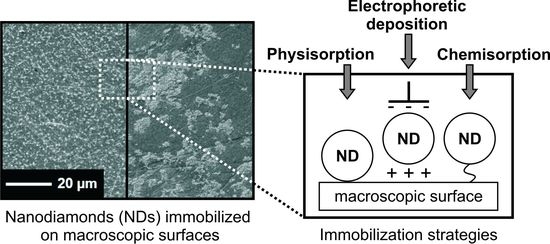
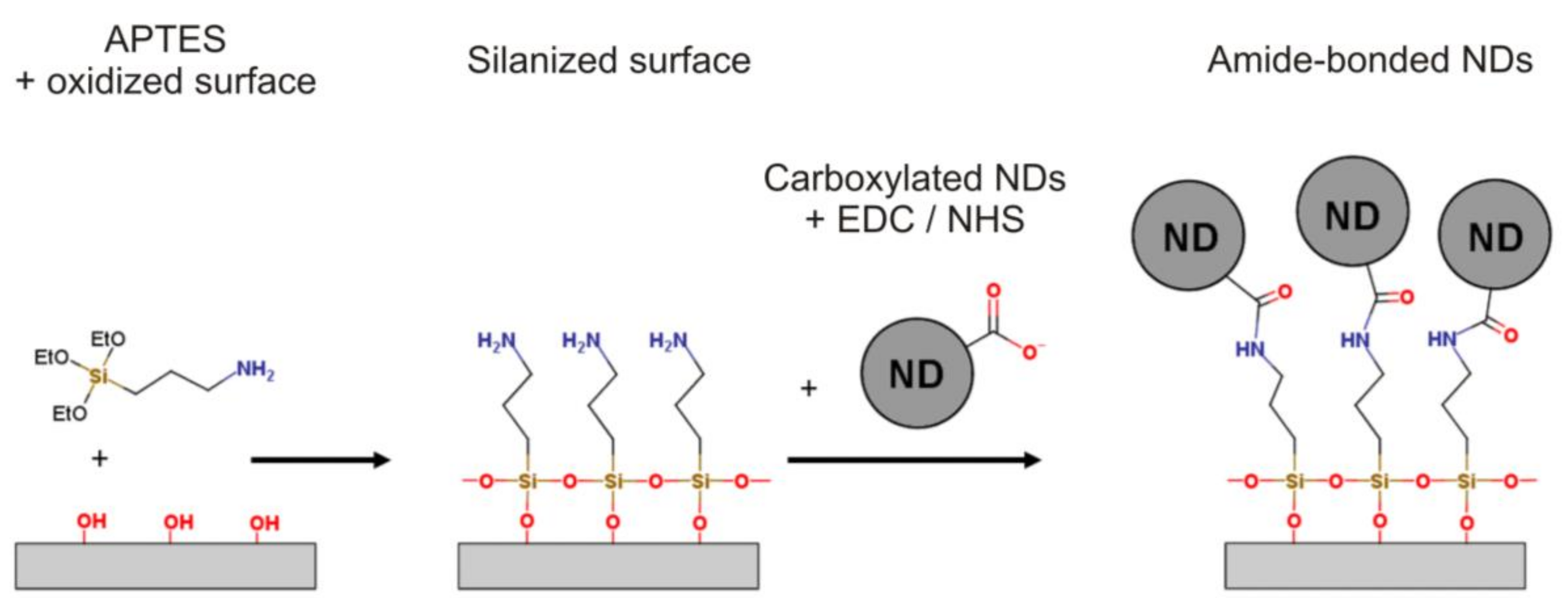
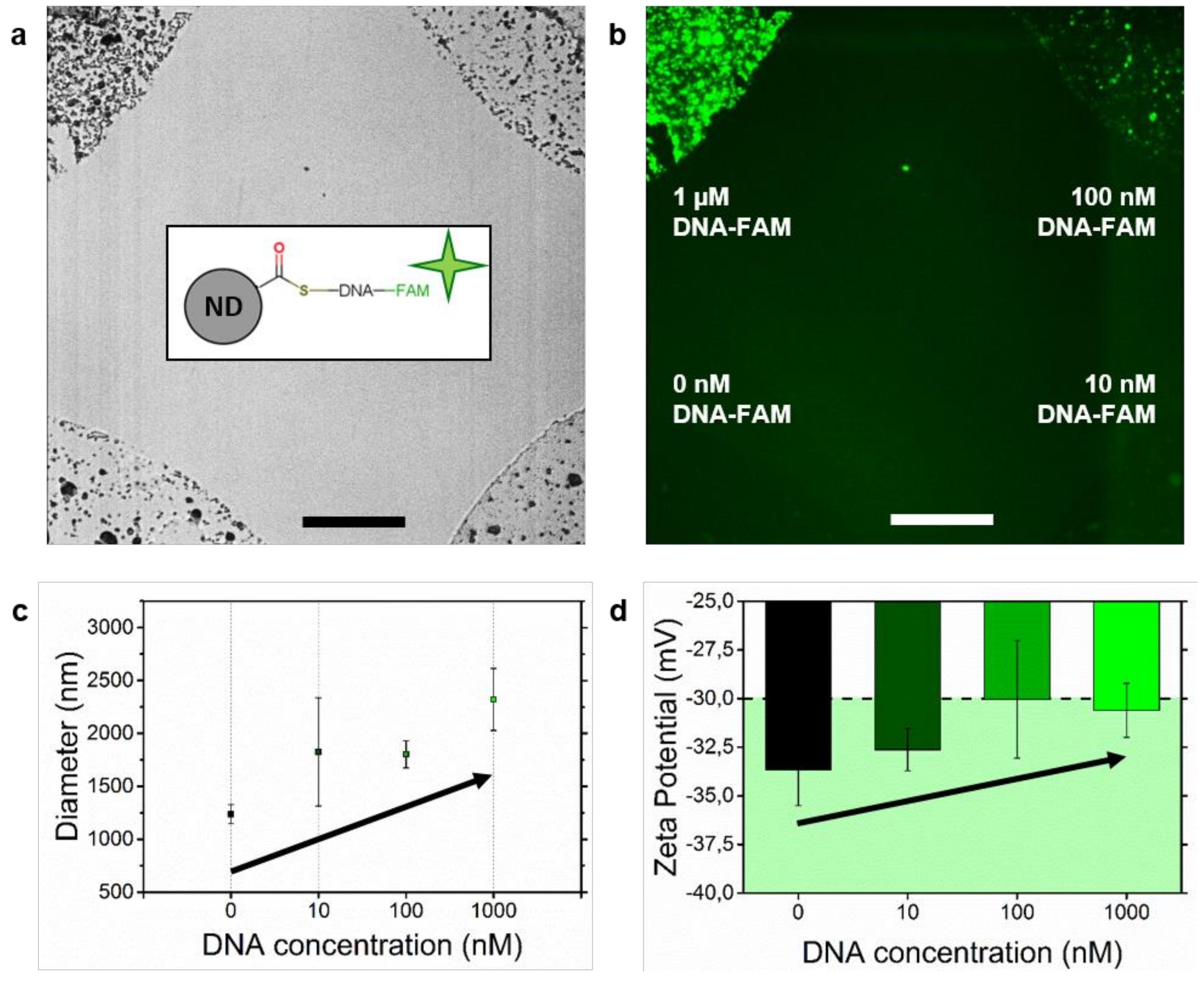
Immobilization of Detonation Nanodiamonds on Macroscopic Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. De-Agglomeration of Nanodiamonds
2.2. Colloidal Stability of Nanodiamonds
2.3. Macroscopic Surface Modification Techniques
2.4. Imaging and Analysing
2.5. Functionalisation of Nanodiamonds
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Niinomi, M. Recent research and development in titanium alloys for biomedical applications and healthcare goods. Sci. Technol. Adv. Mater. 2003, 4, 445–454. [Google Scholar] [CrossRef]
- Albrektsson, T.; Brånemark, P.-I.; Hansson, H.-A.; Lindström, J. Osseointegrated Titanium Implants: Requirements for Ensuring a Long-Lasting, Direct Bone-to-Implant Anchorage in Man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Le Gu’ehennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. J. 2007, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Curto, B.D.; Pedeferri, M. Anodic oxidation of titanium: From technical aspects to biomedical applications. J. Appl. Biomater. Biomech. 2011, 9, 55–69. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Properties of Osteoconductive Biomaterials: Calcium Phosphates. Clin. Orthop. Relat. Res. 2002, 395, 81–98. [Google Scholar] [CrossRef]
- Hench, L.L. The story of Bioglass. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Roy, R.K.; Lee, K.-R. Biomedical Applications of Diamond-Like Carbon Coatings: A Review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 83B, 72–84. [Google Scholar] [CrossRef]
- Chowdhury, S.; Hillman, D.A. Synthesis of ultrasmooth nanostructured diamond films by microwave plasma chemical vapor deposition using a He/H2/CH4/ N2 gas mixture. J. Mater. Res. 2006, 21, 2675–2682. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, C.; Guo, S. Novel Diamond Films Synthesis Strategy: Methanol and Argon Atmosphere by Microwave Plasma CVD Method Without Hydrogen. Nanoscale Res. Lett. 2016, 11, 415. [Google Scholar] [CrossRef]
