From Neural Plate to Cortical Arousal—A Neuronal Network Theory of Sleep Derived from in Vitro “Model” Systems for Primordial Patterns of Spontaneous Bioelectric Activity in the Vertebrate Central Nervous System
Abstract
:“In biological as well as physical-chemical sciences, one should reduce phenomena to as simple as possible experimental conditions.”—Claude Bernard [1]
1. Introduction
2. Motorically Active (“Rhombencephalic”) Sleep
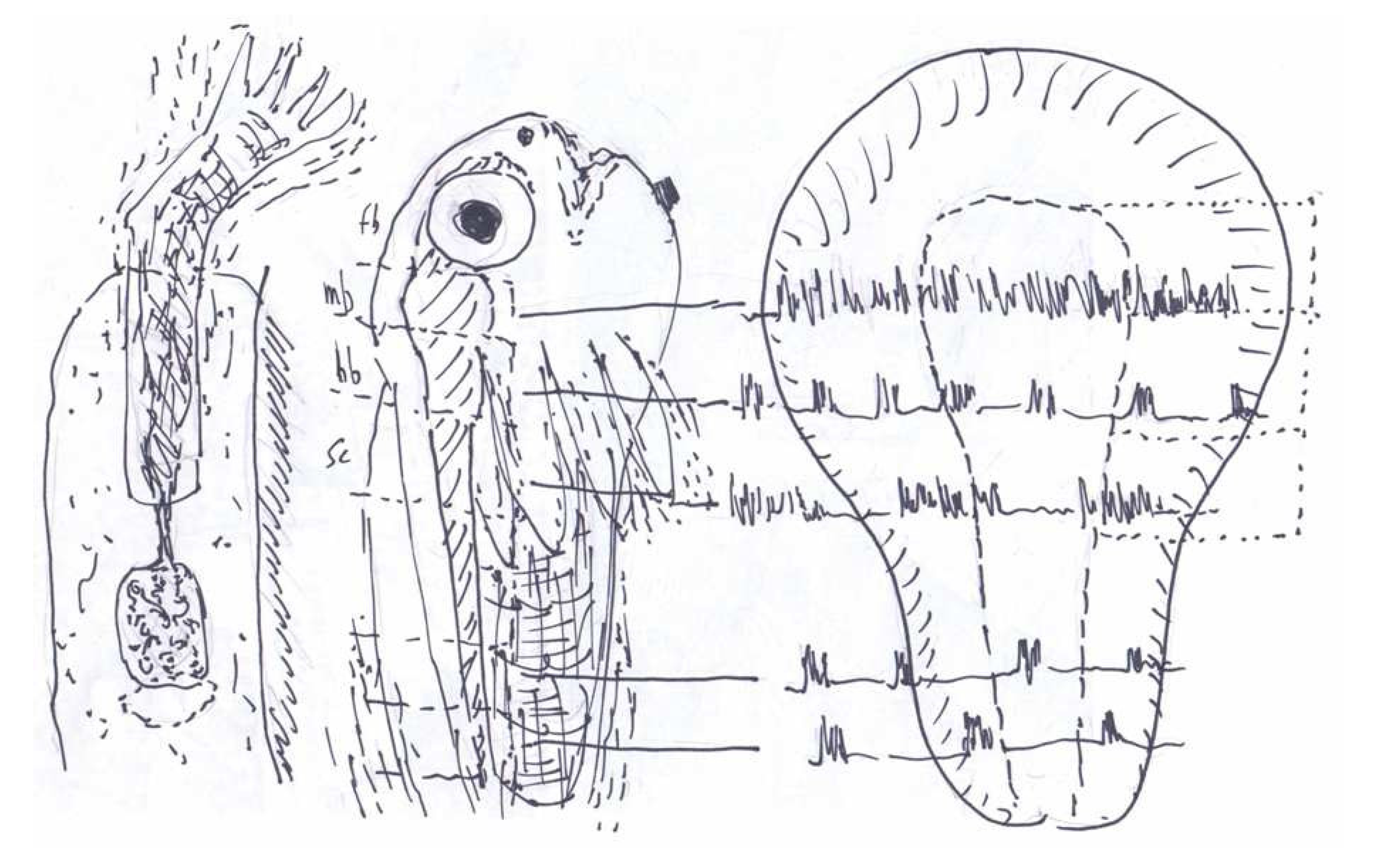
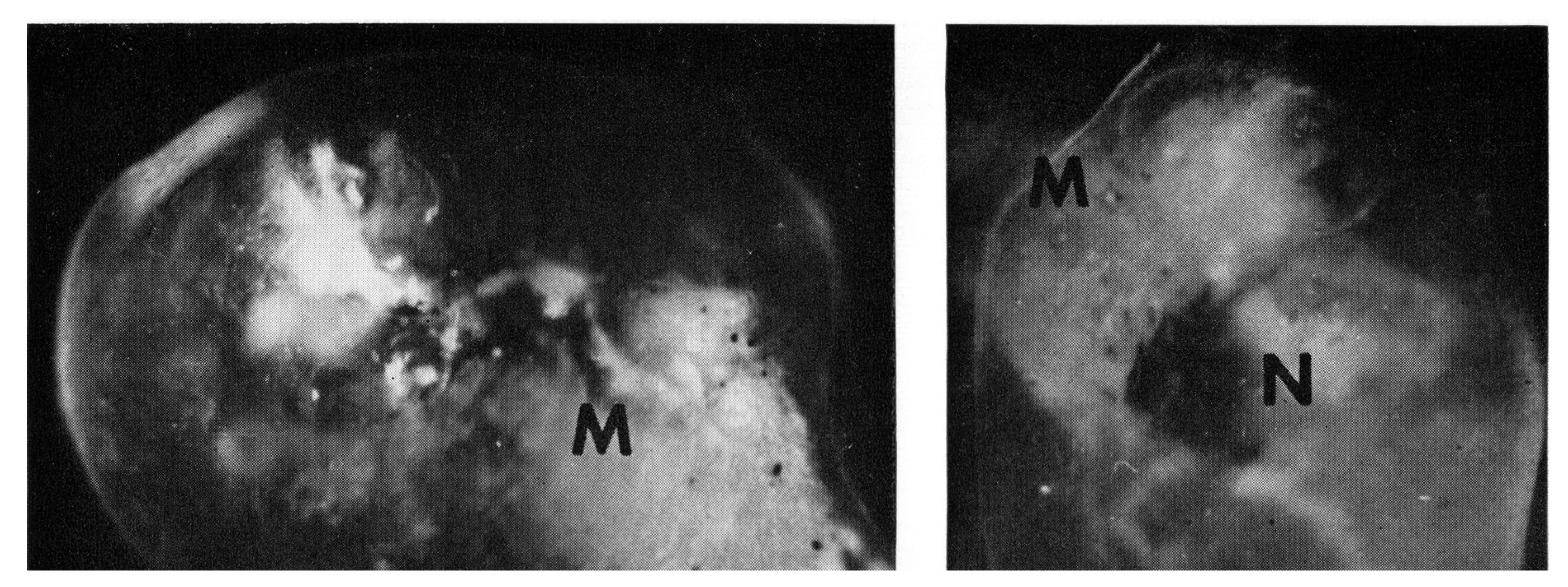
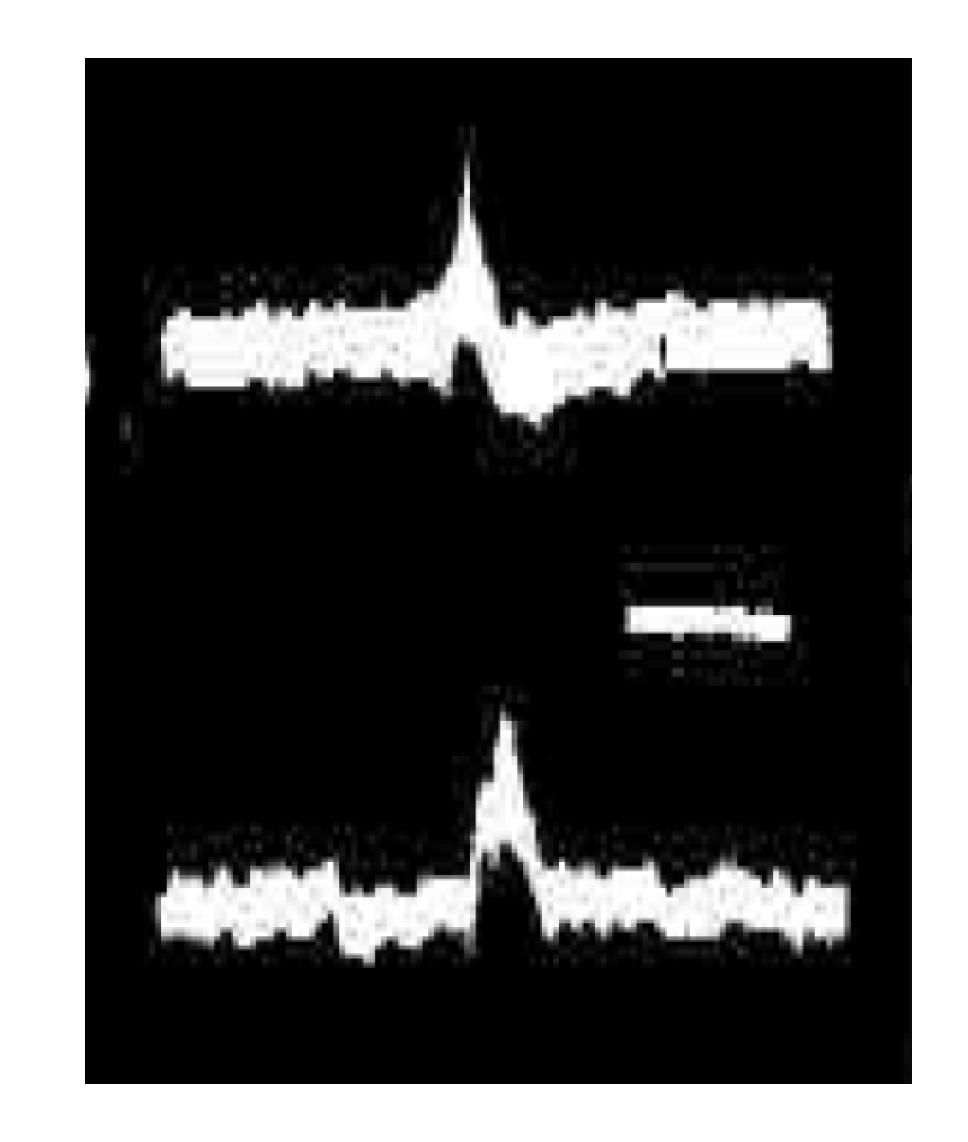

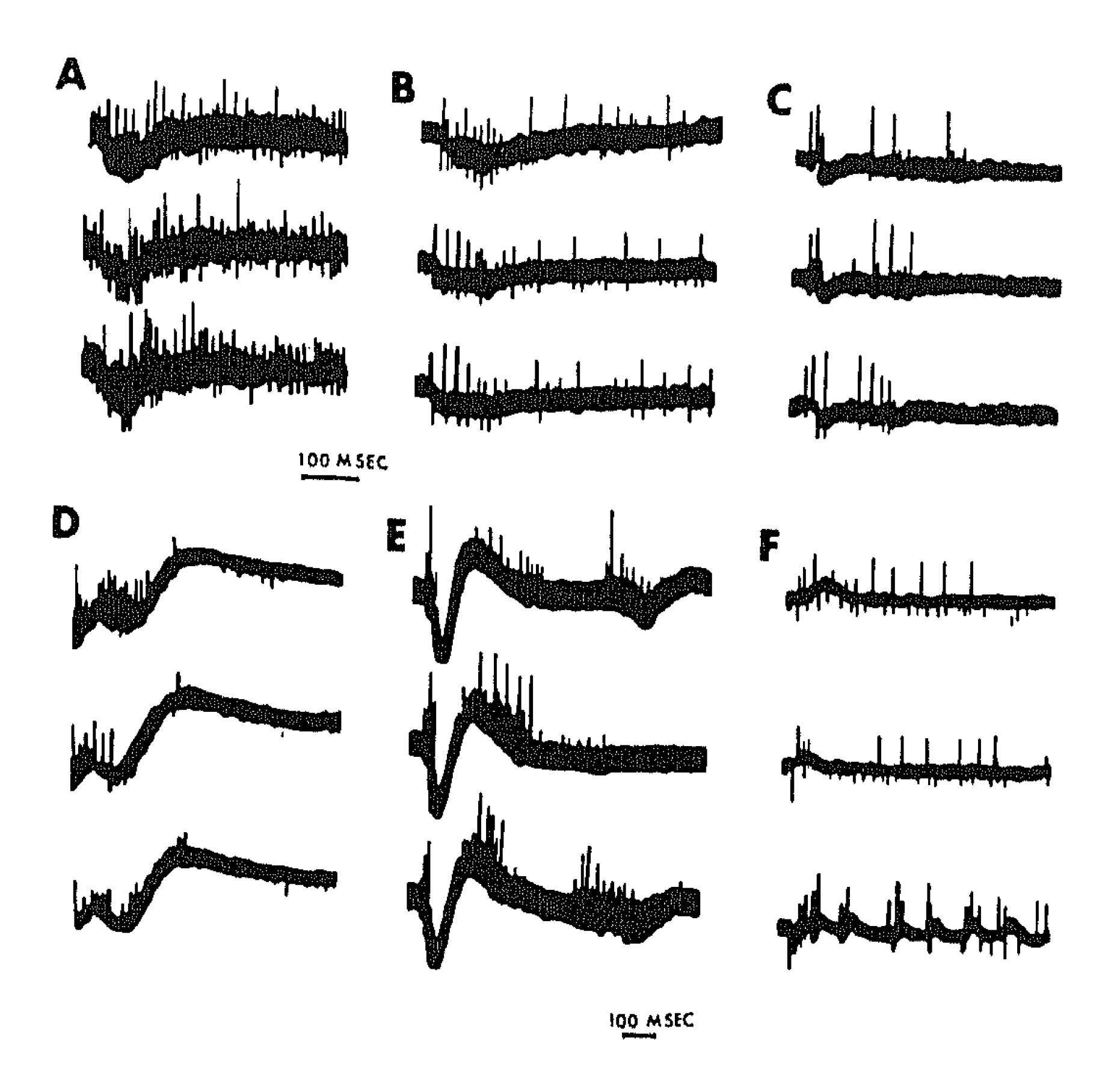
3. Synchronous Slow-Wave (“Telencephalic”) Sleep

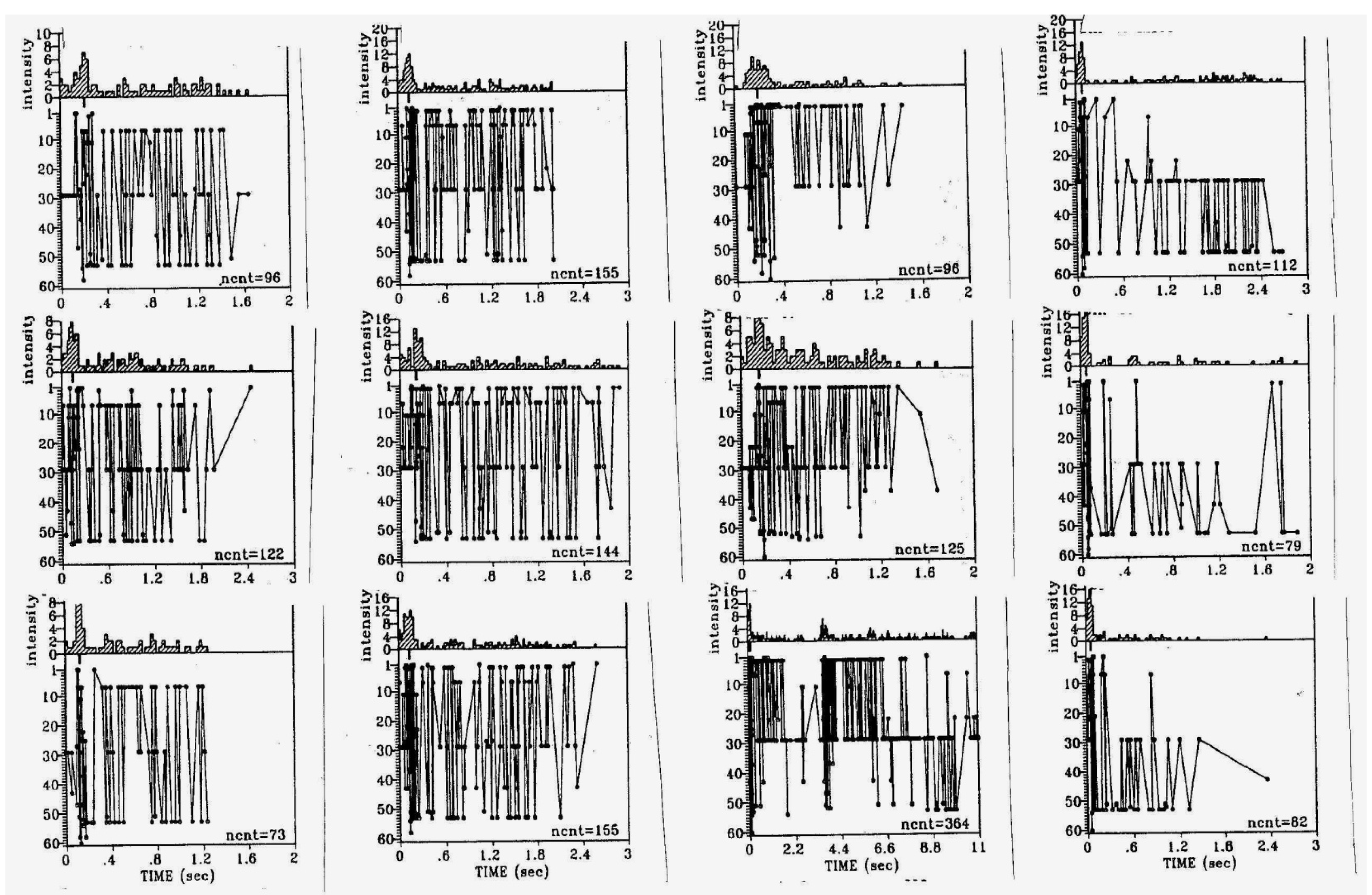
4. Cholinergic “Arousal”: Acute and Long-Lasting Effects on Neocortical Function and Plasticity


| Bursts | Rate/min | Duration (ms) | Intensity (sp/s) | Spikes-inter (%) |
|---|---|---|---|---|
| Ratio of in-carbachol (first 2 h) to pre-carbachol mean values (2 h) | ||||
| CON (6) | 0.54 (0.72) 0.84 * | 1.35 (1.44) 1.46 ^ | 0.69 (0.70) 0.73 ^ | 1.26 (1.41) 1.50 ^ |
| TTX (6) | 0.42 (0.50) 0.77 * | 1.08 (1.19) 1.27 * | 0.72 (0.83) 0.93 ^ | 1.11 (1.20) 1.26 ^ |
| Ratio of post-carbachol (first 2 h) to pre-carbachol mean values (2 h) | ||||
| CON (6) | 0.42 (0.66) 0.90 * | 1.04 (1.11) 1.16 | 0.80 (0.82) 0.94 | 1.09 (1.33) 1.62 |
| TTX (6) | 0.21 (0.31) 0.51 ^ | 1.00 (1.07) 1.11 | 0.81 (0.86) 0.99 | 1.00 (1.08) 1.32 |
| Ratio of post-carbachol (last 2 h) to pre-carbachol mean values (2 h) | ||||
| CON (3) | 0.66 (0.69) 0.75 ** | 0.95 (1.04) 1.16 | 0.91 (1.00) 1.06 | 0.96 (1.00) 1.0 |
| TTX (6) | 0.32 (0.42) 0.80 ^ | 1.00 (1.04) 1.09 | 0.85 (0.87) 0.95 | 1.07 (1.20) 1.37 ^ |
5. Theoretical Implications
Acknowledgments
“It’s not enough to dislike an idea … it must also be shown to be wrong!”—Weiner, N. [96].
“My main qualification for carrying out this study was that nobody else seemed interested in doing it.”—Myrdal, J. [97].
Conflict of Interest
References
- Introduction à l’Étude de la Médicine Expérimentale. 1865. Available online: http://www.ac-grenoble.fr/PhiloSophie/file/bernard_medecine_exp.pdf (accessed on 19 March 2013).
- Weiss, P.A. Autonomous vs. reflexogenous activity of the central nervous system. Proc. Am. Phil. Soc. 1941, 84, 53–64. [Google Scholar]
- Jouvet, M. The Paradox of Sleep; MIT Press: Cambridge, MA, USA, 1999; pp. 27–61. [Google Scholar]
- Siegel, J.M.; Manger, P.R.; Nienhuis, R.; Fahringer, H.M.; Pettigrew, J.D. Monotremes and the evolution of rapid eye movement sleep. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1998, 353, 1147–1157. [Google Scholar] [CrossRef]
- Hamburger, V.; Balaban, M. Observations and experiments on spontaneous rhythmical behavior in the chick embryo. Dev. Biol. 1963, 7, 533–545. [Google Scholar] [CrossRef]
- Corner, M.A. Rhythmicity in the early swimming of anuran larvae. J. Embryol. Exp. Morphol. 1964, 12, 665–671. [Google Scholar]
