Development of Brain EEG Connectivity across Early Childhood: Does Sleep Play a Role?
Abstract
:1. Introduction
2. Results and Discussion
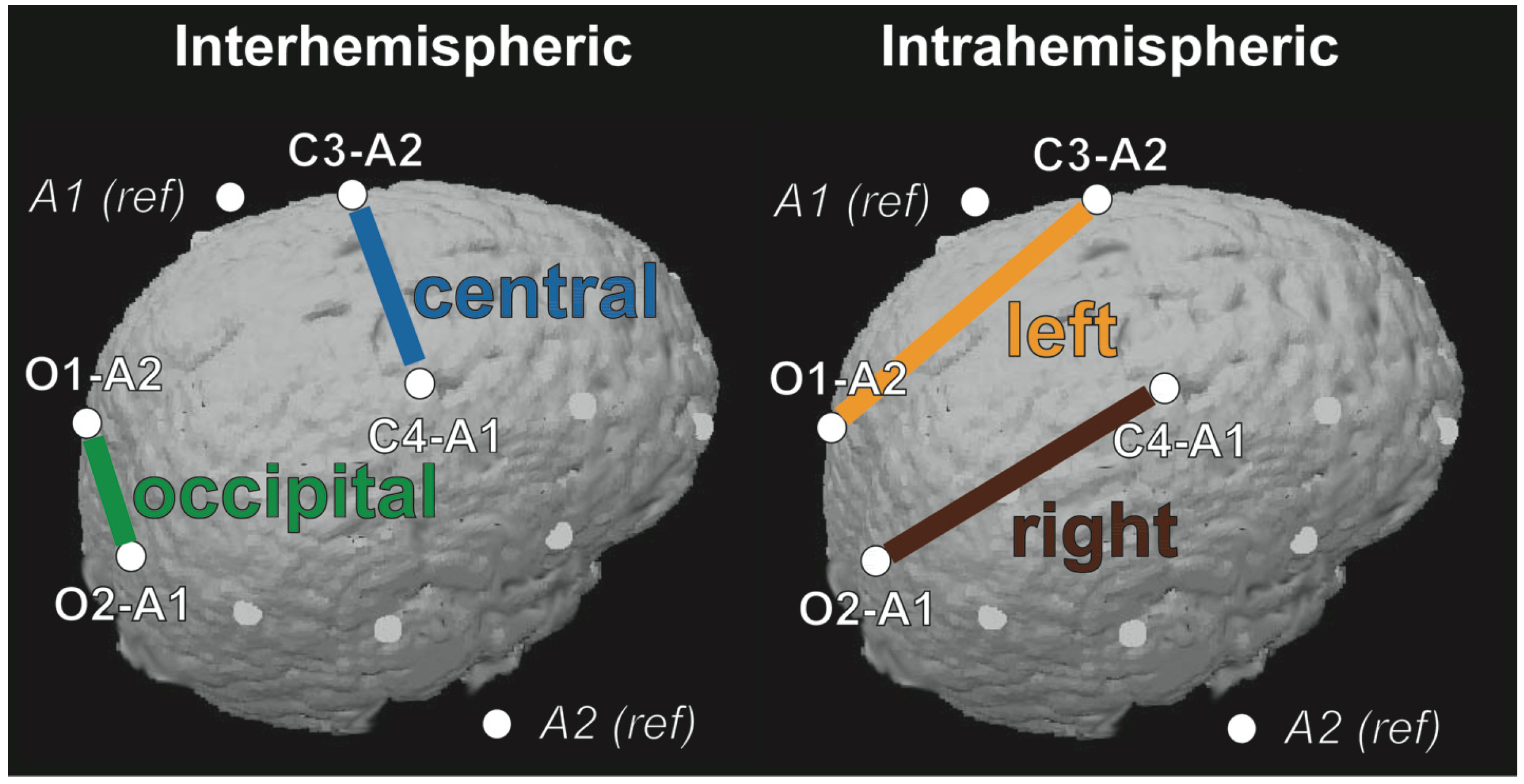
2.1. Connectivity Changes across Development
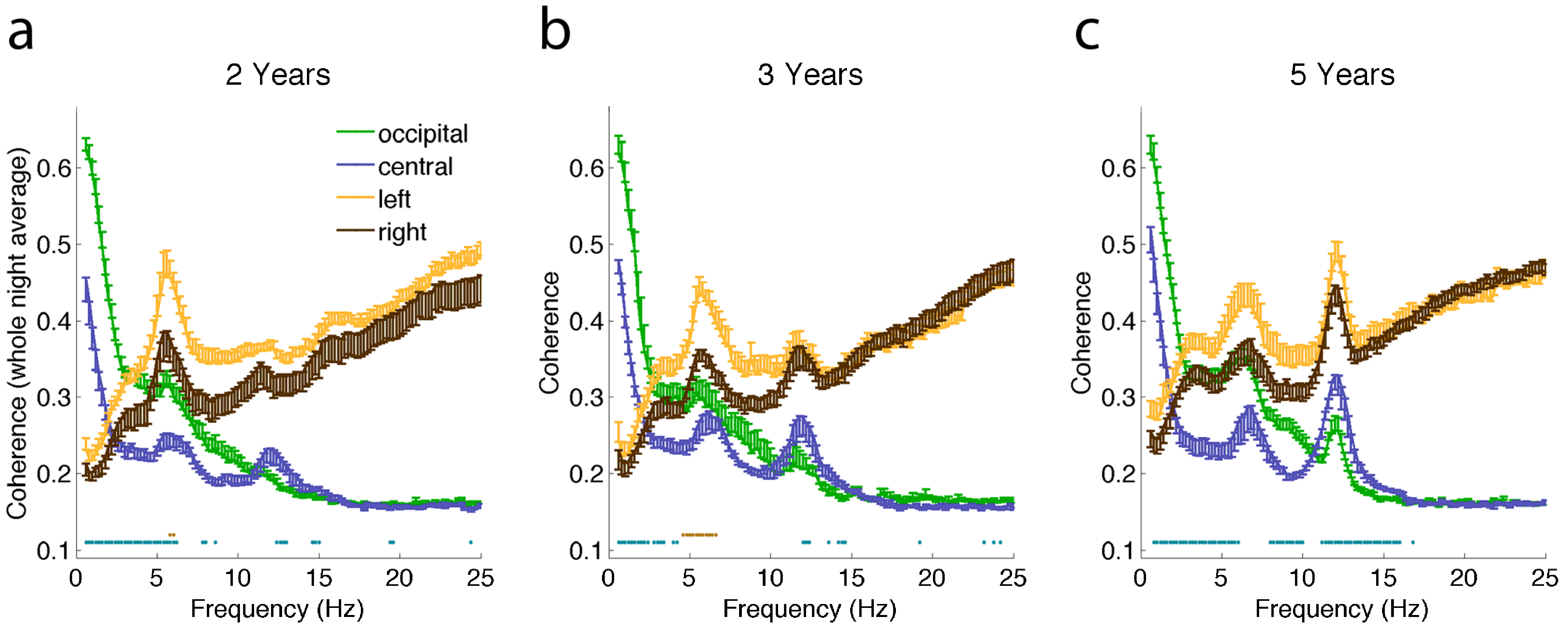
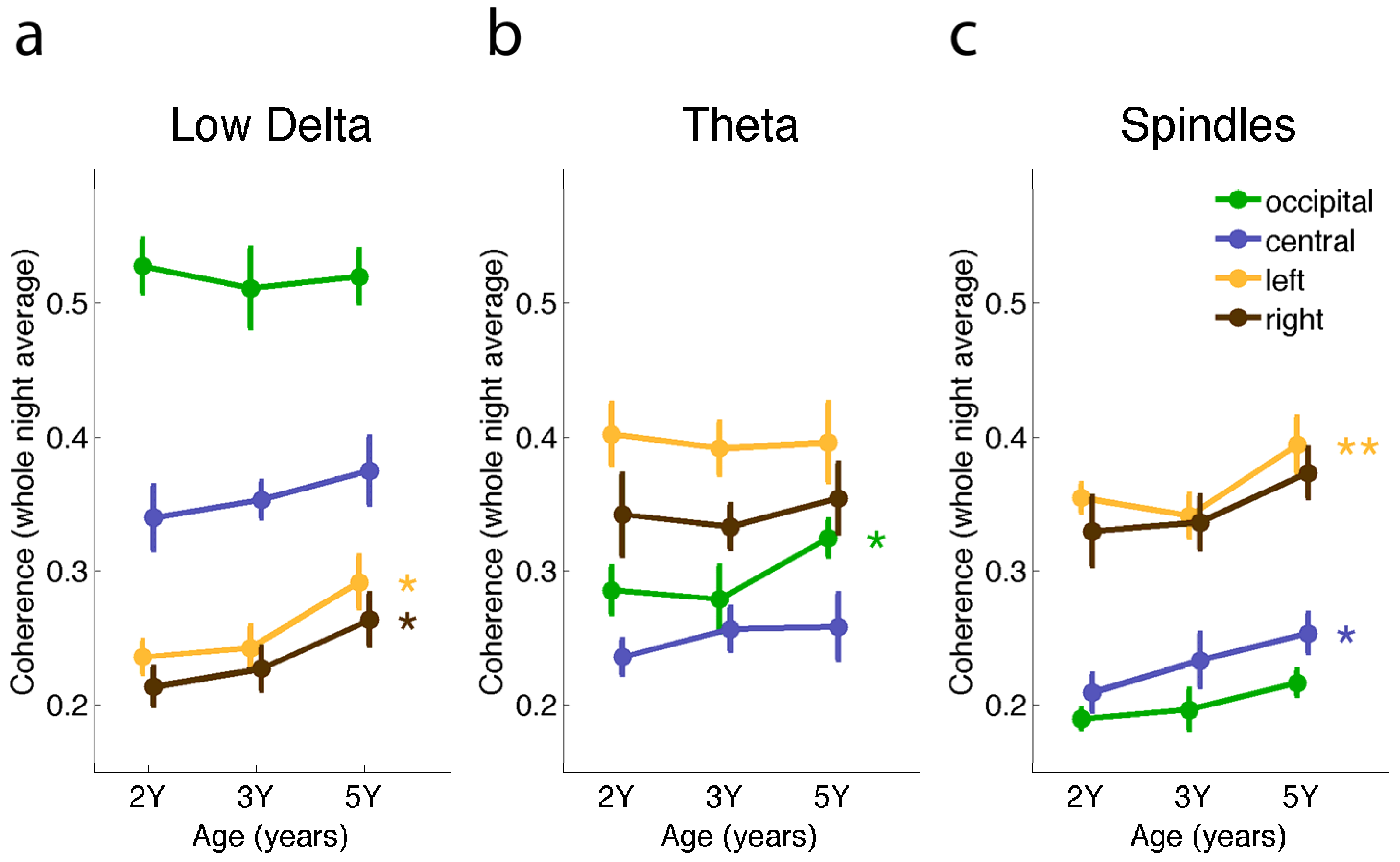
2.2. Connectivity Changes across the Night
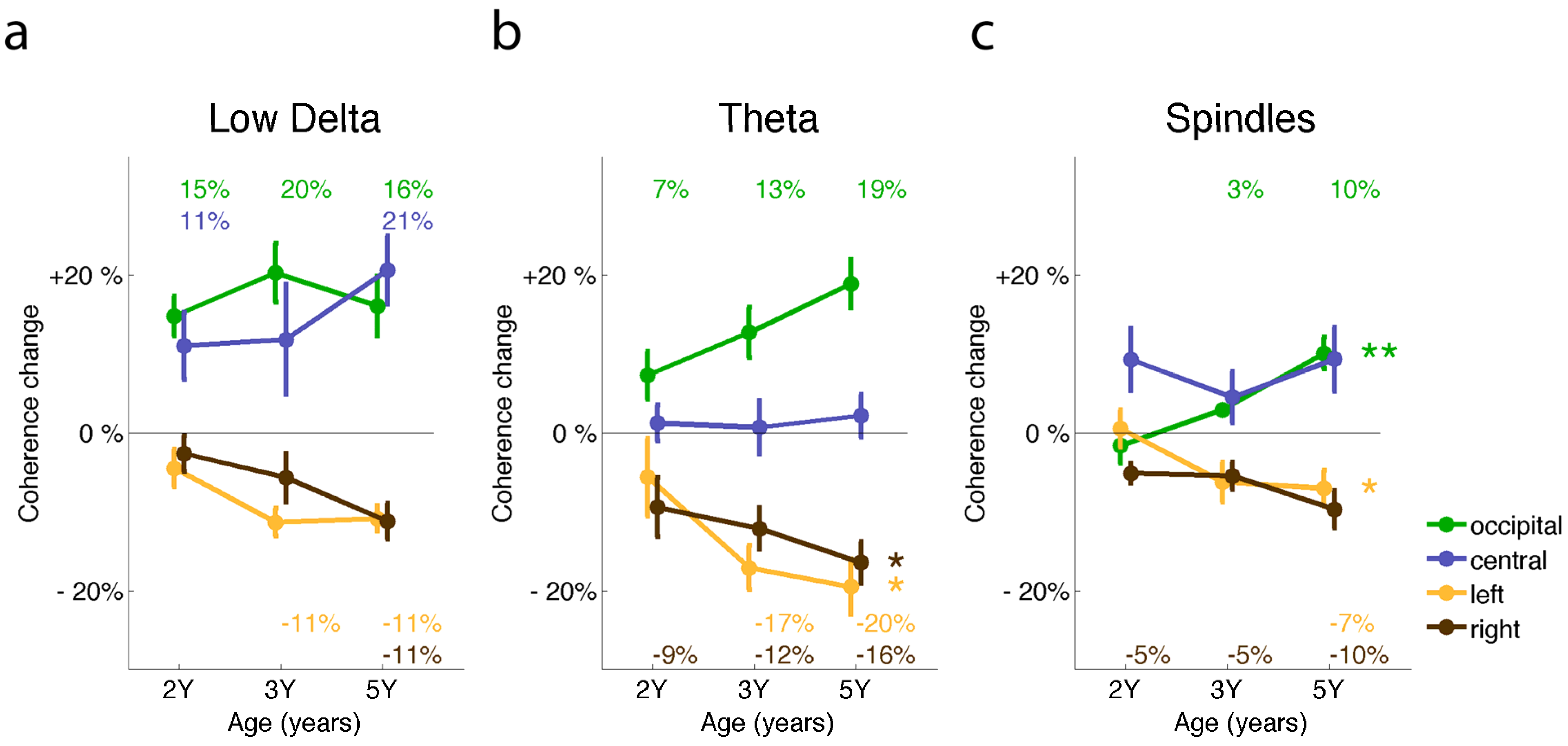
2.3. Sleep EEG Coherence and Neuronal Networks
2.4. Regional Variation in Coherence
2.5. Neuronal Network Refinement
3. Experimental Section
| Age | Duration (min) | Timing (min after Sleep Onset) | ||
|---|---|---|---|---|
| First | Last | First | Last | |
| 2Y | 66.8 ± 4.8 | 41.4 ± 5.6 | 33.4 ± 2.4 | 595.1 ± 8.1 |
| 3Y | 69.2 ± 3.0 | 41.3 ± 4.5 | 34.6 ± 1.5 | 578.5 ± 20.4 |
| 5Y | 76.9 ± 5.4 | 40.2 ± 4.5 | 38.5 ± 2.7 | 575.0 ± 9.5 |
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Deoni, S.C.; Dean, D.C., III; O’Muircheartaigh, J.; Dirks, H.; Jerskey, B.A. Investigating white matter development in infancy and early childhood using myelin water faction and relaxation time mapping. Neuroimage 2012, 63, 1038–1053. [Google Scholar] [CrossRef]
- Huttenlocher, P.R. Synaptic density in human frontal cortex—Developmental changes and effects of aging. Brain Res. 1979, 163, 195–205. [Google Scholar] [CrossRef]
- Achermann, P.; Borbely, A.A. Temporal evolution of coherence and power in the human sleep electroencephalogram. J. Sleep Res. 1998, 7, 36–41. [Google Scholar] [CrossRef]
- Tarokh, L.; Carskadon, M.A.; Achermann, P. Developmental changes in brain connectivity assessed using the sleep EEG. Neuroscience 2010, 171, 622–634. [Google Scholar] [CrossRef]
- Frank, M.G.; Issa, N.P.; Stryker, M.P. Sleep enhances plasticity in the developing visual cortex. Neuron 2001, 30, 275–287. [Google Scholar] [CrossRef]
- Jha, S.K.; Jones, B.E.; Coleman, T.; Steinmetz, N.; Law, C.T.; Griffin, G.; Hawk, J.; Dabbish, N.; Kalatsky, V.A.; Frank, M.G. Sleep-dependent plasticity requires cortical activity. J. Neurosci. 2005, 25, 9266–9274. [Google Scholar] [CrossRef]
