Altered Intrinsic Functional Connectivity in Language-Related Brain Regions in Association with Verbal Memory Performance in Euthymic Bipolar Patients
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cognitive Testing and Individual Psychopathology
2.2. Seed Region: HG Bilateral
| PAT Mean (SD) | CON Mean (SD) | Significance | |
|---|---|---|---|
| Number | 21 | 20 | - |
| Gender | 9 f/12 m | 8 f/12 m | χ2 = 0.03, p = 0.55 |
| Age (years) | 35.67 (10.68) | 36.90 (11.06) | t = 0.36, p = 0.72 |
| MWT-B | 29.86 (3.31) | 31.80 (2.98) | z = −1.83, p = 0.06 |
| Handedness | all right handed | all right handed | - |
| BDI | 9.85 (9.00) | 2.00 (3.57) | t = 3.64, p = 0.001 |
| BRMAS | 0.38 (0.59) | 0.25 (0.44) | t = 0.80, p = 0.43 |
| PANAS PA | 25.62 (8.34) | 35.75 (4.61) | t = −4.84, p < 0.01 |
| PANAS NA | 15.71 (5.25) | 14.20 (4.01) | t = 1.03, p = 0.31 |
| CVLT LS | 53.90 (10.27) | 61.45 (7.21) | t = −2.73, p = 0.01 |
| CVLT DFR I | 11.29 (2.76) | 13.55 (1.88) | t = −3.06, p =0.004 |
| CVLT DFR II | 12.33 (3.02) | 14.05 (1.70) | t = −2.26, p = 0.031 |
| CVLT YNR | 15.10 (1.14) | 15.60 (0.68) | t = −1.74, p = 0.092 |
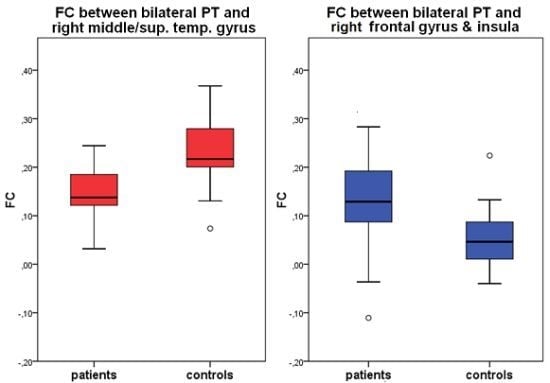
2.3. Seed Region: PT Bilateral
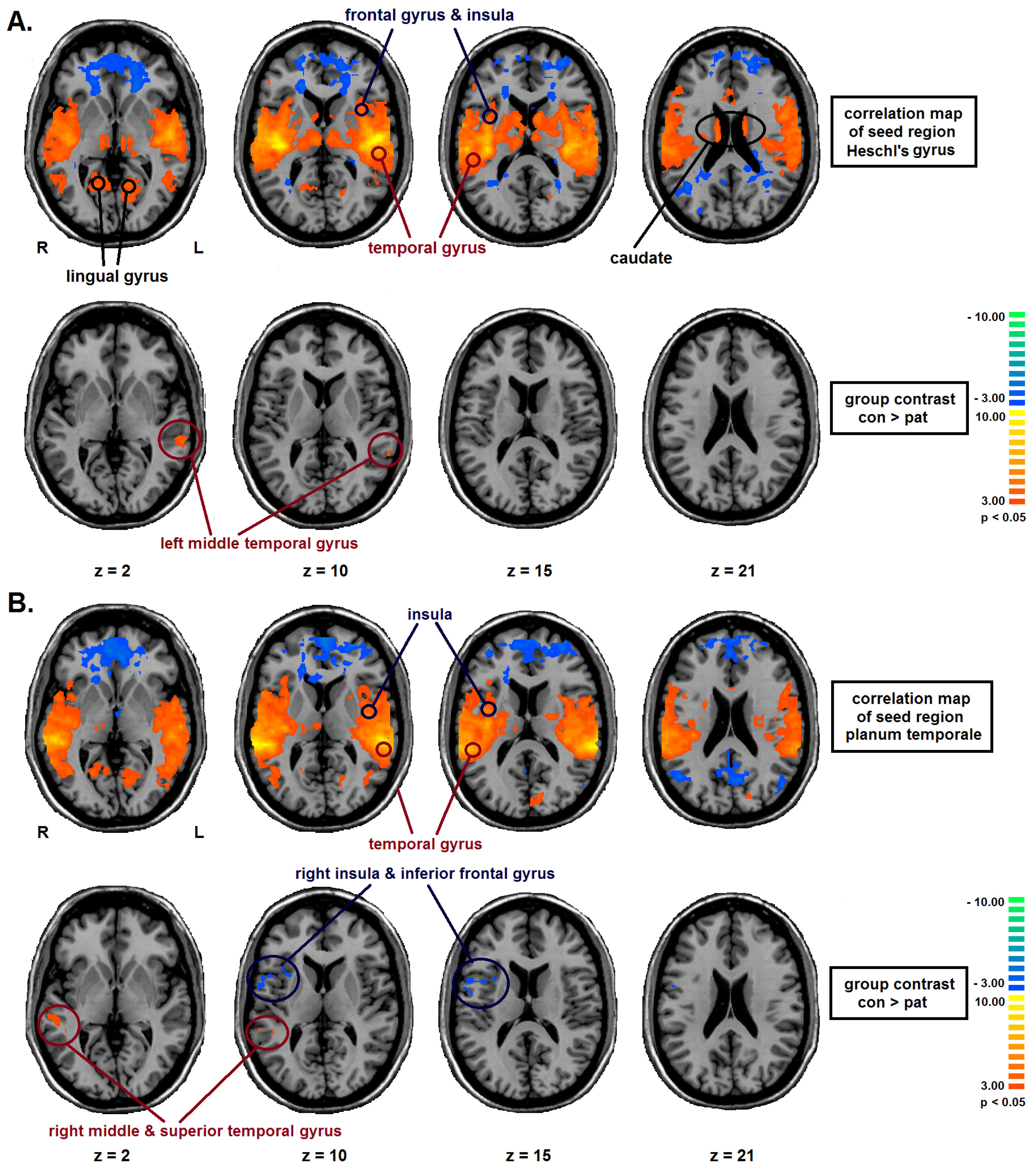
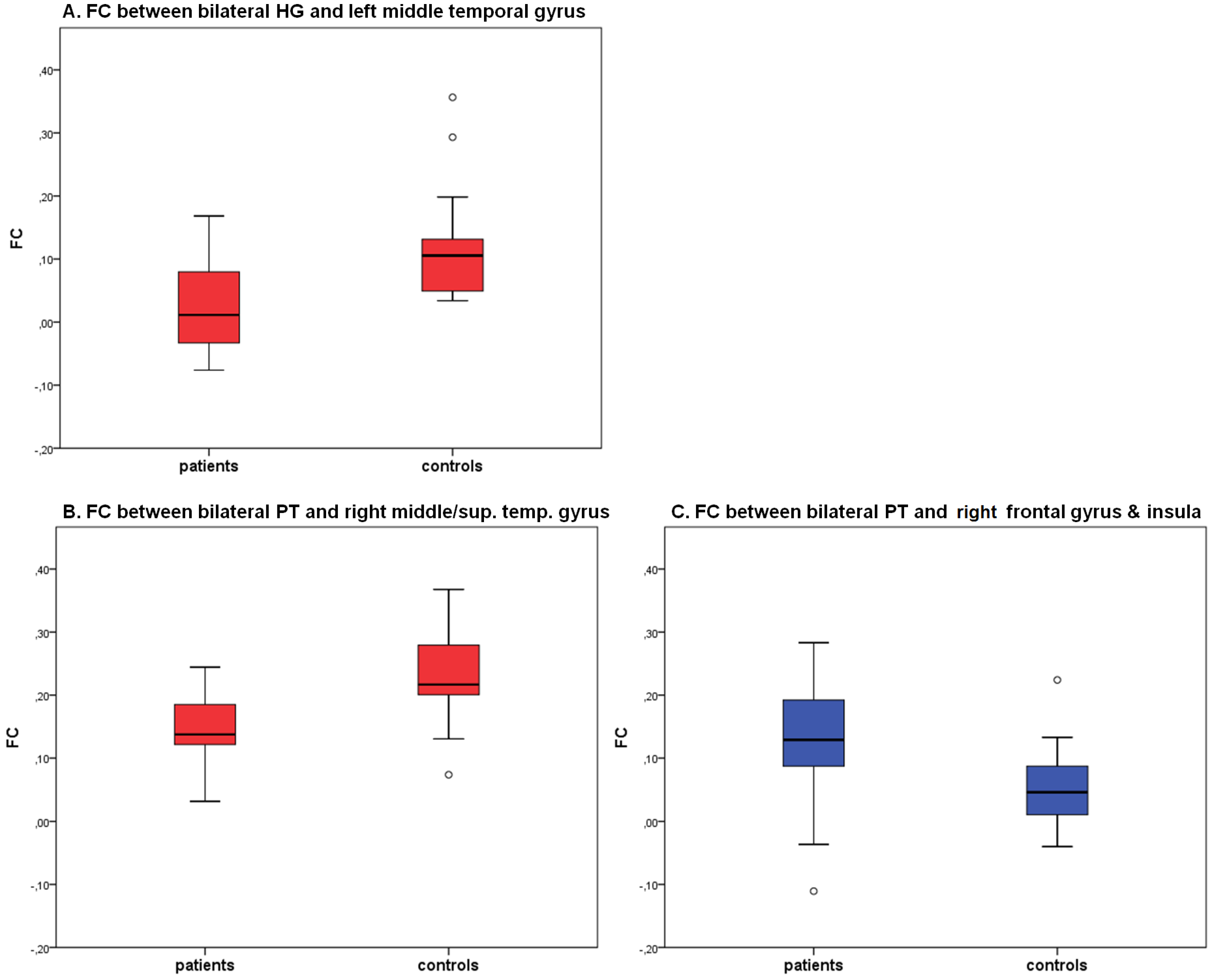
| Seed-Region | Area | BA | TAL-Coordinates | Nr of voxels | group | FC | T | P |
|---|---|---|---|---|---|---|---|---|
| bilateral HG | center middle temporal gyrus | 22 | −58, −36, 5 | 929 | CON | 0.12 (0.09) | 3.94 | <0.01 |
| PAT | 0.02 (0.07) | |||||||
| bilateral PT | right middle & superior temporal gyrus | 22 | 51, −35, 4 | 949 | CON | 0.23 (0.07) | 4.35 | <0.01 |
| PAT | 0.14 (0.06) | |||||||
| right inferior frontal gyrus, precentral gyrus, insula | 13, 44 | 45, 7, 11 | 1406 | CON | 0.05 (0.06) | −3,20 | <0.01 | |
| PAT | 0.13 (0.10) |
2.4. Correlation Analysis