- Corner, M.A. Localization of capacities for functional development in the neural plate of Xenopus laevis. J. Comp. Neurol. 1964, 123, 243–256. [Google Scholar] [CrossRef]
- Schenck, C.H. Paradox lost: Midnight in the battleground of sleep and dreams. J. Neurol. Neurosurg. Psychiatry 2006, 77, 1387. [Google Scholar]
- Corner, M.A. Morphogenetic field properties of the forebrain area of the neural plate in an anuran. Experientia 1966, 22, 188–190. [Google Scholar] [CrossRef]
- Corner, M.A.; Crain, S.M. Spontaneous contractions and bioelectric activity after differentiation in culture of presumptive neuromuscular tissues of the early frog embryo. Experientia 1965, 21, 422–424. [Google Scholar] [CrossRef]
- Corner, M.A.; Crain, S.M. Patterns of spontaneous bioelectric activity during maturation in cultures of fetal rodent medulla and spinal cord tissues. J. Neurobiol. 1972, 3, 25–45. [Google Scholar] [CrossRef]
- Tarrade, T.; Crain, S.M. Regional localization of patterned spontaneous discharges during maturation in culture of fetal mouse medulla and spinal cord explants. Dev. Neurosci. 1978, 1, 119–132. [Google Scholar] [CrossRef]
- Takizawa, N. Integral multiple interspike intervals of spontaneous activity in isolated medulla oblongata of frogs. Brain Res. 1981, 212, 466–469. [Google Scholar] [CrossRef]
- Corner, M.; van der Togt, C. No phylogeny without ontogeny—A comparative and developmental search for the sources of sleep-like neural and behavioral rhythms. Neurosci. Bull. 2012, 28, 25–38. [Google Scholar] [CrossRef]
- Crain, S.M. Bioelectric interactions between cultured fetal rodent spinal cord and skeletal muscle after innervation in vitro. J. Exp. Zool. 1970, 173, 353–370. [Google Scholar] [CrossRef]
- Corner, M.A. The sleep-like nature of early mammalian behavioral rhythms. Behav. Neurosci. 2010, 124, 175–178. [Google Scholar] [CrossRef]
- Crain, S.M.; Bornstein, M.B. Organotypic bioelectric activity in cultured reaggregates of dissociated rodent brain cells. Science 1972, 176, 182–184. [Google Scholar]
- Ham, M.L.; Bettencourt, L.M.; McDaniel, F.D.; Gross, G.W. Spontanous coordinated activity in cultured networks: Analysis of multiple ignition sites, primary circuits, and burst phase delay distributions. J. Comput. Neurosci. 2008, 23, 346–357. [Google Scholar]
- Corner, M.A.; Crain, S.M. The development of spontaneous bioelectrical activities and strychnine sensitivity during maturation in culture of embryonic chick and rodent central nervous tissues. Arch. Int. Pharmacodyn. Ther. 1969, 182, 404–406. [Google Scholar]
- Tabak, J.; O’Donovan, M.J.; Rinzel, J. Differential control of active and silent phases in relaxation models of neuronal rhythms. J. Comput. Neurosci. 2006, 21, 307–328. [Google Scholar] [CrossRef]
