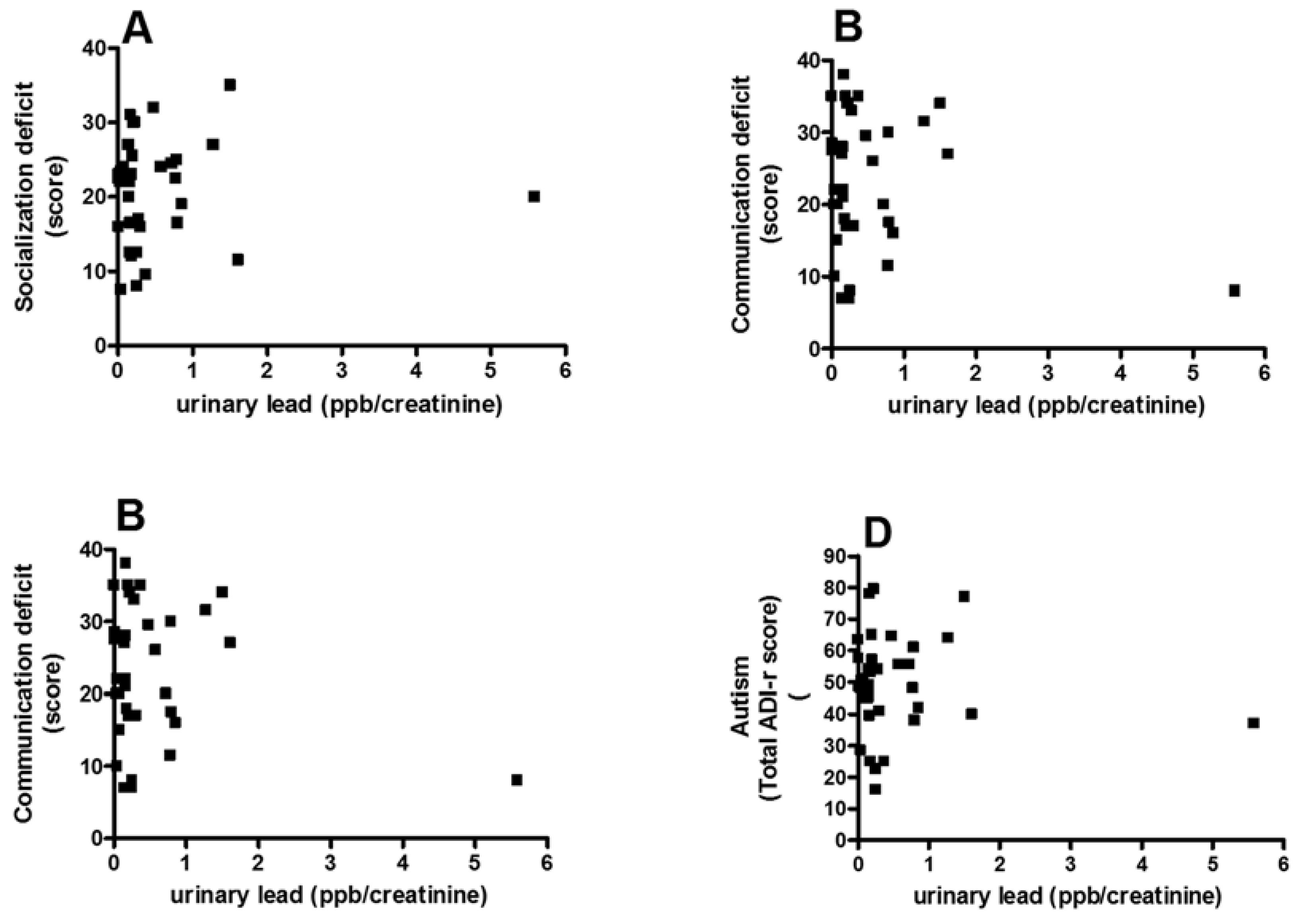
3.3. Correlation between Pb and Core Autism Spectrum Disorder Symptoms
3.4. Analysis of Possible Confounding Factors
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Connolly, J.J.; Hakonarson, H. Etiology of autism spectrum disorder: A genomics perspective. Curr. Psychiatry Rep. 2014, 16. [Google Scholar] [CrossRef]
- Tordjman, S.; Somogyi, E.; Coulon, N.; Kermarrec, S.; Cohen, D.; Bronsard, G.; Bonnot, O.; Weismann-Arcache, C.; Botbol, M.; Lauth, B.; et al. Gene × Environment interactions in autism spectrum disorders: Role of epigenetic mechanisms. Front. Psychiatry 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Golub, N.; Winters, P.; van Wijngaarden, E. A population based study of blood lead levels in relation to depression in the United States. Int. Arch. Occup. Environ. Health 2010, 83, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Weisskopf, M.G.; Proctor, S.P.; Wright, R.O.; Schwarts, J.; Spiro, A.; Sparrow, D.; Nie, H.; Hu, H. Comulative lead exposure and cognitive performance among elderly men. Epidemiology 2007, 18, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Weisskopf, M.G.; Wright, R.O.; Schwartz, J.; Spiro, A.; Aro, A.; Hu, H. Cumulative lead exposure and prospective change in cognition among elderly men: The VA normative aging study. Am. J. Epilemiol. 2004, 160, 1184–1193. [Google Scholar] [CrossRef]
- Wright, R.O.; Tsaih, S.W.; Schwart, J.; Spiro, A.; McDonald, K.; Weiss, S.T.; Hu, H. Lead exposure biomarkers and mini-mental status exam socres in older men. Epidemiology 2003, 14, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Muldoon, S.B.; Cauley, J.A.; Kuller, L.H.; Morrow, L.; Needleman, H.L.; Scott, J.; Hooper, F.J. Effects of blood lead levels on cognitive function of older women. Neuroepidemiology 1996, 15, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Weuve, J.; Korrick, S.A.; Weisskopt, M.G.; Ryan, L.M.; Schwartz, J.; Nie, H.; Grodstein, F.; Hu, H. Cumulative exposure to lead in relation to cognitive function in older women. Environ. Health Perspect. 2009, 117, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Bellinguer, D.C. Lead neurotoxicity and socioeconomic status: Conceptual and analytical issues. Neurotoxicology 2008, 29, 828–832. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, G.; Rosner, D. “Cater to the children”: The role of the lead industry in a public health tragedy, 1900–1955. Am. J. Public Health 2000, 90, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Martí-Cid, R.; Llobet, J.M.; Castell, V.; Domingo, J.L. Dietary intake of arsenic, cadmium, mercury ande lead by the population of Catalonia, Spain. Biol. Trace Elem. Res. 2008, 125, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Martorell, I.; Perelló, G.; Martí-Cid, R.; Llobet, J.M.; Castell, V.; Domingo, J.L. Human exposure to arsenic, cadmium, mercury and lead from foods in Catalonia, Spain: Temporal trend. Biol. Trace Elem. Res. 2011, 142, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Rubio, C.; González-Iglesias, T.; Revert, C.; Reguera, J.I.; Gutiérrez, A.J.; Hardisson, A. Lead dietary intake in a Spanish population (Canary Islands). J. Agric. Food Chem. 2005, 53, 6543–6549. