Cooperative Three-View Imaging Optical Coherence Tomography for Intraoperative Vascular Evaluation
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
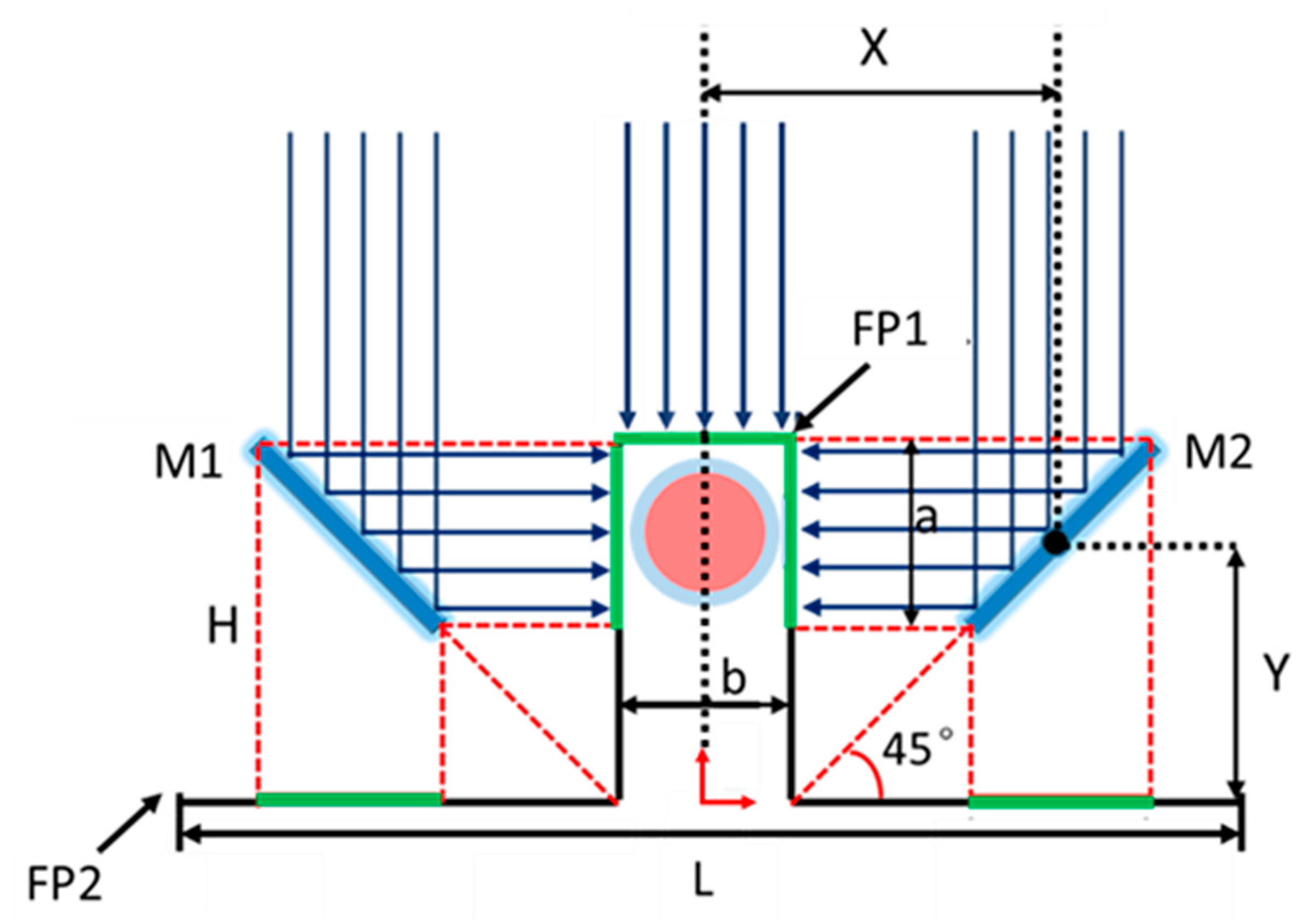