- Wang, H.-D.; Yang, Q.; Niu, C.H. Preparation of films of nanodiamonds by step-by-step deposition approach through hydrogen bonding. Diam. Relat. Mater. 2012, 25, 73–79. [Google Scholar] [CrossRef]
- Forsgren, J.; Engqvist, H. A novel method for local administration of strontium from implant surfaces. J. Mater. Sci. Mater. Med. 2010, 21, 1605–1609. [Google Scholar] [CrossRef] [PubMed]
- Mertens, C.; Steveling, H.G. Early and immediate loading of titanium implants with fluoride-modified surfaces: Results of 5-year prospective study. Clin. Oral Implants Res. 2011, 22, 1354–1360. [Google Scholar] [CrossRef] [PubMed]
- Ostman, P.O.; Wennerberg, A.; Ekestubbe, A.; Albrektsson, T. Immediate occlusal loading of NanoTiteTM tapered implants: A prospective 1-year clinical and radiographic study. Clin. Implant Dent. Relat. Res. 2013, 15, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Pistilli, R.; Felice, P.; Piattelli, M.; Gessaroli, M.; Soardi, E.; Barausse, C.; Buti, J.; Corvino, V. Posterior atrophic jaws rehabilitated with prostheses supported by 5 × 5 mm implants with a novel nanostructured calciumincorporated titanium surface or by longer implants in augmented bone: One-year results from a randomized controlled trial. Eur. J. Oral Implantol. 2013, 6, 343–357. [Google Scholar] [PubMed]
- Kim, H.-S.; Kim, Y.-J.; Jang, J.-H.; Park, J.-W. Surface Engineering of Nanostructured Titanium Implants with Bioactive Ions. J. Dent. Res. 2016, 95, 558–565. [Google Scholar] [CrossRef]
- Li, B.; Li, Y.; Li, J.; Fu, X. Improvement of biological properties of titanium by anodic oxidation and ultraviolet irradiation. Appl. Surf. Sci. 2014, 307, 202–208. [Google Scholar] [CrossRef]
- Portan, D.V.; Kroustalli, A.A.; Deligianni, D.D.; Papanicolaou, G.C. On the biocompatibility between TiO2 nanotubes layer and human osteoblasts. J. Biomed. Mater. Res. 2012, 100A, 2546–2553. [Google Scholar] [CrossRef]
- Voznyakovskii, A.P.; Shugalei, I.V. Surface Characterization of Detonation Nanodiamond Particles. Russ. J. Gen. Chem. 2012, 82, 2256–2258. [Google Scholar] [CrossRef]
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2011, 7, 11–23. [Google Scholar] [CrossRef]