- Streit, J.; Tscherter, A.; Heuschkel, M.O.; Renaud, P. The generation of rhythmic activity in dissociated cultures of rat spinal cord. Eur. J. Neurosci. 2001, 14, 191–202. [Google Scholar] [CrossRef]
- Crain, S.M.; Bornstein, M.B. Early onset of inhibitory functions during synaptogenesis in fetal mouse brain cultures. Brain Res. 1974, 68, 351–357. [Google Scholar] [CrossRef]
- Provine, R.R. Embryonic spinal cord: synchrony and spatial distribution of polyneuronal burst discharges. Brain Res. 1971, 29, 155–158. [Google Scholar] [CrossRef]
- Rosato-Siri, M.; Zoccolan, D.; Furlan, F.; Ballerini, L. Interneurone bursts are spontaneously associated with muscle contractions only during early phases of mouse spinal network development: A study in organotypic cultures. Eur. J. Neurosci. 2004, 20, 2697–2710. [Google Scholar] [CrossRef]
- Czarnecki, A.; Magloire, V.; Streit, J. Local oscillations of spiking in organotypic spinal cord slice explants. Eur. J. Neurosci. 2008, 27, 2076–2088. [Google Scholar] [CrossRef]
- Valatx, J.L.; Jouvet, D.; Jouvet, M. Évolution électroencéphalographique des différents états de sommeil chez le chaton. Electroenceph. Clin. Neurophysiol. 1964, 17, 218–233. [Google Scholar] [CrossRef]
- Corner, M.A. Call it sleep—What animals without backbones can tell us about the phylogeny of intrinsically generated behavioral rhythms during early development. Neurosci. Bull. 2013, 29. in press. [Google Scholar] [CrossRef]
- Spitzer, N.C. Electrical activity in early neuronal development. Nature 2006, 444, 707–712. [Google Scholar] [CrossRef]
- Blumberg, M.S. Beyond dreams: Do sleep-related movements contribute to brain development? Front. Neurol. 2010, 1, 140. [Google Scholar]
- Van Den Pol, A.N.; Obrietan, K.; Belousov, A.B. Glutamate hyperexcitability and seizure-like activity throughout the brain and spinal cord upon relief from chronic glutamate receptor blockade in culture. Neuroscience 1996, 74, 653–674. [Google Scholar] [CrossRef]
- Galante, M.; Nishi, A.; Ballerini, L. Opposite changes in synaptic activity of organotypic rat spinal cord cultures after chronic block of AMPA/kainate or glycine and GABA-A receptors. J. Physiol. (Lond.) 2000, 523, 639–651. [Google Scholar] [CrossRef]
- Gonzales-Islas, C.; Wenner, P. Spontaneous network activity in the embryonic spinal cord regulates AMPAergic and GABAergic synaptic strength. Neuron 2006, 49, 563–575. [Google Scholar] [CrossRef]
- Baker, R.E.; Habets, A.M.; Brenner, E.; Corner, M.A. Influence of growth medium, age in vitro and spontaneous bioelectric activity on the distribution of sensory ganglion-evoked activity in spinal cord explants. Brain Res. 1982, 281, 329–341. [Google Scholar]
- Baker, R.E.; Corner, M.A.; Habets, A.M.C. Effects of chronic suppression of bioelectric activity on the development of sensory ganglion evoked responses in spinal cord explants. J. Neurosci. 1984, 4, 1187–1192. [Google Scholar]