- Buchmann, A.; Ringli, M.; Kurth, S.; Schaerer, M.; Geiger, A.; Jenni, O.G.; Huber, R. EEG sleep slow-wave activity as a mirror of cortical maturation. Cereb. Cortex 2011, 21, 607–615. [Google Scholar] [CrossRef]
- Kurth, S.; Ringli, M.; Geiger, A.; LeBourgeois, M.; Jenni, O.G.; Huber, R. Mapping of cortical activity in the first two decades of life: A high-density sleep electroencephalogram study. J. Neurosci. 2010, 30, 13211–13219. [Google Scholar] [CrossRef] [Green Version]
- Maret, S.; Faraguna, U.; Nelson, A.B.; Cirelli, C.; Tononi, G. Sleep and waking modulate spine turnover in the adolescent mouse cortex. Nat. Neurosci. 2011, 14, 1418–1420. [Google Scholar] [CrossRef]
- Lante, F.; Toledo-Salas, J.C.; Ondrejcak, T.; Rowan, M.J.; Ulrich, D. Removal of synaptic Ca2+-permeable ampa receptors during sleep. J. Neurosci. 2011, 31, 3953–3961. [Google Scholar] [CrossRef]
- Birtoli, B.; Ulrich, D. Firing mode-dependent synaptic plasticity in rat neocortical pyramidal neurons. J. Neurosci. 2004, 24, 4935–4940. [Google Scholar] [CrossRef]
- Vyazovskiy, V.V.; Harris, K.D. Sleep and the single neuron: The role of global slow oscillations in individual cell rest. Nat. Rev. Neurosci. 2013, 14, 443–451. [Google Scholar]
- Contreras, D.; Steriade, M. Cellular basis of EEG slow rhythms: A study of dynamic corticothalamic relationships. J. Neurosci. 1995, 15, 604–622. [Google Scholar]
- Timofeev, I.; Steriade, M. Low-frequency rhythms in the thalamus of intact-cortex and decorticated cats. J. Neurophysiol. 1996, 76, 4152–4168. [Google Scholar]
- Nir, Y.; Staba, R.J.; Andrillon, T.; Vyazovskiy, V.V.; Cirelli, C.; Fried, I.; Tononi, G. Regional slow waves and spindles in human sleep. Neuron 2011, 70, 153–169. [Google Scholar]
- Vyazovskiy, V.V.; Olcese, U.; Lazimy, Y.M.; Faraguna, U.; Esser, S.K.; Williams, J.C.; Cirelli, C.; Tononi, G. Cortical firing and sleep homeostasis. Neuron 2009, 63, 865–878. [Google Scholar]