2.4.1. Individual Psychopathology
2.4.2. Cognitive Performance
| Seed-Region | Area | group | Significant correlations |
|---|---|---|---|
| bilateral HG | center middle temporal gyrus | CON | - |
| PAT | - | ||
| bilateral PT | right middle & superior temporal gyrus | CON | - |
| PAT | CVLT LS: r = −0.477, p = 0.029 | ||
| right inferior frontal gyrus, precentral gyrus, insula | CON | CVLT YNR: r = −0.479, p = 0.033 | |
| PAT | - |
2.4.3. Medication
2.5. Summary of Results
2.6. Association with Language-Related Memory Performance
2.7. Association with Individual Psychopathology
2.8. Limitations
3. Experimental Section
3.1. Participants
3.2. Cognitive Testing
3.3. Individual Psychopathology
3.4. Imaging: Data Acquisition
3.5. Imaging: Data Preprocessing
3.6. Imaging: Statistical Analysis
3.7. Correlation Analyses
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Craddock, N.; Owen, M.J. Molecular genetics and the relationship between epilepsy and psychosis. Br. J. Psychiatry 2010, 197, 75–76, author reply 76. [Google Scholar] [CrossRef]
- Aleman, A.; Hijman, R.; de Haan, E.H.; Kahn, R.S. Memory impairment in schizophrenia: A meta-analysis. Am. J. Psychiatry 1999, 156, 1358–1366. [Google Scholar]
- Torres, I.J.; Boudreau, V.G.; Yatham, L.N. Neuropsychological functioning in euthymic bipolar disorder: A meta-analysis. Acta Psychiatr. Scand. Suppl. 2007. [Google Scholar] [CrossRef]
- Arts, B.; Jabben, N.; Krabbendam, L.; van Os, J. Meta-analyses of cognitive functioning in euthymic bipolar patients and their first-degree relatives. Psychol. Med. 2008, 38, 771–785. [Google Scholar]
- Bora, E.; Yucel, M.; Pantelis, C. Cognitive endophenotypes of bipolar disorder: A meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. J. Affect. Disord. 2009, 113, 1–20. [Google Scholar] [CrossRef]