- Corner, M.A.; Habets, A.M.; Baker, R.E. Bioelectric activity is required for regional specificity of sensory ganglion projections to spinal cord explants cultured in vitro. Roux Arch. Dev. Biol. 1987, 196, 133–136. [Google Scholar] [CrossRef]
- Rhoades, B.K.; Gross, G.W. Potassium and calcium-channel dependence of bursting in cultured neuronal networks. Brain Res. 1994, 643, 310–318. [Google Scholar] [CrossRef]
- Czarnecki, A.; Magloire, V.; Streit, J. Modulation of intrinsic spiking in spinal cord neurons. J. Neurophysiol. 2009, 102, 2441–2452. [Google Scholar] [CrossRef]
- Ben-Ari, Y. Developing networks play a similar melody. Trends Neurosci. 2001, 24, 353–360. [Google Scholar] [CrossRef]
- Momose-Sato, Y.; Mochida, H.; Kinoshita, M. Origin of the earliest correlated neuronal activity in the chick embryo revealed by optical imaging with voltage-sensitive dyes. Eur. J. Neurosci. 2009, 29, 1–13. [Google Scholar] [CrossRef]
- Ren, J.; Greer, J.J. Ontogeny of rhythmic motor patterns generated in the embryonic rat spinal cord. J. Neurophysiol. 2003, 89, 1187–1195. [Google Scholar]
- Corner, M.A.; van Pelt, J.; Wolters, P.S.; Baker, R.E.; Nuytinck, R.H. Physiological effects of sustained blockade of excitatory synaptic transmission on spontaneously active developing neuronal networks—An enquiry into the reciprocal linkage between intrinsic biorhythms and neuroplasticity in early ontogeny. Neurosci. Biobehav. Rev. 2002, 26, 127–185. [Google Scholar] [CrossRef]
- Crain, S.M.; Bornstein, M.B.; Peterson, E.R. Maturation of cultured embryonic CNS tissues during chronic exposure to agents which prevent bioelectric activity. Brain Res. 1968, 8, 363–372. [Google Scholar] [CrossRef]
- Siegel, J.M. Do all animals sleep? Trends Neurosci. 2008, 31, 208–213. [Google Scholar]
- Corner, M.A.; Roholl, P.; Bot, A.P.C. Effects of glutamate on spontaneous electrical activity in the embryonic chick cerebrum. Exp. Neurol. 1974, 44, 229–245. [Google Scholar] [CrossRef]
- Jongkind, J.F.; Corner, M.A.; Bruntink, R. Glycolytic substrate utilization and energy consumption in the cerebral hemispheres of the chick embryo during the period of EEG development. J. Neurochem. 1972, 19, 389–94. [Google Scholar] [CrossRef]
- Corner, M.A.; Bot, A.P.C. Electrical activity in the isolated forebrain of the chick embryo. Brain Res. 1969, 12, 473–476. [Google Scholar] [CrossRef]
- Mirmiran, M.; Corner, M.A. Neuronal discharge patterns in the occipital cortex of developing rats during active and quiet sleep. Dev. Brain Res. 1982, 3, 37–48. [Google Scholar] [CrossRef]
- Habets, A.M.; van Dongen, A.M.; van Huizen, F.; Corner, M.A. Spontaneous neuronal firing patterns in fetal rat cortical networks during development in vitro: A quantitative analysis. Exp. Brain Res. 1987, 69, 43–52. [Google Scholar]
