The Electronic Properties of Silicon Nanowires during Their Dissolution under Simulated Physiological Conditions
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
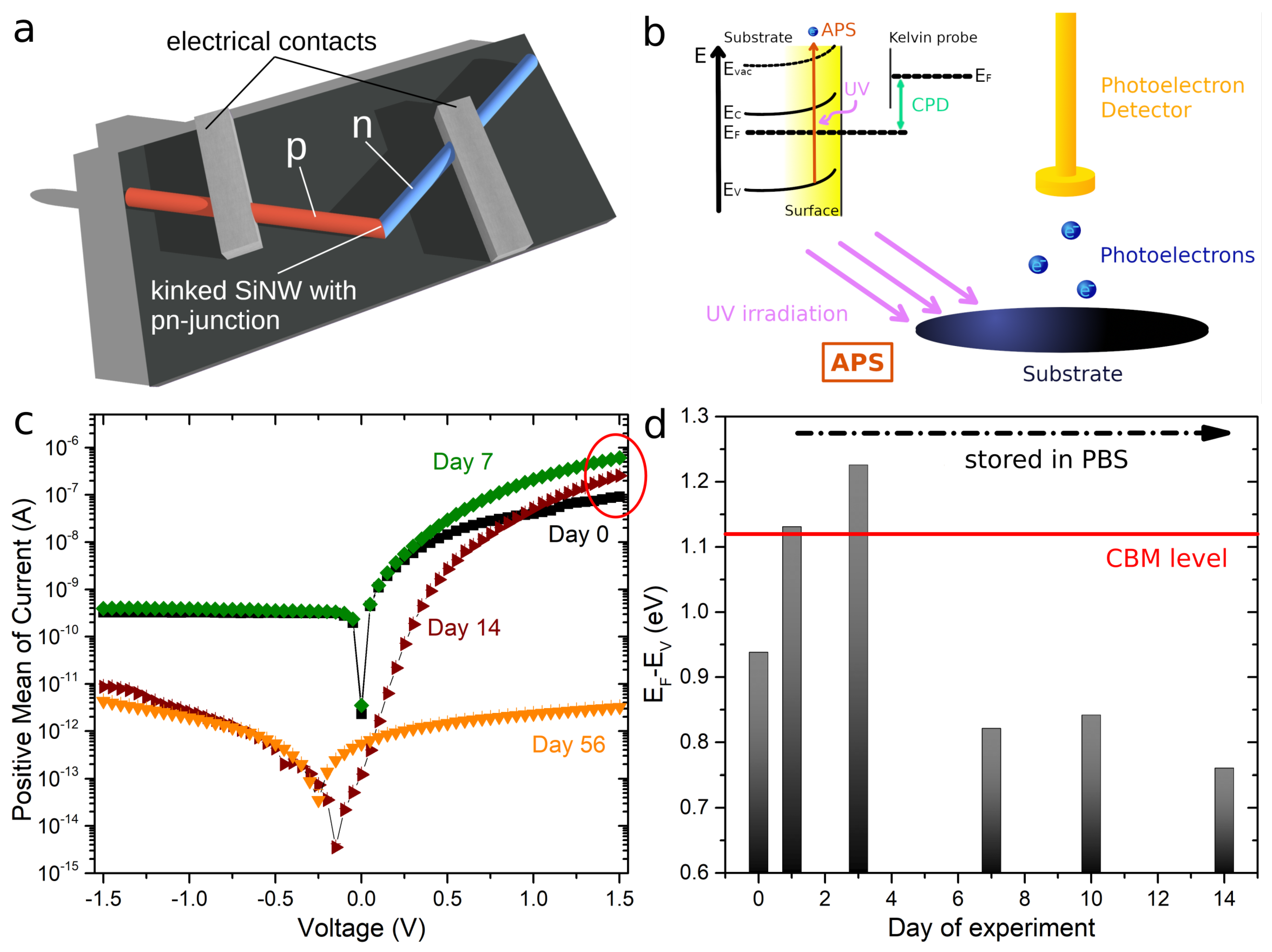
2.1.1. Kinked p–n Junctions for Measurements of Single SiNWs
2.1.2. Samples Densely Covered with SiNWs
2.2. Sample Immersion
2.2.1. Electron Microscopy
2.2.2. Electrical Characterization
2.2.3. AFM Measurements
2.2.4. Reconstruction of the Nanowire Surface Band Structure
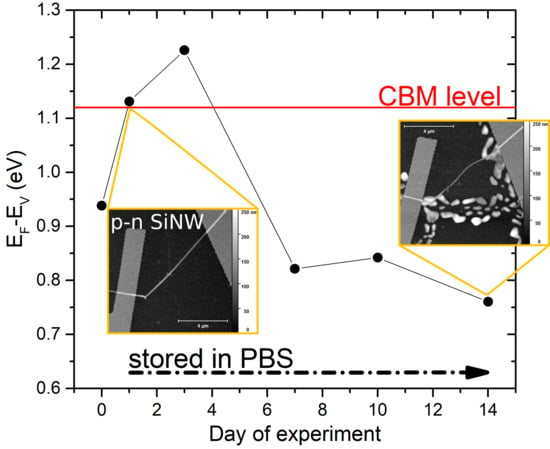
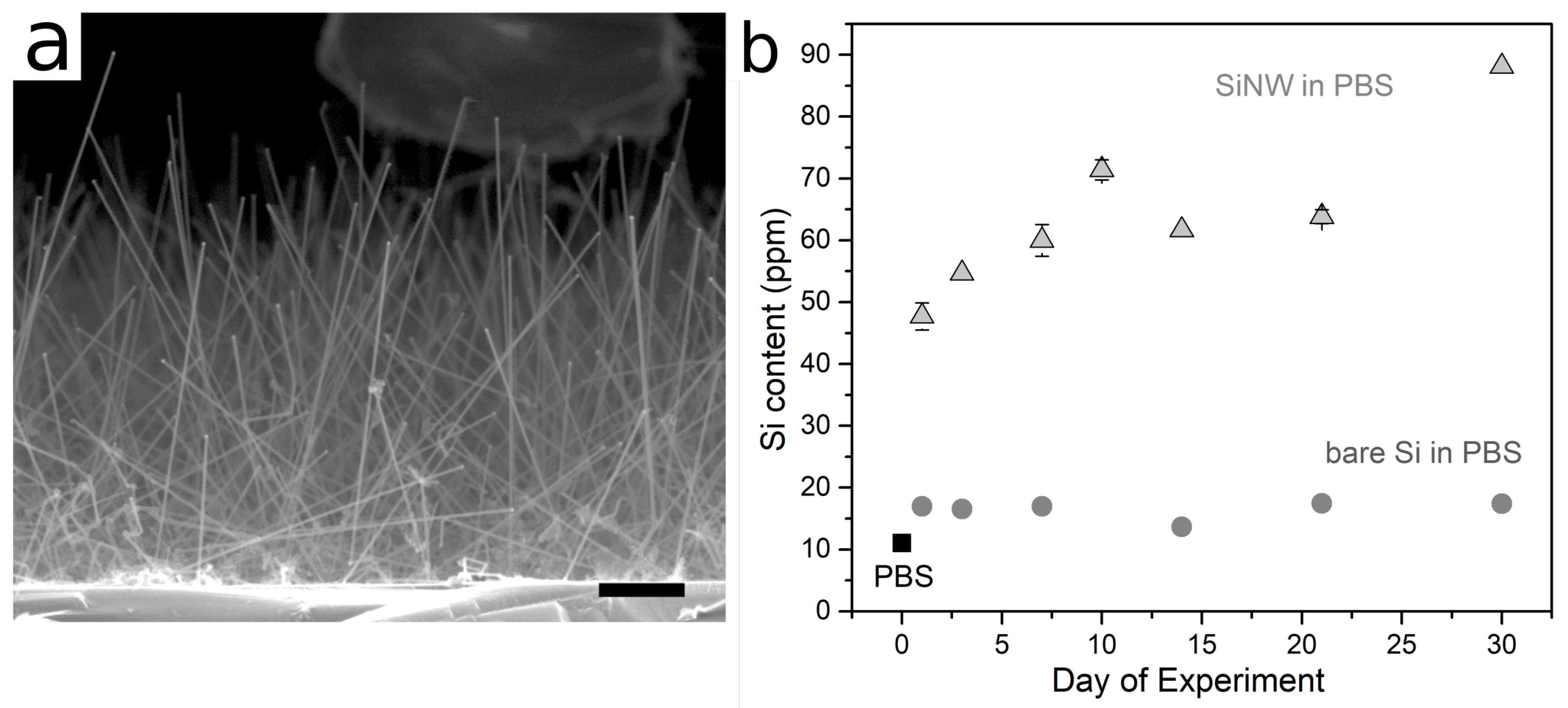
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | atomic force microscopy |
| ALD | atomic layer deposition |
| APS | ambient pressure photoemission spectroscopy |
| AuNP | gold nanoparticle |
| CBM | conduction band minimum |
| CMOS | complementary metal-oxide-semiconductor |
| CPD | contact potential difference |
| CVD | chemical vapor deposition |
| E | Fermi level |
| E | valence band energy level |
| FET | field-effect transistor |
| ICP-AES | inductively coupled plasma atomic emission spectroscopy |
| PBS | phosphate buffered saline |
| SEM | scanning electron microscopy |
| SiNW | silicon nanowire |
| TEM | transmission electron microscopy |
| TMA | trimethyl aluminium |
| VBM | valence band maximum |
| VLS | vapor–liquid–solid |
References
- Cui, Y.; Wei, Q.; Park, H.; Lieber, C.M. Nanowire Nanosensors for Highly Sensitive and Selective Detection of Biological and Chemical Species. Science 2001, 293, 1289–1292. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.I.; Li, B.R.; Chen, Y.T. Silicon nanowire field-effect transistor-based biosensors for biomedical diagnosis and cellular recording investigation. Nano Today 2011, 6, 131–154. [Google Scholar] [CrossRef]
- Hahm, J.I.; Lieber, C.M. Direct Ultrasensitive Electrical Detection of DNA and DNA Sequence Variations Using Nanowire Nanosensors. Nano Lett. 2004, 4, 51–54. [Google Scholar] [CrossRef]