- Tononi, G.; Cirelli, C. Sleep function and synaptic homeostasis. Sleep Med. Rev 2006, 10, 49–62. [Google Scholar]
- Crunelli, V.; Hughes, S.W. The slow (<1 Hz) rhythm of non-rem sleep: A dialogue between three cardinal oscillators. Nat. Neurosci. 2010, 13, 9–17. [Google Scholar]
- Kemp, N.; Bashir, Z.I. Long-term depression: A cascade of induction and expression mechanisms. Prog. Neurobiol. 2001, 65, 339–365. [Google Scholar]
- Jaspers, M.; de Winter, A.F.; Huisman, M.; Verhulst, F.C.; Ormel, J.; Stewart, R.E.; Reijneveld, S.A. Trajectories of psychosocial problems in adolescents predicted by findings from early well-child assessments. J. Adolesc. Health 2012, 51, 475–483. [Google Scholar]
- Bellesi, M.; Pfister-Genskow, M.; Maret, S.; Keles, S.; Tononi, G.; Cirelli, C. Effects of sleep and wake on oligodendrocytes and their precursors. J. Neurosci. 2013, 33, 14288–14300. [Google Scholar] [CrossRef]
- Rechtschaffen, A.; Kales, A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Electroencephalogr. Clin. Neurophysiol. 1969, 26. [Google Scholar] [CrossRef]
- Scholle, S.; Zwacka, G.; Scholle, H.C. Sleep spindle evolution from infancy to adolescence. Clin. Neurophysiol. 2007, 118, 1525–1531. [Google Scholar] [CrossRef]
- Kurth, S.; Ringli, M.; Lebourgeois, M.K.; Geiger, A.; Buchmann, A.; Jenni, O.G.; Huber, R. Mapping the electrophysiological marker of sleep depth reveals skill maturation in children and adolescents. Neuroimage 2012, 63, 959–965. [Google Scholar] [CrossRef]
- Campbell, I.G.; Grimm, K.J.; de Bie, E.; Feinberg, I. Sex, puberty, and the timing of sleep EEG measured adolescent brain maturation. Proc. Natl. Acad. Sci. USA 2012, 109, 5740–5743. [Google Scholar] [CrossRef]
- Borbely, A.A. A two process model of sleep regulation. Hum. Neurobiol. 1982, 1, 195–204. [Google Scholar]
- Wang, X.J. Neurophysiological and computational principles of cortical rhythms in cognition. Physiol. Rev. 2010, 90, 1195–1268. [Google Scholar] [CrossRef]