- Liu, K.-K.; Cheng, C.-L.; Chang, C.-C.; Chao, J.-I. Biocompatible and detectable carboxylated nanodiamond on human cell. Nanotechnology 2007, 18, 325102. [Google Scholar] [CrossRef]
- Ho, D.; Wang, C.-H.K.; Chow, E.K.-H. Nanodiamonds: The intersection of nanotechnology, drug development, and personalized medicine. Sci. Adv. 2015, 1, e1500439. [Google Scholar] [CrossRef]
- Chow, E.K.; Zhang, X.-Q.; Chen, M.; Lam, R.; Robinson, E.; Huang, H.; Schaffer, D.; Osawa, E.; Goga, A.; Ho, D. Nanodiamond Therapeutic Delivery Agents Mediate Enhanced Chemoresistant Tumor Treatment. Sci. Transl. Med. 2011, 3, 73ra21. [Google Scholar] [CrossRef]
- Schrand, A.M.; Huang, H.; Carlson, C.; Schlager, J.J. Are Diamond Nanoparticles Cytotoxic? J. Phys. Chem. B 2007, 111, 2–7. [Google Scholar] [CrossRef]
- Chao, J.-I.; Perevedentseva, E.; Chung, P.-H.; Liu, K.-K. Nanometer-Sized Diamond Particle as a Probe for Biolabeling. Biophys. J. 2007, 93, 2199–2208. [Google Scholar] [CrossRef]
- El-Say, K.M. Nanodiamond as a drug delivery system: Applications and prospective. J. Appl. Pharm. Sci. 2012, 1, 29–39. [Google Scholar]
- Vial, S.; Mansuy, C.; Sagan, S.; Irinopoulou, T.; Burlina, F.; Boudou, J.-P.; Chassaing, G.; Lavielle, S. Peptide-Grafted Nanodiamonds: Preparation, Cytotoxicity and Uptake in Cells. ChemBioChem 2008, 9, 2113–2119. [Google Scholar] [CrossRef]
- Puzyr, A.P.; Baron, A.V.; Purtov, K.V.; Bortnikov, E.V. Nanodiamonds with novel properties: A biological study. Diam. Relat. Mater. 2007, 16, 2124–2128. [Google Scholar] [CrossRef]
- Bradac, C.; Say, J.M.; Rastogi, I.D.; Cordina, N.M. Nano-assembly of nanodiamonds by conjugation to actin filaments. J. Biophotonics 2016, 9, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Yeap, W.S.; Tan, Y.Y.; Loh, K.P. Using Detonation Nanodiamond for the Specific Capture of Glycoproteins. Anal. Chem. 2008, 80, 4659–4665. [Google Scholar] [CrossRef]
- Hens, S.C.; Cunningham, G.; Tyler, T.; Moseenkov, S.; Kuznetsov, V.; Shenderova, O. Nanodiamond bioconjugate probes and their collection by electrophoresis. Diam. Relat. Mater. 2008, 17, 1858–1866. [Google Scholar] [CrossRef]