- Deckersbach, T.; McMurrich, S.; Ogutha, J.; Savage, C.R.; Sachs, G.; Rauch, S.L. Characteristics of non-verbal memory impairment in bipolar disorder: The role of encoding strategies. Psychol. Med. 2004, 34, 823–832. [Google Scholar] [CrossRef]
- Robinson, L.J.; Thompson, J.M.; Gallagher, P.; Goswami, U.; Young, A.H.; Ferrier, I.N.; Moore, P.B. A meta-analysis of cognitive deficits in euthymic patients with bipolar disorder. J. Affect. Disord. 2006, 93, 105–115. [Google Scholar] [CrossRef]
- Penfield, W.; Perot, P. The brain’s record of auditory and visual experience. A final summary and discussion. Brain 1963, 86, 595–696. [Google Scholar] [CrossRef]
- Chance, S.A.; Casanova, M.F.; Switala, A.E.; Crow, T.J. Auditory cortex asymmetry, altered minicolumn spacing and absence of ageing effects in schizophrenia. Brain 2008, 131, 3178–3192. [Google Scholar] [CrossRef]
- Oertel, V.; Knochel, C.; Rotarska-Jagiela, A.; Schonmeyer, R.; Lindner, M.; van de Ven, V.; Haenschel, C.; Uhlhaas, P.; Maurer, K.; Linden, D.E. Reduced laterality as a trait marker of schizophrenia—evidence from structural and functional neuroimaging. J. Neurosci. 2010, 30, 2289–2299. [Google Scholar] [CrossRef]
- Sommer, I.E.; Ramsey, N.F.; Mandl, R.C.; van Oel, C.J.; Kahn, R.S. Language activation in monozygotic twins discordant for schizophrenia. Br. J. Psychiatry 2004, 184, 128–135. [Google Scholar] [CrossRef]
- Sumich, A.; Chitnis, X.A.; Fannon, D.G.; O’Ceallaigh, S.; Doku, V.C.; Faldrowicz, A.; Sharma, T. Unreality symptoms and volumetric measures of heschl’s gyrus and planum temporal in first-episode psychosis. Biol. Psychiatry 2005, 57, 947–950. [Google Scholar] [CrossRef]
- Chen, H.H.; Nicoletti, M.A.; Hatch, J.P.; Sassi, R.B.; Axelson, D.; Brambilla, P.; Monkul, E.S.; Keshavan, M.S.; Ryan, N.D.; Birmaher, B.; et al. Abnormal left superior temporal gyrus volumes in children and adolescents with bipolar disorder: A magnetic resonance imaging study. Neurosci. Lett. 2004, 363, 65–68. [Google Scholar] [CrossRef]