- Butts, D.A.; Kanold, P.O. The applicability of spike time dependent plasticity to development. Front. Synaptic Neurosci. 2010, 2. [Google Scholar] [CrossRef]
- De Gennaro, L.; Marzano, C.; Fratello, F.; Moroni, F.; Pellicciari, M.C.; Ferlazzo, F.; Costa, S.; Couyoumdjian, A.; Curcio, G.; Sforza, E.; et al. The electroencephalographic fingerprint of sleep is genetically determined: A twin study. Ann. Neurol. 2008, 64, 455–460. [Google Scholar] [CrossRef]
- Buckelmuller, J.; Landolt, H.P.; Stassen, H.H.; Achermann, P. Trait-like individual differences in the human sleep electroencephalogram. Neuroscience 2006, 138, 351–356. [Google Scholar] [CrossRef]
- Finelli, L.A.; Achermann, P.; Borbely, A.A. Individual “fingerprints” in human sleep EEG topography. Neuropsychopharmacology 2001, 25, S57–S62. [Google Scholar] [CrossRef]
- Tarokh, L.; Carskadon, M.A.; Achermann, P. Trait-like characteristics of the sleep EEG across adolescent development. J. Neurosci. 2011, 31, 6371–6378. [Google Scholar] [CrossRef]
- Piantoni, G.; Poil, S.S.; Linkenkaer-Hansen, K.; Verweij, I.M.; Ramautar, J.R.; van Someren, E.J.; van der Werf, Y.D. Individual differences in white matter diffusion affect sleep oscillations. J. Neurosci. 2013, 33, 227–233. [Google Scholar] [CrossRef]
- Fogel, S.M.; Smith, C.T. The function of the sleep spindle: A physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci. Biobehav. Rev. 2011, 35, 1154–1165. [Google Scholar] [CrossRef]
- Cirelli, C. A molecular window on sleep: Changes in gene expression between sleep and wakefulness. Neuroscientist 2005, 11, 63–74. [Google Scholar] [CrossRef]
- Persson, J.; Nyberg, L.; Lind, J.; Larsson, A.; Nilsson, L.G.; Ingvar, M.; Buckner, R.L. Structure-function correlates of cognitive decline in aging. Cereb. Cortex 2006, 16, 907–915. [Google Scholar]
- Sasson, E.; Doniger, G.M.; Pasternak, O.; Assaf, Y. Structural correlates of memory performance with diffusion tensor imaging. Neuroimage 2010, 50, 1231–1242. [Google Scholar] [CrossRef]