- Zhang, G.J.; Zhang, L.; Huang, M.J.; Luo, Z.H.H.; Tay, G.K.I.; Lim, E.J.A.; Kang, T.G.; Chen, Y. Silicon nanowire biosensor for highly sensitive and rapid detection of Dengue virus. Sens. Actuators B Chem. 2010, 146, 138–144. [Google Scholar] [CrossRef]
- Wu, J. Semiconducting silicon nanowire array fabrication for high throughput screening in the biosciences. In Semiconducting Silicon Nanowires for Biomedical Applications; Coffer, J.L., Ed.; Woodhead Publishing: Sawston, UK, 2014; Chapter 9; pp. 171–191. [Google Scholar]
- Luo, L.; Jie, J.; Zhang, W.; He, Z.; Wang, J.; Yuan, G.; Zhang, W.; Wu, L.C.M.; Lee, S.T. Silicon nanowire sensors for Hg2+ and Cd2+ ions. Appl. Phys. Lett. 2009, 94, 193101. [Google Scholar] [CrossRef]
- Elfström, N.; Juhasz, R.; Sychugov, I.; Engfeldt, T.; Karlström, A.E.; Linnros, J. Surface Charge Sensitivity of Silicon Nanowires: Size Dependence. Nano Lett. 2007, 7, 2608–2612. [Google Scholar] [CrossRef] [PubMed]
- Lis, D.; Backus, E.H.G.; Hunger, J.; Parekh, S.H.; Bonn, M. Liquid flow along a solid surface reversibly alters interfacial chemistry. Science 2014, 344, 1138–1142. [Google Scholar] [CrossRef] [PubMed]
- Iler, R. The Chemistry of Silica—Solubility, Polymerization, Colloid and Surface Properties, and Biochemistry; Wiley-Interscience: Hoboken, NJ, USA, 1979. [Google Scholar]
- Morita, M.; Ohmi, T.; Hasegawa, E.; Kawakami, M.; Ohwada, M. Growth of native oxide on a silicon surface. J. Appl. Phys. 1990, 68, 1272–1281. [Google Scholar] [CrossRef]
- Zhou, W.; Dai, X.; Fu, T.M.; Xie, C.; Liu, J.; Lieber, C.M. Long Term Stability of Nanowire Nanoelectronics in Physiological Environments. Nano Lett. 2014, 14, 1614–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Jiang, Z.; Qing, Q.; Mai, L.; Zhang, Q.; Lieber, C.M. Design and Synthesis of Diverse Functional Kinked Nanowire Structures for Nanoelectronic Bioprobes. Nano Lett. 2013, 13, 746–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, R.S.; Ellis, W.C. Vapor-liquid-solid mechanism of single crystal growth. Appl. Phys. Lett. 1964, 4, 89–90. [Google Scholar] [CrossRef]