- Takahashi, T.; Malhi, G.S.; Wood, S.J.; Yucel, M.; Walterfang, M.; Kawasaki, Y.; Suzuki, M.; Pantelis, C. Gray matter reduction of the superior temporal gyrus in patients with established bipolar I disorder. J. Affect. Disord. 2010, 123, 276–282. [Google Scholar] [CrossRef]
- Qiu, A.; Vaillant, M.; Barta, P.; Ratnanather, J.T.; Miller, M.I. Region-of-interest-based analysis with application of cortical thickness variation of left planum temporale in schizophrenia and psychotic bipolar disorder. Hum. Brain Mapp. 2008, 29, 973–985. [Google Scholar] [CrossRef]
- Oertel-Knochel, V.; Linden, D.E. Cerebral asymmetry in schizophrenia. Neuroscientist 2011, 17, 456–467. [Google Scholar] [CrossRef]
- Bruder, G.E. Cerebral laterality and psychopathology: A review of dichotic listening studies. Schizophr. Bull. 1993, 9, 134–151. [Google Scholar] [CrossRef]
- Bruder, G.E.; Stewart, J.W.; Towey, J.P.; Friedman, D. Abnormal cerebral laterality in bipolar depression: Convergence of behavioral and brain event-related potential findings. Biol. Psychiatry 1992, 32, 33–47. [Google Scholar] [CrossRef]
- Bruder, G.; Fong, R.; Tenke, C.; Leite, P.; Towey, J.; Stewart, J.; McGrath, P.; Quitkin, F. Regional brain asymmetries in major depression with or without an anxiety disorder: A quantitative electroencephalographic study. Biol. Psychiatry 1997, 41, 939–948. [Google Scholar] [CrossRef]
- Kato, T.; Shioiri, T.; Murashita, J.; Hamakawa, H.; Takahashi, Y.; Inubushi, T.; Takahashi, S. Lateralized abnormality of high energy phosphate metabolism in the frontal lobes of patients with bipolar disorder detected by phase-encoded 31P-MRS. Psychol. Med. 1995, 25, 557–566. [Google Scholar] [CrossRef]