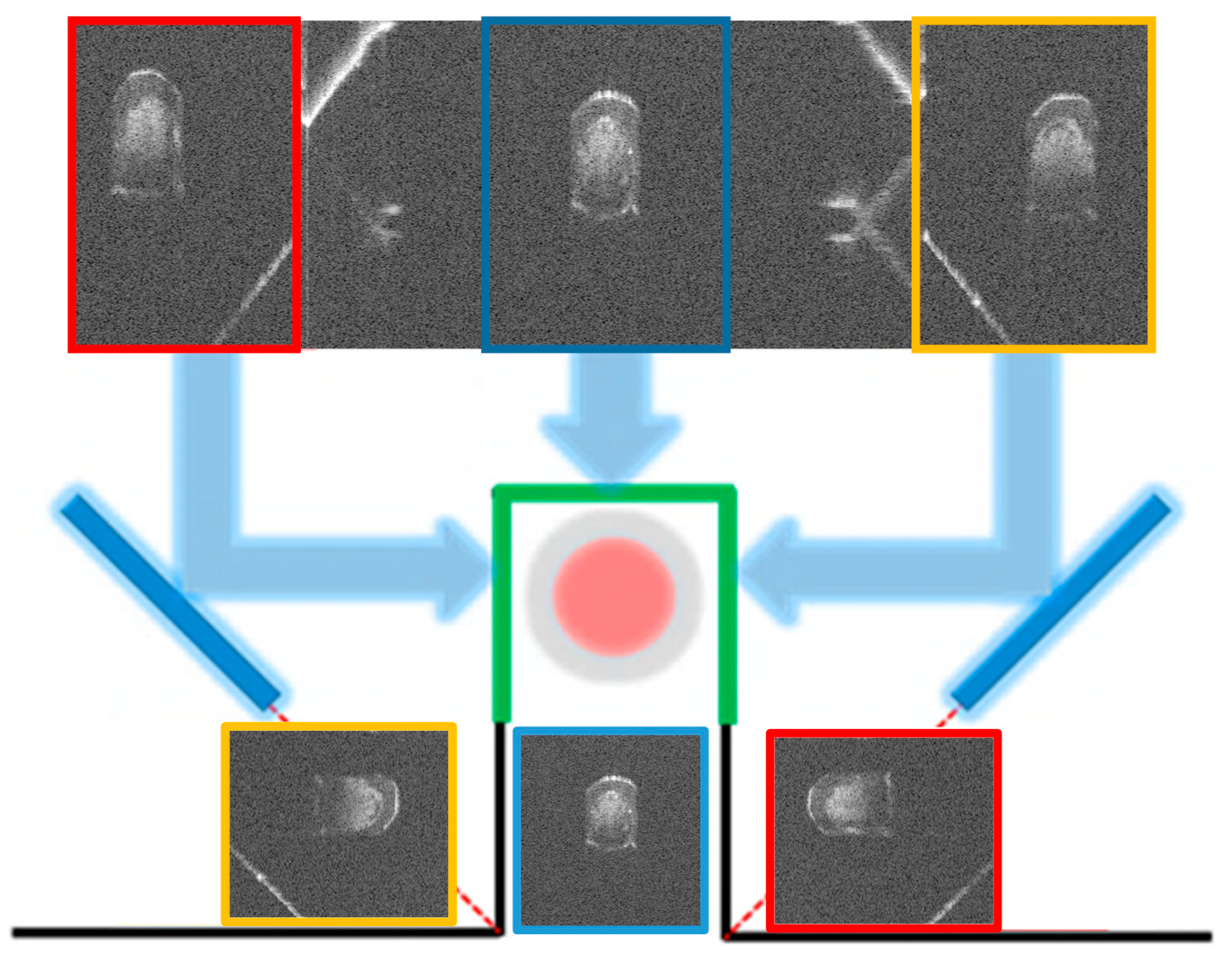
2.1. Principle of Cooperative Three-View Imaging