- Cui, Y.; Lauhon, L.J.; Gudiksen, M.S.; Wang, J.; Lieber, C.M. Diameter-controlled synthesis of single-crystal silicon nanowires. Appl. Phys. Lett. 2001, 78, 2214–2216. [Google Scholar] [CrossRef]
- Wu, Y.; Cui, Y.; Huynh, L.; Barrelet, C.J.; Bell, D.C.; Lieber, C.M. Controlled Growth and Structures of Molecular-Scale Silicon Nanowires. Nano Lett. 2004, 4, 433–436. [Google Scholar] [CrossRef]
- Steinbach, A.M.; Sandner, T.; Mizaikoff, B.; Strehle, S. Gas phase silanization for silicon nanowire sensors and other lab-on-a-chip systems. Phys. Status Solidi C 2015, 13, 135–141. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Challinger, S.; Baikie, I.; Birdwell, A.G.; Strehle, S. Construction of the Energy Band Diagram of Hydrogen Terminated Diamond and Silicon Nanowires. Phys. Status Solidi C 2017, 14, 1700152. [Google Scholar]
- Baikie, I.D.; Grain, A.C.; Sutherland, J.; Law, J. Ambient pressure photoemission spectroscopy of metal surfaces. Appl. Surf. Sci. 2014, 323, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Iler, R.K. The Occurrence, Dissolution, and Deposition of Silica. In The Chemistry of Silica—Solubility, Polymerization, Colloid and Surface Properties, and Biochemistry; Wiley-Interscience: Hoboken, NJ, USA, 1979; Chapter 1; pp. 3–115. [Google Scholar]
- Philipp, H.; Taft, E. An optical characterization of native oxides and thin thermal oxides on silicon. J. Appl. Phys. 1982, 53, 5224–5229. [Google Scholar] [CrossRef]
- Mizsei, J. Fermi-level pinning and passivation on the oxide-covered and bare silicon surfaces and interfaces. Vacuum 2002, 67, 59–67. [Google Scholar] [CrossRef]
- Helms, C.R.; Poindexter, E.H. The silicon-silicon dioxide system: Its microstructure and imperfections. Rep. Prog. Phys. 1994, 57, 791. [Google Scholar] [CrossRef]
- Schmidt, V.; Senz, S.; Gösele, U. Influence of the Si/SiO2 interface on the charge carrier density of Si nanowires. Appl. Phys. A 2007, 86, 187–191. [Google Scholar] [CrossRef]