- Reite, M.; Teale, P.; Rojas, D.C.; Reite, E.; Asherin, R.; Hernandez, O. Meg auditory evoked fields suggest altered structural/functional asymmetry in primary but not secondary auditory cortex in bipolar disorder. Bipolar Disord. 2009, 11, 371–381. [Google Scholar] [CrossRef]
- Van den Heuvel, M.P.; Hulshoff Pol, H.E. Exploring the brain network: A review on resting-state fMRI functional connectivity. Eur. Neuropsychopharmacol. 2010, 20, 519–534. [Google Scholar] [CrossRef]
- Anand, A.; Li, Y.; Wang, Y.; Lowe, M.J.; Dzemidzic, M. Resting state corticolimbic connectivity abnormalities in unmedicated bipolar disorder and unipolar depression. Psychiatry Res. 2009, 171, 189–198. [Google Scholar] [CrossRef]
- Chepenik, L.G.; Raffo, M.; Hampson, M.; Lacadie, C.; Wang, F.; Jones, M.M.; Pittman, B.; Skudlarski, P.; Blumberg, H.P. Functional connectivity between ventral prefrontal cortex and amygdala at low frequency in the resting state in bipolar disorder. Psychiatry Res. 2010, 182, 207–210. [Google Scholar] [CrossRef]
- Ongur, D.; Lundy, M.; Greenhouse, I.; Shinn, A.K.; Menon, V.; Cohen, B.M.; Renshaw, P.F. Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res. 2010, 183, 59–68. [Google Scholar] [CrossRef]
- Chai, X.J.; Whitfield-Gabrieli, S.; Shinn, A.K.; Gabrieli, J.D.; Nieto Castanon, A.; McCarthy, J.M.; Cohen, B.M.; Ongur, D. Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology 2011, 36, 2009–2017. [Google Scholar] [CrossRef]
- Liu, C.H.; Ma, X.; Wu, X.; Zhang, Y.; Zhou, F.C.; Li, F.; Tie, C.L.; Dong, J.; Wang, Y.J.; Yang, Z.; et al. Regional homogeneity of resting-state brain abnormalities in bipolar and unipolar depression. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2012, 41, 52–59. [Google Scholar]
- Hautzinger, M.; Keller, F.; Kühner, C. Das Beck Depressionsinventar II. Deutsche Bearbeitung und Handbuch Zum Bdi II; Harcourt Test Services: Frankfurt, Germany, 2006. [Google Scholar]
- Bech, P. Rating scales for affective disorders: Their validity and consistency. Acta Psychiatr. Scand. 1981, 295, 1–101. [Google Scholar]