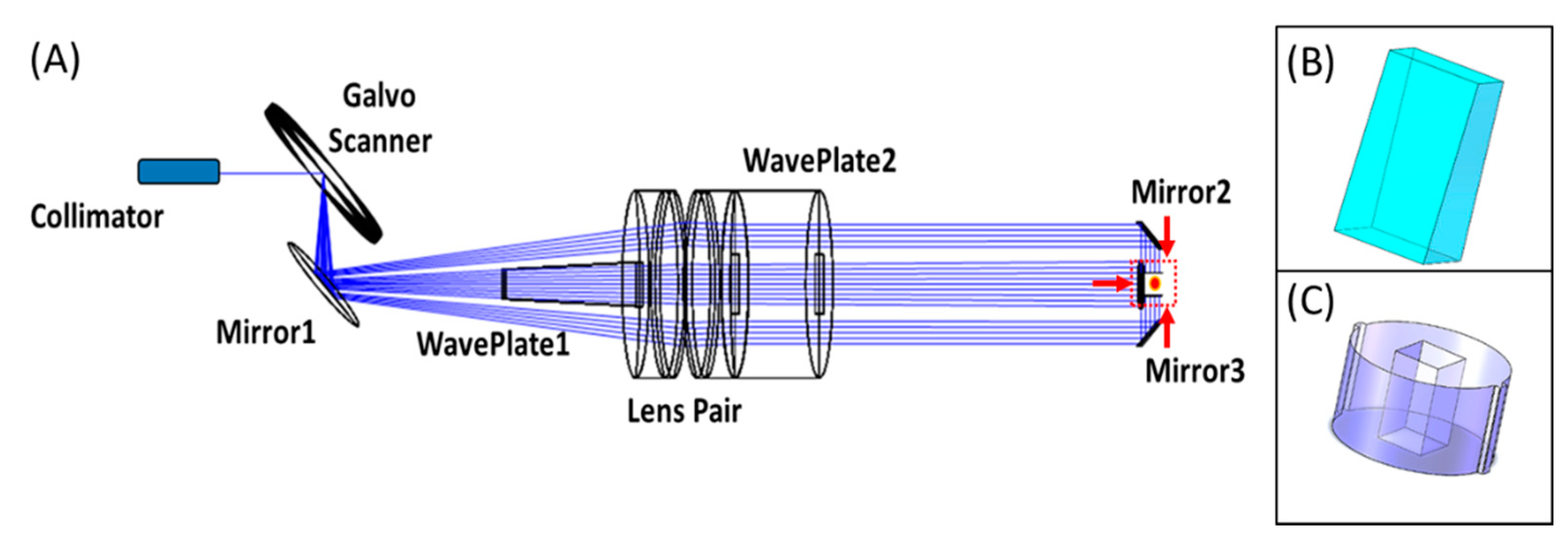
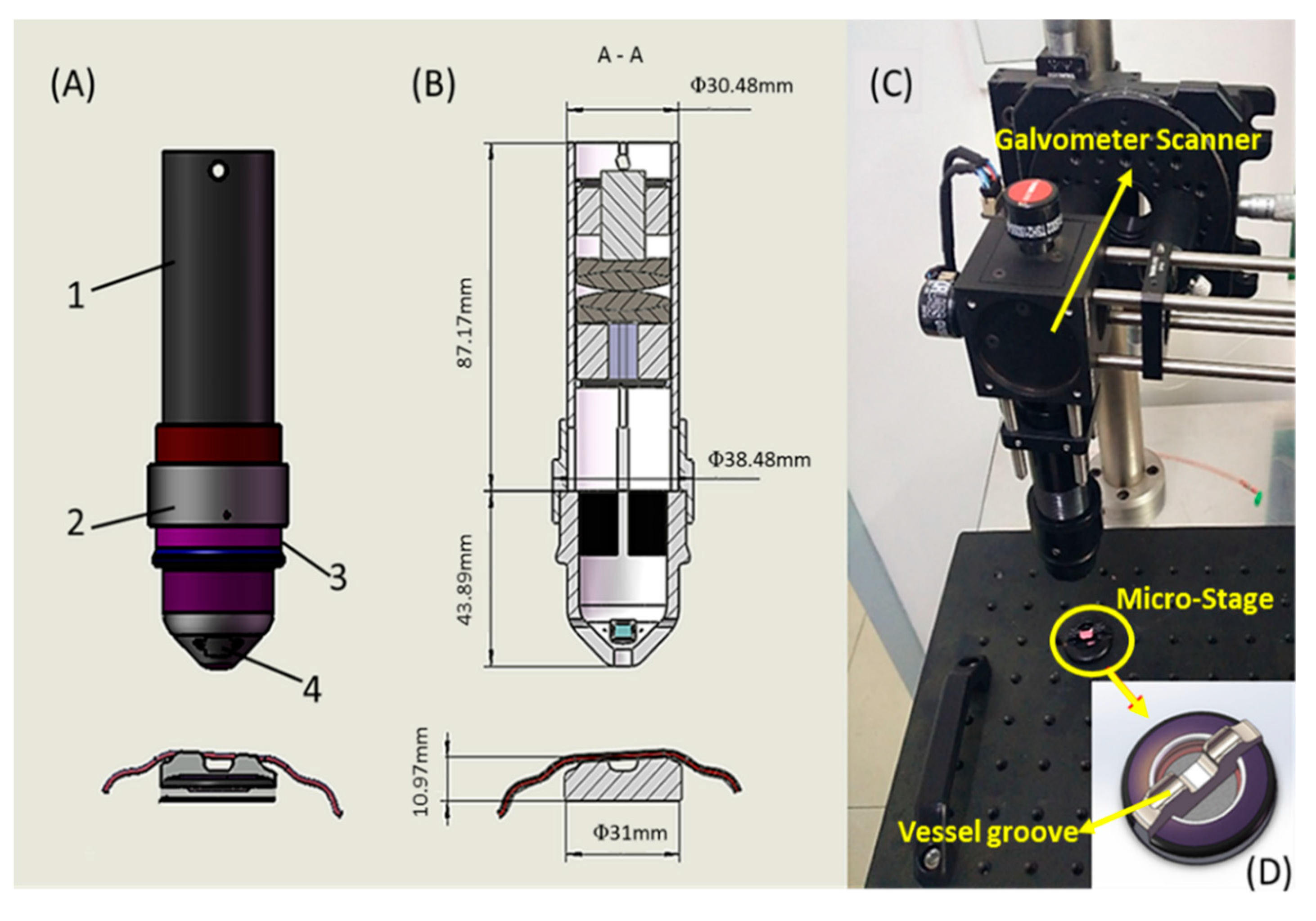
2.2. Optical Design of The Sample Arm