- Kang, S.; Hwang, S.; Cheng, H.; Yu, S.; Kim, B.H.; Kim, J.; Huang, Y.; Rogers, J.A. Dissolution Behaviors and Applications of Silicon Oxides and Nitrides in Transient Electronics. Adv. Funct. Mater. 2014, 24, 4427–4434. [Google Scholar] [CrossRef]
- Fang, H.; Zhao, J.; Yu, K.J.; Song, E.; Farimani, A.B.; Chiang, C.H.; Jin, X.; Xue, Y.; Xu, D.; Du, W.; et al. Ultrathin, transferred layers of thermally grown silicon dioxide as biofluid barriers for biointegrated flexible electronic systems. Proc. Natl. Acad. Sci. USA 2016, 113, 11682–11687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peled, A.; Pevzner, A.; Peretz Soroka, H.; Patolsky, F. Morphological and chemical stability of silicon nanostructures and their molecular overlayers under physiological conditions: Towards long-term implantable nanoelectronic biosensors. J. Nanobiotechnol. 2014, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Glunz, S.W.; Biro, D.; Rein, S.; Warta, W. Field-effect passivation of the SiO2Si interface. J. Appl. Phys. 1999, 86, 683–691. [Google Scholar] [CrossRef]
- Velusamy, T.; Mitra, S.; Macias-Montero, M.; Svrcek, V.; Mariotti, D. Varying Surface Chemistries for p-Doped and n-Doped Silicon Nanocrystals and Impact on Photovoltaic Devices. ACS Appl. Mater. Interfaces 2015, 7, 28207–28214. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Yu, K.J.; Kim, Y.; Yoon, Y.; Xie, Z.; Song, E.; Luan, H.; Feng, X.; Huang, Y.; Rogers, J.A. Kinetics and Chemistry of Hydrolysis of Ultrathin, Thermally Grown Layers of Silicon Oxide as Biofluid Barriers in Flexible Electronic Systems. ACS Appl. Mater. Interfaces 2017, 9, 42633–42638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.J.; Ning, Y. Silicon nanowire biosensor and its applications in disease diagnostics: A review. Anal. Chim. Acta 2012, 749, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.K.; Koo, J.; Lee, Y.K.; Rogers, J.A. Advanced Materials and Devices for Bioresorbable Electronics. Acc. Chem. Res. 2018, 51, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.W.; Tao, H.; Kim, D.H.; Cheng, H.; Song, J.K.; Rill, E.; Brenckle, M.A.; Panilaitis, B.; Won, S.M.; Kim, Y.S.; et