- Wierzbicki, M.; Sawosz, E.; Grodzik, M.; Hotowy, A. Carbon nanoparticles downregulate expression of basic fibroblast growth factor in the heart during embryogenesis. Int. J. Nanomed. 2013, 8, 3427–3435. [Google Scholar]
- Thomas, V.; Halloran, B. In vitro studies on the effect of particle size on macrophage responses to nanodiamond wear debris. Acta Biomater. 2012, 8, 1939–1947. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.C.; González, C.H.; Miller, B.S.; Edgington, R.J.; Ferretti, P.; Jackman, R.B. Surface functionalisation of nanodiamonds for human neural stem cell adhesion and proliferation. Sci. Rep. 2017, 7, 7307. [Google Scholar] [CrossRef] [PubMed]
- Edgington, R.J.; Thalhammer, A.; Welch, J.O.; Bongrain, A.; Bergonzo, P.; Scorsone, E.; Jackman, R.B.; Schoepfer, R. Patterned neuronal networks using nanodiamonds and the effect of varying nanodiamond properties on neuronal adhesion and outgrowth. J. Neural Eng. 2013, 10, 056022. [Google Scholar] [CrossRef]
- Huang, H.; Pierstorff, E.; Osawa, E.; Ho, D. Protein-Mediated Assembly of Nanodiamond Hydrogels into a Biocompatible and Biofunctional Multilayer Nanofilm. ACS Nano 2008, 2, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Stehlik, S.; Varga, M.; Stenclova, P.; Ondic, L.; Ledinsky, M.; Pangrac, J.; Vanek, O.; Lipov, J.; Kromka, A.; Rezek, B. Ultrathin Nanocrystalline Diamond Films with Silicon Vacancy Color Centers via Seeding by 2 nm Detonation Nanodiamonds. ACS Appl. Mater. Interfaces 2017, 9, 38842–38853. [Google Scholar] [CrossRef]
- Domonkos, M.; Ižák, T.; Varga, M.; Potocký, Š.; Demo, P.; Kromka, A. Diamond nucleation and growth on horizontally and vertically aligned Si substrates at low pressure in a linear antenna microwave plasma system. Diam. Relat. Mater. 2018, 82, 41–49. [Google Scholar] [CrossRef]
- Majchrowicz, D.; Kosowska, M.; Sankaran, K.J.; Struk, P.; Wasowicz, M.; Sobaszek, M.; Haenen, K.; Jedrzejewska-Szczerska, M. Nitrogen-Doped Diamond Film for Optical Investigation of Hemoglobin Concentration. Materials 2018, 11, 109. [Google Scholar] [CrossRef]
- Shavel, A.; Gaponik, N.; Eychmueller, A. Covalent Linking of CdTe Nanocrystals to Amino-Functionalized Surfaces. ChemPhysChem 2005, 6, 449–451. [Google Scholar] [CrossRef]