2.3. Mechanical Design and Fabrication
2.4. SD-OCT System Configuration
2.5. Ethical Considerations
3. Results
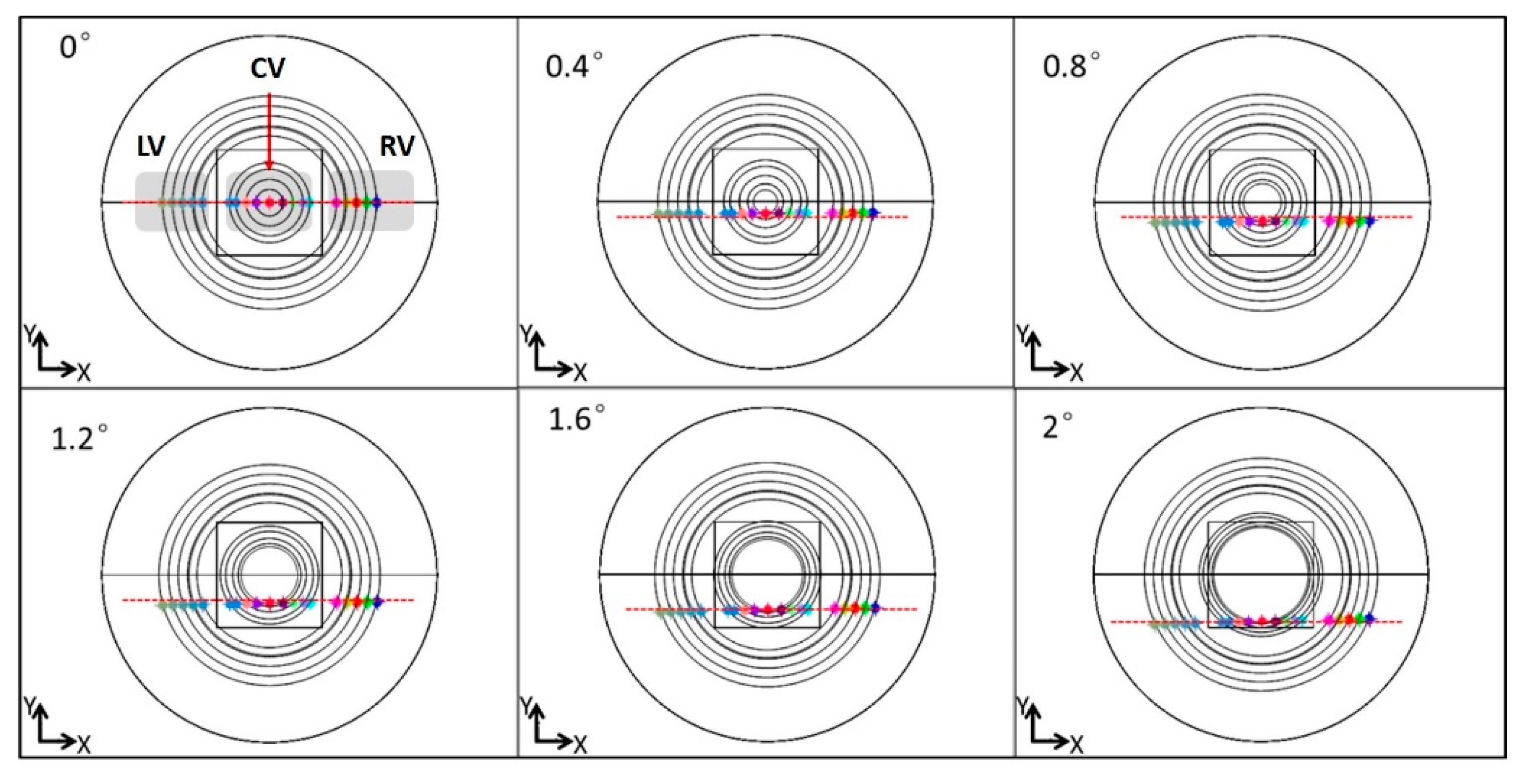
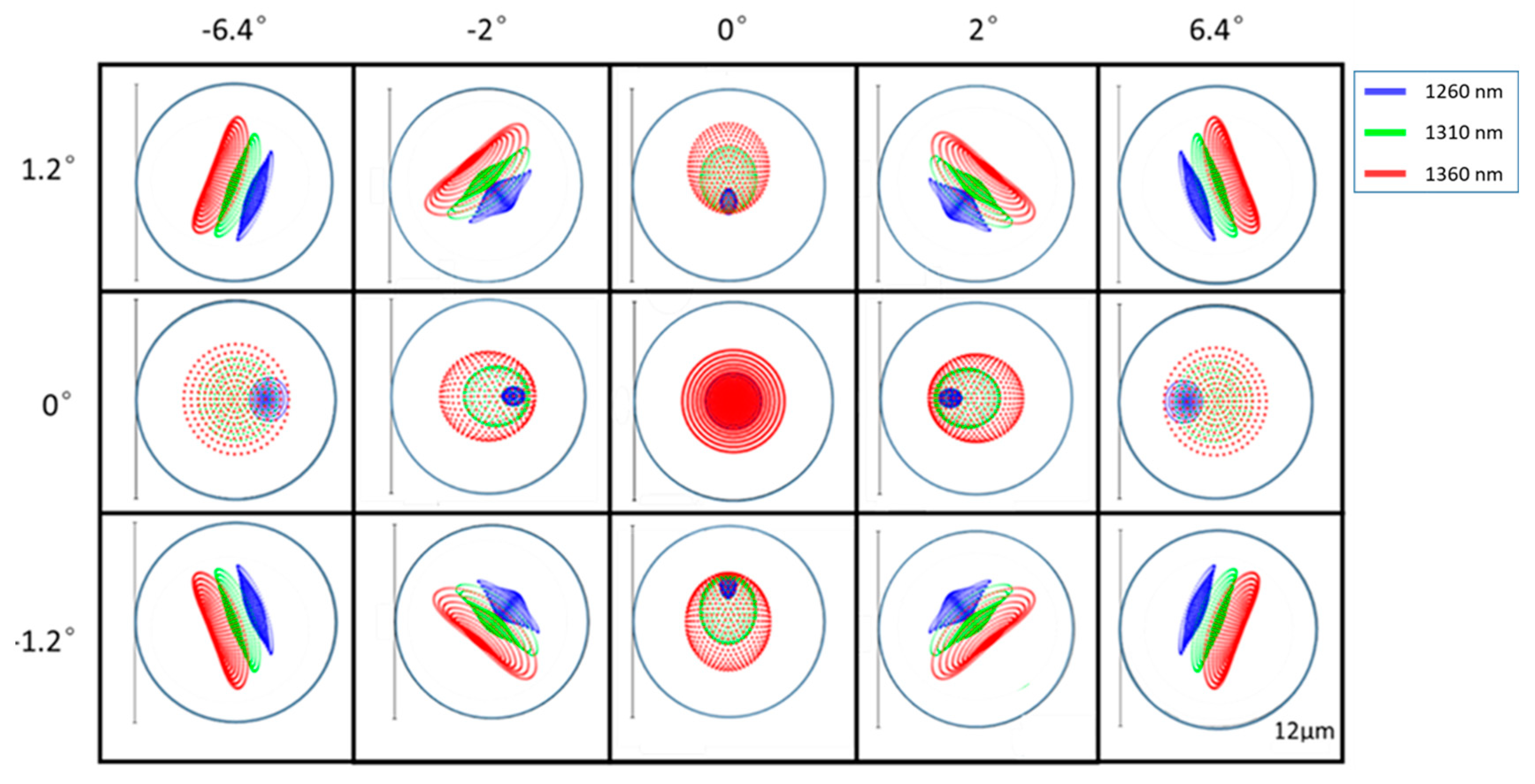