- Karunanayaka, P.R.; Holland, S.K.; Schmithorst, V.J.; Solodkin, A.; Chen, E.E.; Szaflarski, J.P.; Plante, E. Age-related connectivity changes in fmri data from children listening to stories. Neuroimage 2007, 34, 349–360. [Google Scholar] [CrossRef]
- Kikuchi, M.; Shitamichi, K.; Yoshimura, Y.; Ueno, S.; Remijn, G.B.; Hirosawa, T.; Munesue, T.; Tsubokawa, T.; Haruta, Y.; Oi, M.; et al. Lateralized theta wave connectivity and language performance in 2- to 5-year-old children. J. Neurosci. 2011, 31, 14984–14988. [Google Scholar] [CrossRef]
- Thatcher, R.W.; Walker, R.A.; Giudice, S. Human cerebral hemispheres develop at different rates and ages. Science 1987, 236, 1110–1113. [Google Scholar]
- Hayes, T.L.; Lewis, D.A. Hemispheric differences in layer III pyramidal neurons of the anterior language area. Arch. Neurol. 1993, 50, 501–505. [Google Scholar] [CrossRef]
- Johnston, J.M.; Vaishnavi, S.N.; Smyth, M.D.; Zhang, D.; He, B.J.; Zempel, J.M.; Shimony, J.S.; Snyder, A.Z.; Raichle, M.E. Loss of resting interhemispheric functional connectivity after complete section of the corpus callosum. J. Neurosci. 2008, 28, 6453–6458. [Google Scholar] [CrossRef]
- Nir, Y.; Mukamel, R.; Dinstein, I.; Privman, E.; Harel, M.; Fisch, L.; Gelbard-Sagiv, H.; Kipervasser, S.; Andelman, F.; Neufeld, M.Y.; et al. Interhemispheric correlations of slow spontaneous neuronal fluctuations revealed in human sensory cortex. Nat. Neurosci. 2008, 11, 1100–1108. [Google Scholar] [CrossRef]
- Gazzaniga, M.S. Cerebral specialization and interhemispheric communication: Does the corpus callosum enable the human condition? Brain 2000, 123, 1293–1326. [Google Scholar] [CrossRef]
- Eliassen, J.C.; Baynes, K.; Gazzaniga, M.S. Direction information coordinated via the posterior third of the corpus callosum during bimanual movements. Exp. Brain Res. 1999, 128, 573–577. [Google Scholar] [CrossRef]