- Krüger, A.; Kataoka, F.; Ozawa, M.; Fujino, T.; Suzukic, Y.; Aleksenskii, A.E.; YaVul’, A.; Ōsawa, E. Unusually tight aggregation in detonation nanodiamond: Identification and disintegration. Carbon 2005, 43, 1722. [Google Scholar] [CrossRef]
- Gaillard, C.; Girard, H.A.; Falck, C.; Paget, V.; Simic, V.; Ugolin, N.; Bergonzo, P.; Chevillard, S.; Arnault, J.C. Peptide nucleic acid-nanodiamonds: Covalent and stable conjugates for DNA targeting. RSC Adv. 2014, 4, 3566. [Google Scholar] [CrossRef]
- Akiel, R.D.; Zhang, X.; Abeywardana, C.; Stepanov, V.; Qin, P.Z.; Takahashi, S. Investigating Functional DNA Grafted on Nanodiamond Surface Using Site-Directed Spin Labeling and Electron Paramagnetic Resonance Spectroscopy. J. Phys. Chem. B 2016, 120, 4003–4008. [Google Scholar] [CrossRef]
- Zhang, T.; Neumann, A.; Lindlau, J.; Wu, Y.; Pramanik, G.; Naydenov, B.; Jelezko, F.; Schueder, F.; Huber, S.; Huber, M.; et al. DNA-Based Self-Assembly of Fluorescent Nanodiamonds. J. Am. Chem. Soc. 2015, 137, 9776–9779. [Google Scholar] [CrossRef]
- Purtov, K.V.; Burakova, L.P.; Puzyr, A.P.; Bondar, V.S. The interaction of linear and ring forms of DNA molecules with nanodiamonds synthesized by detonation. Nanotechnology 2008, 19, 325101. [Google Scholar] [CrossRef]
- Basu, R.; Skaggs, N.; Shalov, S.; Brereton, P. Evidence of nanodiamond-self-assembly in a liquid crystal, and the consequent impacts on the liquid crystal properties. AIP Adv. 2017, 7, 075008. [Google Scholar] [CrossRef]
- Ryu, T.-K.; Baek, S.W.; Lee, G.-J.; Rhee, C.; Choi, S.-W. Targeted Tumor Therapy Based on Nanodiamonds Decorated with Doxorubicin and Folic Acid. Macromol. Biosci. 2017, 17, 1600180. [Google Scholar] [CrossRef]
- Beutner, R.; Michael, J.; Schwenzer, B.; Scharnweber, D. Biological nano-functionalization of titanium-based biomaterial surfaces: A flexible toolbox. J. R. Soc. Interface 2010, 7, 93–105. [Google Scholar] [CrossRef]
- Balasaheb Nimse, S.; Song, K.; Sonawane, M.D.; Sayyed, D.R.; Kim, T. Immobilization Techniques for Microarray: Challenges and Applications. Sensors 2014, 14, 22208–22229. [Google Scholar] [CrossRef]