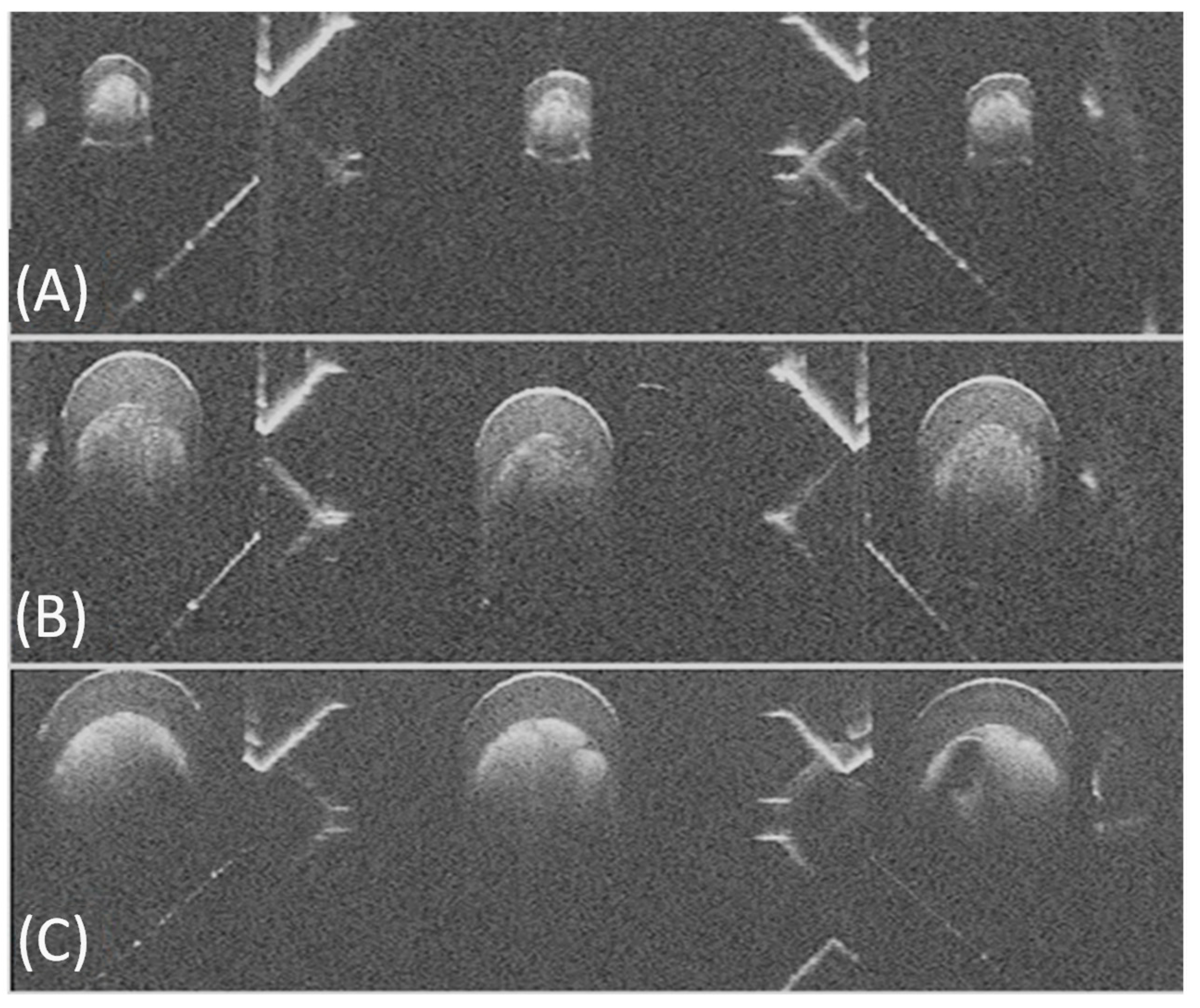
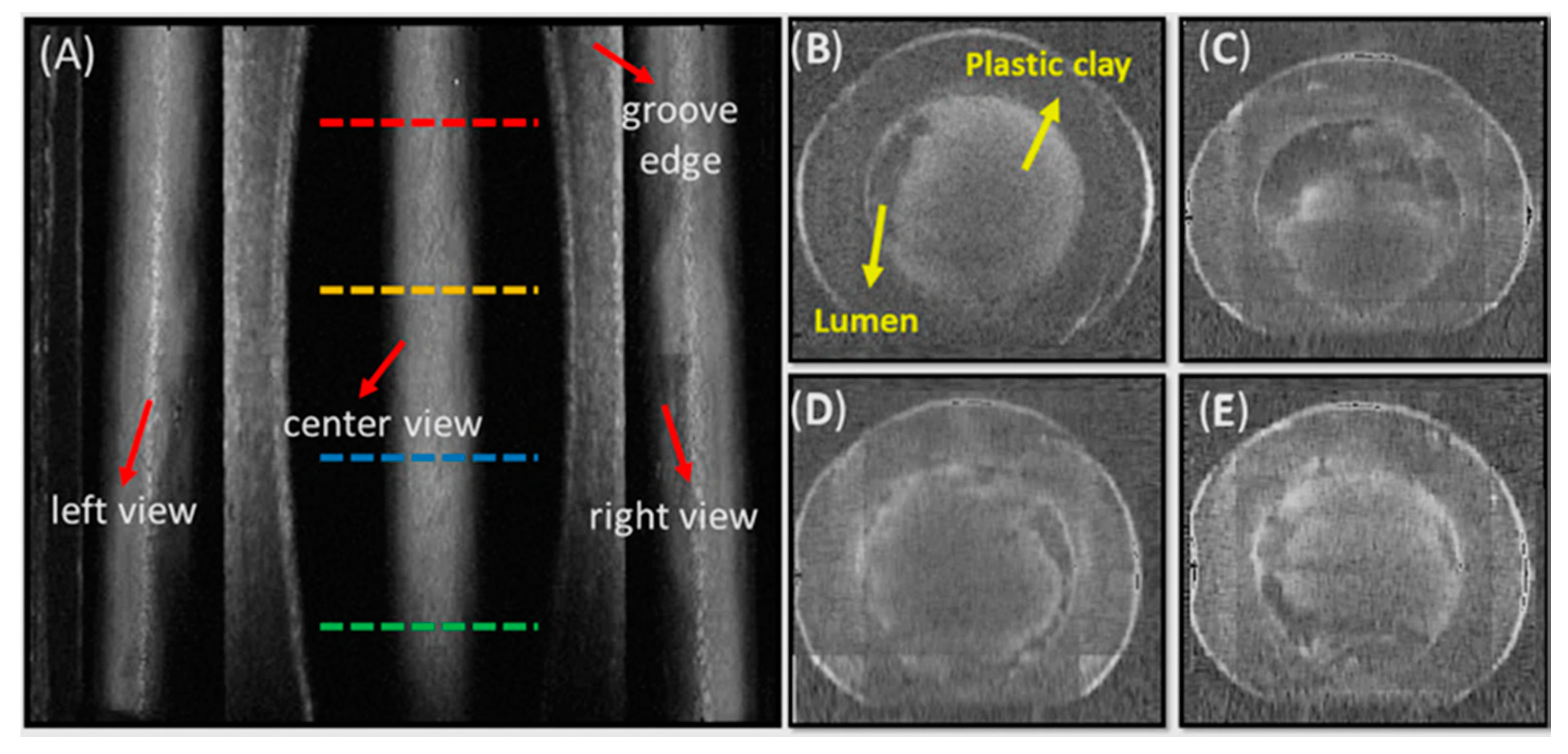