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.L.; Kim, D.; Galeano, M.A.; Paul, C.J.; Morgan, S.P. The relationship between early childhood blood lead levels and performance on end-of-grade test. Environ. Health Perspect. 2007, 115, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Olivero-Verbel, J.; Padilla-Bottet, C.; de la Rosa, O. Relationships between phsycochemical parameters and the toxicity of leachates from a municipal solid waste landfill. Ecotoxicol. Environ. Saf. 2008, 70, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Llop, S.; Ibarlucea, J.; Sunyer, J.; Ballester, F. Current dietary exposure to mercury during pregnancy and childhood and public health recommendations. Gac. Sanit. 2013, 27, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Goodlad, J.K.; Marcus, D.K.; Fulton, J.J. Lead and attention-deficit/hyperactivity disorder (ADHD) symptoms: A meta-analysis. Clin. Psychol. Rev. 2013, 33, 147–128. [Google Scholar] [CrossRef]
- Eubig, P.A.; Aguiar, A.; Schantz, S.L. Lead and PCBs as risk factors for attention deficit/hiperactivity disorder. Environ. Health Perspect. 2010, 118, 1654–1667. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Green, P.G.; Stamova, B.; Hertz-Picciotto, I.; Pessah, I.N.; Hansen, R.; Yang, X.; Gregg, J.P.; Ashwood, P.; Jickling, G.; et al. Correlations of gene expression with blood lead levels in children wiht autism compared to typically develping controls. Neurotox. Res. 2011, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yorbik, O.; Kurt, I.; Hasimi, A.; Oztürk, O. Chromium, cadmium and lead levels in urine of children wiht autism and typically develping controls. Biol. Trace Elem. Res. 2010, 135, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Lord, C. Follow-up of two-year-olds referred for possible autism. J. Child Psychol. Psychiatry 1995, 36, 1365–1382. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, M.T.; Naghizadeh, B.; López-Larrubia, P.; Cauli, O. Gender-dependent behavioural impairment and brain metabolites in young adult rats after short term exposure to Pb acetate. Toxicol. Lett. 2012, 210, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Erfurth, E.M.; Gerhardsson, L.; Nilsson, A.; Rylander, L.; Schütz, A.; Skerfving, S.; Börjesson, J. Effects of lead on the endocrine system in lead smelter workers. Arch. Environ. Health 2001, 56, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Paschal, D.C.; Ting, B.G.; Morrow, J.C.; Pirkle, J.L.; Jackson, R.J.; Sampson, E.J.; Miller, D.T.; Caldwell, K.L. Trace metals in urine of United States residents: Reference range concentrations. Environ. Res. 1998, 76, 53–59. [Google Scholar] [CrossRef]