- Brain Development Cooperative Group. Total and regional brain volumes in a population-based normative sample from 4 to 18 years: The NIH MRI study of normal brain development. Cereb. Cortex 2012, 22, 1–12. [Google Scholar] [CrossRef]
- Huttenlocher, P.R.; Dabholkar, A.S. Regional differences in synaptogenesis in human cerebral cortex. J. Comp. Neurol. 1997, 387, 167–178. [Google Scholar] [CrossRef]
- Shaw, P.; Kabani, N.J.; Lerch, J.P.; Eckstrand, K.; Lenroot, R.; Gogtay, N.; Greenstein, D.; Clasen, L.; Evans, A.; Rapoport, J.L.; et al. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 2008, 28, 3586–3594. [Google Scholar] [CrossRef]
- Teller, D.Y. The development of visual-acuity in human and monkey infants. Trends Neurosci. 1981, 4, 21–24. [Google Scholar] [CrossRef]
- Steriade, M.; Deschenes, M.; Domich, L.; Mulle, C. Abolition of spindle oscillations in thalamic neurons disconnected from nucleus reticularis thalami. J. Neurophysiol. 1985, 54, 1473–1497. [Google Scholar]
- Steriade, M.; Timofeev, I. Neuronal plasticity in thalamocortical networks during sleep and waking oscillations. Neuron 2003, 37, 563–576. [Google Scholar] [CrossRef]
- Geiger, A.; Huber, R.; Kurth, S.; Ringli, M.; Jenni, O.G.; Achermann, P. The sleep EEG as a marker of intellectual ability in school age children. Sleep 2011, 34, 181–189. [Google Scholar]
- Gray, J.R.; Chabris, C.F.; Braver, T.S. Neural mechanisms of general fluid intelligence. Nat. Neurosci. 2003, 6, 316–322. [Google Scholar] [CrossRef]
- Kane, M.J.; Engle, R.W. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: An individual-differences perspective. Psychonomic Bull. Rev. 2002, 9, 637–671. [Google Scholar] [CrossRef]
- Fair, D.A.; Cohen, A.L.; Dosenbach, N.U.; Church, J.A.; Miezin, F.M.; Barch, D.M.; Raichle, M.E.; Petersen, S.E.; Schlaggar, B.L. The maturing architecture of the brain’s default network. Proc. Natl. Acad. Sci. USA 2008, 105, 4028–4032. [Google Scholar] [CrossRef]
- Fransson, P.; Skiold, B.; Horsch, S.; Nordell, A.; Blennow, M.; Lagercrantz, H.; Aden, U. Resting-state networks in the infant brain. Proc. Natl. Acad. Sci. USA 2007, 104, 15531–15536. [Google Scholar]