al. A Physically Transient Form of Silicon Electronics. Science 2012, 337, 1640–1644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen-Karni, T.; Casanova, D.; Cahoon, J.F.; Qing, Q.; Bell, D.C.; Lieber, C.M. Synthetically Encoded Ultrashort-Channel Nanowire Transistors for Fast, Pointlike Cellular Signal Detection. Nano Lett. 2012, 12, 2639–2644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, B.; Cohen-Karni, T.; Qing, Q.; Duan, X.; Xie, P.; Lieber, C.M. Three-Dimensional, Flexible Nanoscale Field-Effect Transistors as Localized Bioprobes. Science 2010, 329, 830–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qing, Q.; Jiang, Z.; Xu, L.; Gao, R.; Mai, L.; Lieber, C.M. Free-standing kinked nanowire transistor probes for targeted intracellular recording in three dimensions. Nat. Nanotechnol. 2013, 9, 142–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, R.; Strehle, S.; Tian, B.; Cohen-Karni, T.; Xie, P.; Duan, X.; Qing, Q.; Lieber, C.M. Outside Looking In: Nanotube Transistor Intracellular Sensors. Nano Lett. 2012, 12, 3329–3333. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steinbach, A.M.; Sandner, T.; Nilsen, M.; Hua, X.; Sivakumar, R.; Geiger, D.; Moeinian, A.; Strehle, S. The Electronic Properties of Silicon Nanowires during Their Dissolution under Simulated Physiological Conditions. Appl. Sci. 2019, 9, 804. https://doi.org/10.3390/app9040804
Steinbach AM, Sandner T, Nilsen M, Hua X, Sivakumar R, Geiger D, Moeinian A, Strehle S. The Electronic Properties of Silicon Nanowires during Their Dissolution under Simulated Physiological Conditions. Applied Sciences. 2019; 9(4):804. https://doi.org/10.3390/app9040804
Chicago/Turabian StyleSteinbach, Annina M., Tanja Sandner, Madeleine Nilsen, Ximeng Hua, Ragul Sivakumar, Dorin Geiger, Ardeshir Moeinian, and Steffen Strehle. 2019. "The Electronic Properties of Silicon Nanowires during Their Dissolution under Simulated Physiological Conditions" Applied Sciences 9, no. 4: 804. https://doi.org/10.3390/app9040804