- Tietz, N.W. Clinical Guide to Laboratory Tests, 3rd ed.; W.B. Saunders: Philadelphia, PA, USA, 1995. [Google Scholar]
- Adams, J.B.; Audhya, T.; McDonough-Means, S.; Rubin, R.A.; Quig, D.; Geis, E.; Gehn, E.; Loresto, M.; Mitchell, J.; Atwood, S.; et al. Toxicological status of children with autism vs. neurotypical children and the association with autism severity. Biol. Trace Elem. Res. 2013, 151, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Geier, D.A.; Kern, J.K.; King, P.G.; Sykes, L.K.; Geier, M.R. Hair toxic metal concentrations and autism spectrum disorder severity in young children. Int. J. Environ. Res. Public Health 2012, 9, 4486–4497. [Google Scholar] [CrossRef] [PubMed]
- Valentino, M.; Rapisarda, V.; Santarelli, L.; Bracci, M.; Scorcelletti, M.; di Lorenzo, L.; Cassano, F.; Soleo, L. Effect of lead on the levels of some immunoregulatory cytokines in occupationally exposed workers. Hum. Exp. Toxicol. 2007, 26, 551–556. [Google Scholar] [CrossRef]
- Hertz-Picciotto, I.; Delwiche, L. The rise in autism and the role of age at diagnosis. Epidemiology 2009, 20, 84–90. [Google Scholar] [CrossRef] [PubMed]
- MMWR. Prevalence of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill. Summ. 2010, 63, 1–21. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Arlington, VA, USA, 2012. [Google Scholar]
- Macedoni-Lukšič, M.; Gosar, D.; Bjørklund, G.; Oražem, J.; Kodrič, J.; Lešnik-Musek, P.; Zupančič, M.; France-Štiglic, A.; Sešek-Briški, A.; Neubauer, D.; et al. Levels of Metals in the Blood and Specific Porphyrins in the Urine in Children with Autism Spectrum Disorders. Biol. Trace Elem. Res. 2014, 163, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.M.; Ly, A.R.; Goldberg, W.A.; Clarke-Stewart, K.A.; Dudgeon, J.V.; Mull, C.G.; Chan, T.J.; Kent, E.E.; Mason, A.Z.; Ericson, J.E.; et al. Heavy metal in children’s tooth enamel: Related to autism and disruptive behaviors? J. Autism Dev. Disord. 2012, 42, 929–936. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuentes-Albero, M.; Puig-Alcaraz, C.; Cauli, O. Lead Excretion in Spanish Children with Autism Spectrum Disorder. Brain Sci. 2015, 5, 58-68. https://doi.org/10.3390/brainsci5010058
Fuentes-Albero M, Puig-Alcaraz C, Cauli O. Lead Excretion in Spanish Children with Autism Spectrum Disorder. Brain Sciences. 2015; 5(1):58-68. https://doi.org/10.3390/brainsci5010058
Chicago/Turabian StyleFuentes-Albero, Milagros, Carmen Puig-Alcaraz, and Omar Cauli. 2015. "Lead Excretion in Spanish Children with Autism Spectrum Disorder" Brain Sciences 5, no. 1: 58-68. https://doi.org/10.3390/brainsci5010058