- Moore, A.R.; Zhou, W.-L.; Jakovcevski, I.; Zecevic, N.; Antic, S.D. Spontaneous electrical activity in the human fetal cortex. J. Neurosci. 2011, 31, 2391–2398. [Google Scholar] [CrossRef]
- Compte, A.; Sanchez-Vives, M.V.; McCormick, D.A.; Wang, X.-J. Cellular and network mechanisms of slow oscillatory activity (<1 Hz) and wave propagation in a cortical network model. J. Neurophysiol. 2003, 89, 2707–2725. [Google Scholar] [CrossRef]
- Baker, R.E.; Corner, M.A; van Pelt, J. Spontaneous neuronal discharge patterns in developing organotypic mega-co-cultures of neonatal rat cerebral cortex. Brain Res. 2006, 1101, 29–35. [Google Scholar] [CrossRef]
- Jimbo, Y. MEA-based recording of neuronal activity in vitro. Arch. Ital. Biol. 2007, 145, 289–297. [Google Scholar]
- Hinard, V.; Mikhail, C.; Pradervand, S.; Curie, T.; Houtkooper, R.H.; Auwerx, J.; Franken, P.; Tafti, M. Key electrophysiological, molecular, and metabolic signatures of sleep and wakefulness revealed in primary cortical cultures. J. Neurosci. 2012, 32, 12506–12517. [Google Scholar] [CrossRef]
- Chiappalone, M.A.; Vato, A.; Berdondini, L.; Koudelka-Hep, M.; Martinoia, S. Network dynamics and synchronous activity in cultured cortical neurons. Int. J. Neural Syst. 2007, 17, 87–103. [Google Scholar] [CrossRef]
- Chiappalone, M.A.; Bove, M.; Vato, A.; Tedesco, M.; Martinoia, S. Dissociated cortical networks show spontaneously correlated activity patterns during in vitro development. Brain Res. 2006, 1093, 41–53. [Google Scholar]
- Segal, M.M. Epileptiform activity in microcultures containing one excitatory hippocampal neuron. J. Neurophysiol. 1991, 65, 761–770. [Google Scholar]
- Buzsáki, G. Rhythms of the Brain; Oxford University Press: New York, NY, USA, 2006; pp. 175–205. [Google Scholar]
- Corner, M.A.; Baker, R.E.; van Pelt, J. Physiological consequences of selective suppression of intrinsically generated bioelectric discharges in a living “model” system for slow-wave sleep activity. Neurosci. Biobehav. Rev. 2008, 32, 1569–1600. [Google Scholar] [CrossRef]
- Corner, M.A.; Baker, R.E.; van Pelt, J.; Wolters, P.S. Compensatory physiological responses to chronic blockade of amino acid receptors during early development in spontaneously active organotypic cerebral cortex explants cultured in vitro. Prog. Brain Res. 2005, 147, 231–248. [Google Scholar] [CrossRef]
- Hangya, B.; Tihanyi, B.T.; Entz, L.; Fabo, D.; Eross, L.; Wittner, L.; Jakus, R.; Varga, V.; Freund, T.F.; Ulbet, I. Complex propagation patterns characterize human cortical activity during slow wave sleep. J. Neurosci. 2011, 31, 8770–8779. [Google Scholar] [CrossRef]
- Sun, J.-J.; Kilb, W.; Luhmann, H.J. Self-organization of repetitive spike patterns in developing neuronal networks in-vitro. Eur. J. Neurosci. 2010, 32, 1289–1299. [Google Scholar] [CrossRef]