- Krohne, H.W.; Egloff, B.; Kohlmann, C.-W.; Tausch, A. Untersuchungen mit einer deutschen version der “Positive and negative affect schedule” (PANAS). Diagnostica 1996, 42, 139–156. [Google Scholar]
- Niemann, H.; Sturm, W.; Töhne-Otto, A.I.T.; Wilmes, K. California Verbal Learning Test (CVLT). Deutsche Adaptation; Pearson Assessment & Information: Frankfurt, Germany, 2008. [Google Scholar]
- Almeida, J.R.; Mechelli, A.; Hassel, S.; Versace, A.; Kupfer, D.J.; Phillips, M.L. Abnormally increased effective connectivity between parahippocampal gyrus and ventromedial prefrontal regions during emotion labeling in bipolar disorder. Psychiatry Res. 2009, 30, 195–201. [Google Scholar]
- Rotarska-Jagiela, A.; van de Ven, V.; Oertel-Knochel, V.; Uhlhaas, P.J.; Vogeley, K.; Linden, D.E. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr. Res. 2010, 117, 21–30. [Google Scholar] [CrossRef]
- Vercammen, A.; Knegtering, H.; den Boer, J.A.; Liemburg, E.J.; Aleman, A. Auditory hallucinations in schizophrenia are associated with reduced functional connectivity of the temporo-parietal area. Biol. Psychiatry 2010, 67, 912–918. [Google Scholar] [CrossRef]
- Gavrilescu, M.; Rossell, S.; Stuart, G.W.; Shea, T.L.; Innes-Brown, H.; Henshall, K.; McKay, C.; Sergejew, A.A.; Copolov, D.; Egan, G.F. Reduced connectivity of the auditory cortex in patients with auditory hallucinations: A resting state functional magnetic resonance imaging study. Psychol. Med. 2010, 40, 1149–1158. [Google Scholar] [CrossRef]
- Fleisher, A.S.; Sherzai, A.; Taylor, C.; Langbaum, J.B.; Chen, K.; Buxton, R.B. Resting-state bold networks vs. task-associated functional MRI for distinguishing Alzheimer’s disease risk groups. Neuroimage 2009, 47, 1678–1690. [Google Scholar]
- APA, Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, DC, USA, 1994.