- Supekar, K.; Musen, M.; Menon, V. Development of large-scale functional brain networks in children. PLoS Biol. 2009, 7, e1000157. [Google Scholar] [CrossRef]
- Johnson, M.H.; Munakata, Y. Processes of change in brain and cognitive development. Trends Cogn. Sci. 2005, 9, 152–158. [Google Scholar] [CrossRef]
- Fields, R.D. Imaging learning: The search for a memory trace. Neuroscientist 2011, 17, 185–196. [Google Scholar] [CrossRef]
- Lee, Y.; Morrison, B.M.; Li, Y.; Lengacher, S.; Farah, M.H.; Hoffman, P.N.; Liu, Y.; Tsingalia, A.; Jin, L.; Zhang, P.W.; et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 2012, 487, 443–448. [Google Scholar] [CrossRef]
- Stevens, B.; Porta, S.; Haak, L.L.; Gallo, V.; Fields, R.D. Adenosine: A neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron 2002, 36, 855–868. [Google Scholar] [CrossRef]
- Araque, A.; Navarrete, M. Development. Electrically driven insulation in the central nervous system. Science 2011, 333, 1587–1588. [Google Scholar] [CrossRef]
- Kukley, M.; Capetillo-Zarate, E.; Dietrich, D. Vesicular glutamate release from axons in white matter. Nat. Neurosci. 2007, 10, 311–320. [Google Scholar] [CrossRef]
- Wake, H.; Lee, P.R.; Fields, R.D. Control of local protein synthesis and initial events in myelination by action potentials. Science 2011, 333, 1647–1651. [Google Scholar] [CrossRef]
- Kramer, E.M.; Schardt, A.; Nave, K.A. Membrane traffic in myelinating oligodendrocytes. Microsc. Res. Tech. 2001, 52, 656–671. [Google Scholar] [CrossRef]
- Chiang, M.C.; Barysheva, M.; Shattuck, D.W.; Lee, A.D.; Madsen, S.K.; Avedissian, C.; Klunder, A.D.; Toga, A.W.; McMahon, K.L.; de Zubicaray, G.I.; et al. Genetics of brain fiber architecture and intellectual performance. J. Neurosci. 2009, 29, 2212–2224. [Google Scholar] [CrossRef] [Green Version]
- Dahl, R.E. The impact of inadequate sleep on children’s daytime cognitive function. Semin. Pediatr. Neurol. 1996, 3, 44–50. [Google Scholar] [CrossRef]