- Madhavan, R.; Chao, Z.S.; Potter, S.M. Plasticity of recurring spatiotemporal activity patterns in cortical networks. Phys. Biol. 2007, 4, 181–193. [Google Scholar] [CrossRef]
- Wagenaar, D.A.; Madhavan, R.; Potter, S.M. An extremely rich repertoire of bursting patterns during the development of cortical cultures. BMC Neurosci. 2006, 7, 11. [Google Scholar] [CrossRef]
- Van Pelt, J.; Corner, M.A.; Wolters, P.S.; Rutten, W.L.; Ramakers, G.J. Longterm stability and developmental changes in spontaneous network burst firing patterns in dissociated rat cerebral cortex cell cultures on multielectrode arrays. Neurosci. Lett. 2004, 361, 86–89. [Google Scholar] [CrossRef]
- Frost, J.D.; Gol, A. Computer determination of relationships between EEG activity and single unit discharges in isolated cerebral cortex. Exp. Neurol. 1966, 14, 506–519. [Google Scholar] [CrossRef]
- Grinstein, G.; Linsker, R. Synchronous neural activity in scale-free network models versus random network models. Proc. Natl. Acad. Sci. USA 2005, 102, 9948–9953. [Google Scholar] [CrossRef]
- Downes, J.H.; Hammond, M.W.; Xydas, D.; Spencer, M.C.; Becerra, V.M.; Warwick, K.; Whalley, B.J.; Nasuto, S.J. Emergence of a small-world functional network in cultured neurons. PLoS Comput. Biol. 2012, 8, e1002522. [Google Scholar] [CrossRef]
- Gritsun, T.A.; Le Feber, J.; Stegenga, J.; Rutten, W.L. Network bursts in cortical cultures are best simulated using pacemaker neurons and adaptive synapses. Biol. Cybern. 2012, 102, 293–310. [Google Scholar]
- Corner, M.A. Spontaneous neuronal burst discharges as dependent and independent variables in the maturation of cerebral cortex tissue cultured in vitro: A review of activity-dependent studies in live “model” systems for the development of intrinsically generated bioelectric slow-wave sleep patterns. Brain Res. Rev. 2008, 59, 221–244. [Google Scholar] [CrossRef]
- Van Huizen, F.; Romijn, H.J.; Habets, A.M. Synaptogenesis in rat cerebral cortex cultures is affected during chronic blockade of spontaneous bioelectric activity by tetrodotoxin. Brain Res. 1985, 351, 67–80. [Google Scholar]
- Van Ooyen, A.; van Pelt, J.; Corner, M.A. Implications of activity-dependent neurite outgrowth for neuronal morphology and network development. J. Theor. Biol. 1995, 172, 63–82. [Google Scholar] [CrossRef]
- Ramakers, G.J.; Corner, M.A.; Habets, A.M. Development in the absence of spontaneous bioelectric activity results in increased stereotyped burst firing in cultures of dissociated cerebral cortex. Exp. Brain Res. 1990, 79, 157–166. [Google Scholar]
- Swann, J.W.; Le, J.T.; Lam, T.T.; Owens, J.; Mayer, A.T. The impact of chronic network hyperexcitability on developing glutamatergic synapses. Eur. J. Neurosci. 2007, 26, 975–991. [Google Scholar] [CrossRef]