- Hu, Q.; Katti, P.S.; Gu, Z. Enzyme-Responsive Nanomaterials for Controlled Drug Delivery. Nanoscale 2014, 6, 12273–12286. [Google Scholar] [CrossRef]
- Yassin, M.A.; Mustafa, K.; Xing, Z.; Sun, Y.; Fasmer, K.E.; Waag, T.; Krueger, A.; Steinmüller-Nethl, D.; Finne-Wistrand, A.; Leknes, K.N. A Copolymer Scaffold Functionalized with Nanodiamond Particles Enhances Osteogenic Metabolic Activity and Bone Regeneration. Macromol. Biosci. 2017, 17, 1600427. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Bruschi, M.; Waag, T.; Schweeberg, S.; Tian, Y.; Meinhardt, T.; Stigler, R.; Larsson, K.; Funk, M.; Steinmueller-Nethl, D.; et al. Functionalization of bone implants with nanodiamond particles and angiopoietin-1 to improve vascularization and bone regeneration. J. Mater. Chem. B 2017, 5, 6629–6636. [Google Scholar] [CrossRef]
- Zhanga, W.; Wang, G.; Liu, Y.; Zhao, X.; Zou, D.; Zhu, C.; Jin, Y.; Huang, Q.; Sun, J.; Liu, X.; et al. The synergistic effect of hierarchical micro/nano-topography and bioactive ions for enhanced osseointegration. Biomaterials 2013, 34, 3184–3195. [Google Scholar] [CrossRef] [PubMed]


| Uncoated | Dip Coated | EPD-Treated | Amide-Bonded NDs | |
|---|---|---|---|---|
| Excess surface area (µm2) | 1.0 | 11.7 | 5.5 | 19.6 |
| Ra (nm) | 7.3 | 17.7 | 19.6 | 49.7 |
| RMS (nm) | 10.6 | 24.4 | 25.1 | 66.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balakin, S.; Dennison, N.R.; Klemmed, B.; Spohn, J.; Cuniberti, G.; Römhildt, L.; Opitz, J. Immobilization of Detonation Nanodiamonds on Macroscopic Surfaces. Appl. Sci. 2019, 9, 1064. https://doi.org/10.3390/app9061064
Balakin S, Dennison NR, Klemmed B, Spohn J, Cuniberti G, Römhildt L, Opitz J. Immobilization of Detonation Nanodiamonds on Macroscopic Surfaces. Applied Sciences. 2019; 9(6):1064. https://doi.org/10.3390/app9061064
Chicago/Turabian StyleBalakin, Sascha, Nicholas R. Dennison, Benjamin Klemmed, Juliane Spohn, Gianaurelio Cuniberti, Lotta Römhildt, and Jörg Opitz. 2019. "Immobilization of Detonation Nanodiamonds on Macroscopic Surfaces" Applied Sciences 9, no. 6: 1064. https://doi.org/10.3390/app9061064