- Lehrl, S. Mehrfachwahl-Wortschatz-Intelligenztest MWT-B; Spitta Verlag GmbH: Göttingen, Gremany, 2005. [Google Scholar]
- Wittchen, H.-U.; Wunderlich, U.; Gruschwitz, S.; Zaudig, M. Strukturiertes Klinisches Interview für DSM-IV (SKID); Beltz-Test: Göttingen, Germany, 1996. [Google Scholar]
- Deichmann, R.; Schwarzbauer, C.; Turner, R. Optimisation of the 3D MDEFT sequence for anatomical brain imaging: Technical implications at 1.5 and 3 T. Neuroimage 2004, 21, 757–767. [Google Scholar] [CrossRef]
- Goebel, R.; Esposito, F.; Formisano, E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: From single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum. Brain Mapp. 2006, 27, 392–401. [Google Scholar] [CrossRef]
- Talairach, J.; Tournoux, P. Co-Planar Stereotexic Atlas of the Human Brain; Thieme Medical: New York, NY, USA, 1988. [Google Scholar]
- Kasai, K.; Shenton, M.E.; Salisbury, D.F.; Hirayasu, Y.; Onitsuka, T.; Spencer, M.H.; Yurgelun-Todd, D.A.; Kikinis, R.; Loesz, F.A.; McCarley, R.W. Progressive decrease of left heschl gyrus and planum temporale gray matter volume in first-episode schizophrenia. Arch. Gen. Psychiatry 2003, 60, 766–775. [Google Scholar] [CrossRef]
- Hirayasu, Y.; Tanaka, S.; Shenton, M.E.; Salisbury, D.F.; DeSantis, M.A.; Levitt, J.J.; Wible, C.; Yurgelun-Todd, D.; Kikinis, R.; Jolesz, F.A.; et al. Prefrontal gray matter volume reduction in first episode schizophrenia. Cereb. Cortex 2000, 11, 374–381. [Google Scholar]
- Schonmeyer, R.; Prvulovic, D.; Rotarska-Jagiela, A.; Haenschel, C.; Linden, D.E. Automated segmentation of lateral ventricles from human and primate magnetic resonance images using cognition network technology. Magn. Reson. Imaging 2006, 24, 1377–1387. [Google Scholar] [CrossRef]
- Brain Imaging Center Frankfurt, Germany. Available online: http://www.bic.uni-frankfurt.de/bv-tools/ (accessed on 15 January 2013).
- Birn, R.M.; Diamond, J.B.; Smith, M.A.; Bandettini, P.A. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage 2006, 31, 1536–1548. [Google Scholar] [CrossRef]
- Chai, X.J.; Castanon, A.N.; Ongur, D.; Whitfield-Gabrieli, S. Anticorrelations in resting state networks without global signal regression. Neuroimage 2012, 59, 1420–1428. [Google Scholar] [CrossRef]
- Biswal, B.; Yetkin, F.Z.; Haughton, V.M.; Hyde, J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995, 34, 537–541. [Google Scholar] [CrossRef]
- Fox, M.D.; Raichle, M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. 2007, 8, 700–711. [Google Scholar] [CrossRef]
- Forman, S.D.; Cohen, J.D.; Fitzgerald, M.; Eddy, W.F.; Mintun, M.A.; Noll, D.C. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magn. Reson. Med. 2005, 33, 636–647. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Reinke, B.; Ven, V.V.d.; Matura, S.; Linden, D.E.J.; Oertel-Knöchel, V. Altered Intrinsic Functional Connectivity in Language-Related Brain Regions in Association with Verbal Memory Performance in Euthymic Bipolar Patients. Brain Sci. 2013, 3, 1357-1373. https://doi.org/10.3390/brainsci3031357
Reinke B, Ven VVd, Matura S, Linden DEJ, Oertel-Knöchel V. Altered Intrinsic Functional Connectivity in Language-Related Brain Regions in Association with Verbal Memory Performance in Euthymic Bipolar Patients. Brain Sciences. 2013; 3(3):1357-1373. https://doi.org/10.3390/brainsci3031357
Chicago/Turabian StyleReinke, Britta, Vincent Van de Ven, Silke Matura, David E. J. Linden, and Viola Oertel-Knöchel. 2013. "Altered Intrinsic Functional Connectivity in Language-Related Brain Regions in Association with Verbal Memory Performance in Euthymic Bipolar Patients" Brain Sciences 3, no. 3: 1357-1373. https://doi.org/10.3390/brainsci3031357