- Takada, N.; Yanagawa, Y.; Komatsu, Y. Activity-dependent maturation of excitatory synaptic connections in solitary neuron cultures of mouse neocortex. Eur. J. Neurosci. 2005, 21, 422–430. [Google Scholar] [CrossRef]
- Corner, M.A.; Ramakers, G.J. Spontaneous firing as an epigenetic factor in brain development—Physiological consequences of chronic tetrodotoxin and picrotoxin exposure in cultured rat neocortex neurons. Dev. Brain Res. 1992, 65, 57–64. [Google Scholar] [CrossRef]
- Maffei, A.; Turrigiano, G. The age of plasticity: Developmental regulation of synaptic plasticity in neocortical microcircuits. Prog. Brain Res. 2008, 169, 211–223. [Google Scholar] [CrossRef]
- McCabe, A.K.; Chisholm, S.L.; Picken-Barry, H.L.; Moody, W.J. The self-regulating nature of spontaneous synchronized activity in developing mouse cortical neurones. J. Physiol. (Lond.) 2006, 577, 155–167. [Google Scholar] [CrossRef]
- Stewart, C.V.; Plenz, D. Homeostasis of neuronal avalanches during postnatal cortex development in vitro. J. Neurosci. Meth. 2008, 169, 405–416. [Google Scholar] [CrossRef]
- Wierenga, W.J.; Walsh, M.F.; Turrigiano, G.G. Regulation of the expression locus of homeostatic plasticity. J. Neurophysiol. 2006, 96, 2127–2133. [Google Scholar] [CrossRef]
- Minerbi, A.; Kahana, R.; Goldfeld, L.; Kaufman, M.; Marom, S.; Ziv, N.E. Long-term relationships between synaptic tenacity, synaptic remodelling and network activity. PLoS Biol. 2009, 7, e1000136. [Google Scholar] [CrossRef]
- Dash, M.B.; Douglas, C.L.; Vyazovskiy, V.V.; Cirelli, C.; Tononi, G. Long-term homeostasis of extracellular glutamate in the rat cerebral cortex across sleep and waking states. J. Neurosci. 2009, 29, 620–629. [Google Scholar] [CrossRef]
- Spitzer, N.C.; Borodinsky, L.N.; Root, C.M. Homeostatic activity-dependent paradigm for neurotransmitter specification. Cell Calcium 2005, 37, 417–423. [Google Scholar] [CrossRef]
- Opitz, T.; de Lima, A.D.; Voigt, T. Spontaneous development of synchronous oscillatory activity during maturation of cortical networks in vitro. J. Neurophysiol. 2002, 88, 2196–2206. [Google Scholar] [CrossRef]
- Baker, R.E.; van Pelt, J. Co-cultured but not isolated cortical explants display normal dendritic development: A long-term quantitative study. Dev. Brain Res. 1997, 98, 21–27. [Google Scholar] [CrossRef]
- Corner, M.A. Ontogeny of Brain Sleep Mechanisms. In Brain Mechanisms of Sleep; Raven Press: New York, NY, USA, 1985; pp. 175–197. [Google Scholar]
- Shaffery, J.P.; Roffwarg, H.P. The Ontogenetic Hypothesis of Rapid Eye Movement Sleep Function Revisited. In Current Advances in Sleep Biology (Neuroscience Research Progress Series); Frank, M.G., Ed.; Nova Science: Hauppauge, NY, USA, 2009; pp. 177–216. [Google Scholar]
- Mirmiran, M. The function of fetal/neonatal rapid-eye-movement sleep. Behav. Brain Res. 1995, 69, 13–22. [Google Scholar] [CrossRef]