- Kubota, M.; Miyata, J.; Sasamoto, A.; Sugihara, G.; Yoshida, H.; Kawada, R.; Fujimoto, S.; Tanaka, Y.; Sawamoto, N.; Fukuyama, H.; et al. Thalamocortical disconnection in the orbitofrontal region associated with cortical thinning in schizophrenia. JAMA Psychiatry 2013, 70, 12–21. [Google Scholar] [CrossRef]
- Bushey, D.; Tononi, G.; Cirelli, C. Sleep and synaptic homeostasis: Structural evidence in drosophila. Science 2011, 332, 1576–1581. [Google Scholar] [CrossRef]
- LeBourgeois, M.K.; Rusterholz, T.; Jenni, O.G.; Carskadon, M.A.; Achermann, P. Do the dynamics of sleep homeostasis (process S) change across early childhood? In Presented at the 26th Annual Meeting of the Associated Professional Sleep Societies (APSS), Boston, MA, USA, 9–13 June 2012. Abstract 0050.
- Maris, E.; Oostenveld, R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 2007, 164, 177–190. [Google Scholar] [CrossRef]
Supplementary Files
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kurth, S.; Achermann, P.; Rusterholz, T.; LeBourgeois, M.K. Development of Brain EEG Connectivity across Early Childhood: Does Sleep Play a Role? Brain Sci. 2013, 3, 1445-1460. https://doi.org/10.3390/brainsci3041445
Kurth S, Achermann P, Rusterholz T, LeBourgeois MK. Development of Brain EEG Connectivity across Early Childhood: Does Sleep Play a Role? Brain Sciences. 2013; 3(4):1445-1460. https://doi.org/10.3390/brainsci3041445
Chicago/Turabian StyleKurth, Salome, Peter Achermann, Thomas Rusterholz, and Monique K. LeBourgeois. 2013. "Development of Brain EEG Connectivity across Early Childhood: Does Sleep Play a Role?" Brain Sciences 3, no. 4: 1445-1460. https://doi.org/10.3390/brainsci3041445
APA StyleKurth, S., Achermann, P., Rusterholz, T., & LeBourgeois, M. K. (2013). Development of Brain EEG Connectivity across Early Childhood: Does Sleep Play a Role? Brain Sciences, 3(4), 1445-1460. https://doi.org/10.3390/brainsci3041445