- Kaufman, M.; Corner, M.A.; Ziv, N.E. Long-term relationships between cholinergic tone, synchronous bursting and synaptic remodeling. PLoS One 2012, 7, e40980. [Google Scholar] [CrossRef]
- Wagenaar, D.A.; Madhavan, R.; Pine, J.; Potter, S.M. Controlling bursting in cortical cultures with closed-loop multi-electrode stimulation. J. Neurosci. 2005, 25, 680–688. [Google Scholar] [CrossRef]
- Le Feber, J.; Rutten, W.L.; Stegenga, J.; Wolters, P.S.; Ramakers, G.J.; van Pelt, J. Conditional firing probabilities in cultured neuronal networks: A stable underlying structure in widely varying spontaneous activity patterns. J. Neural Eng. 2007, 4, 54–67. [Google Scholar] [CrossRef]
- Le Feber, J. ; Technical University of Twente, Enschede, The Netherlands. Personal communication, 2012.
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signaling dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef] [Green Version]
- Kavanau, J. REM and NREM sleep as natural accompaniments of the evolution of warm-bloodedness. Neurosci. Biobehav. Rev. 2002, 26, 889–906. [Google Scholar] [CrossRef]
- Krueger, J.M.; Obál, F., Jr. A neuronal group theory of sleep function. J. Sleep Res. 1993, 2, 63–69. [Google Scholar] [CrossRef]
- Schenck, C.H.; Mahowald, M.W. REM sleep behavior disorder: Clinical, developmental and neuroscience perspectives 16 years after its formal identification in sleep. Sleep 2002, 25, 120–138. [Google Scholar]
- Weiner, N.; Central Institute for Brain Research, Amsterdam, The Netherlands. Personal communication, 1963.
- Myrdal, J. Report from a Chinese Village; Random House: New York, NY, USA, 1970. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Corner, M.A. From Neural Plate to Cortical Arousal—A Neuronal Network Theory of Sleep Derived from in Vitro “Model” Systems for Primordial Patterns of Spontaneous Bioelectric Activity in the Vertebrate Central Nervous System. Brain Sci. 2013, 3, 800-820. https://doi.org/10.3390/brainsci3020800
Corner MA. From Neural Plate to Cortical Arousal—A Neuronal Network Theory of Sleep Derived from in Vitro “Model” Systems for Primordial Patterns of Spontaneous Bioelectric Activity in the Vertebrate Central Nervous System. Brain Sciences. 2013; 3(2):800-820. https://doi.org/10.3390/brainsci3020800
Chicago/Turabian StyleCorner, Michael A. 2013. "From Neural Plate to Cortical Arousal—A Neuronal Network Theory of Sleep Derived from in Vitro “Model” Systems for Primordial Patterns of Spontaneous Bioelectric Activity in the Vertebrate Central Nervous System" Brain Sciences 3, no. 2: 800-820. https://doi.org/10.3390/brainsci3020800
APA StyleCorner, M. A. (2013). From Neural Plate to Cortical Arousal—A Neuronal Network Theory of Sleep Derived from in Vitro “Model” Systems for Primordial Patterns of Spontaneous Bioelectric Activity in the Vertebrate Central Nervous System. Brain Sciences, 3(2), 800-820. https://doi.org/10.3390/brainsci3020800