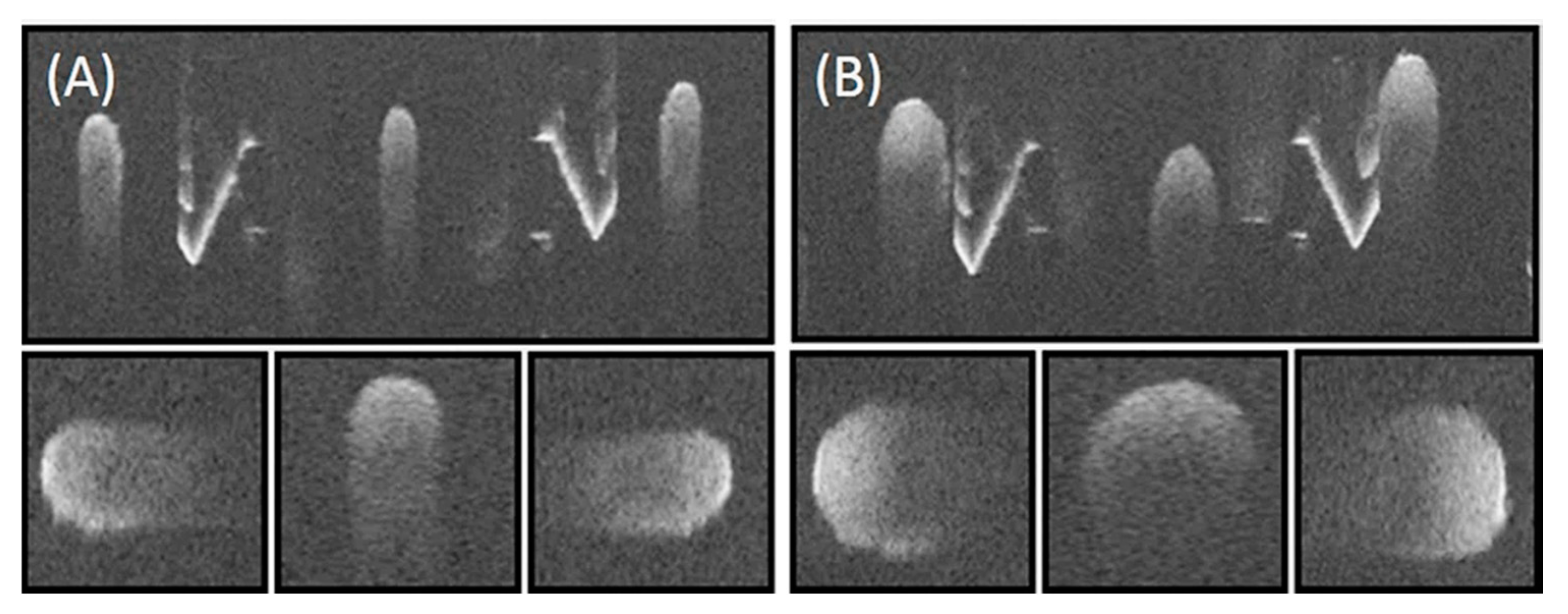
3.1. Phantom Imaging
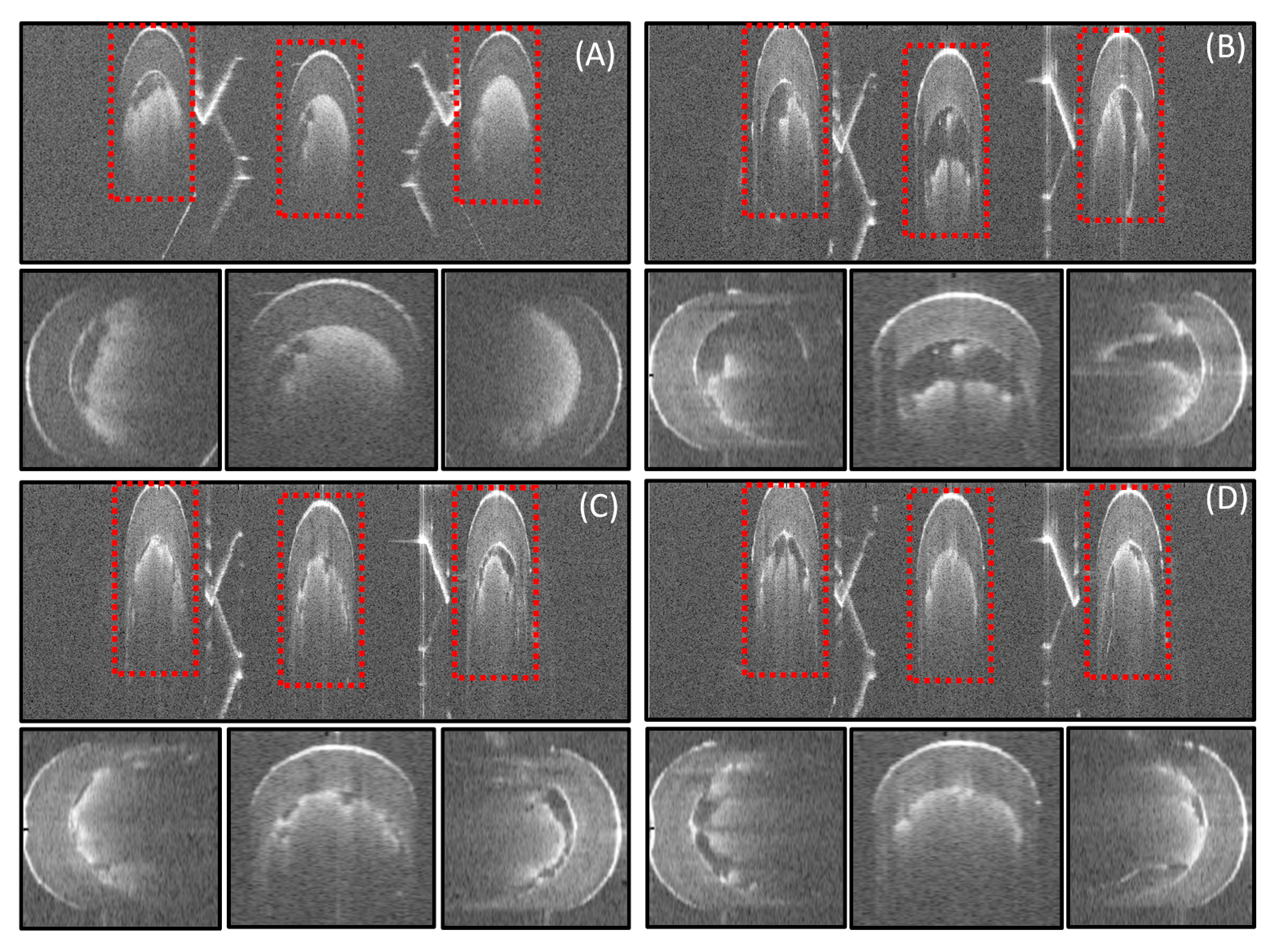
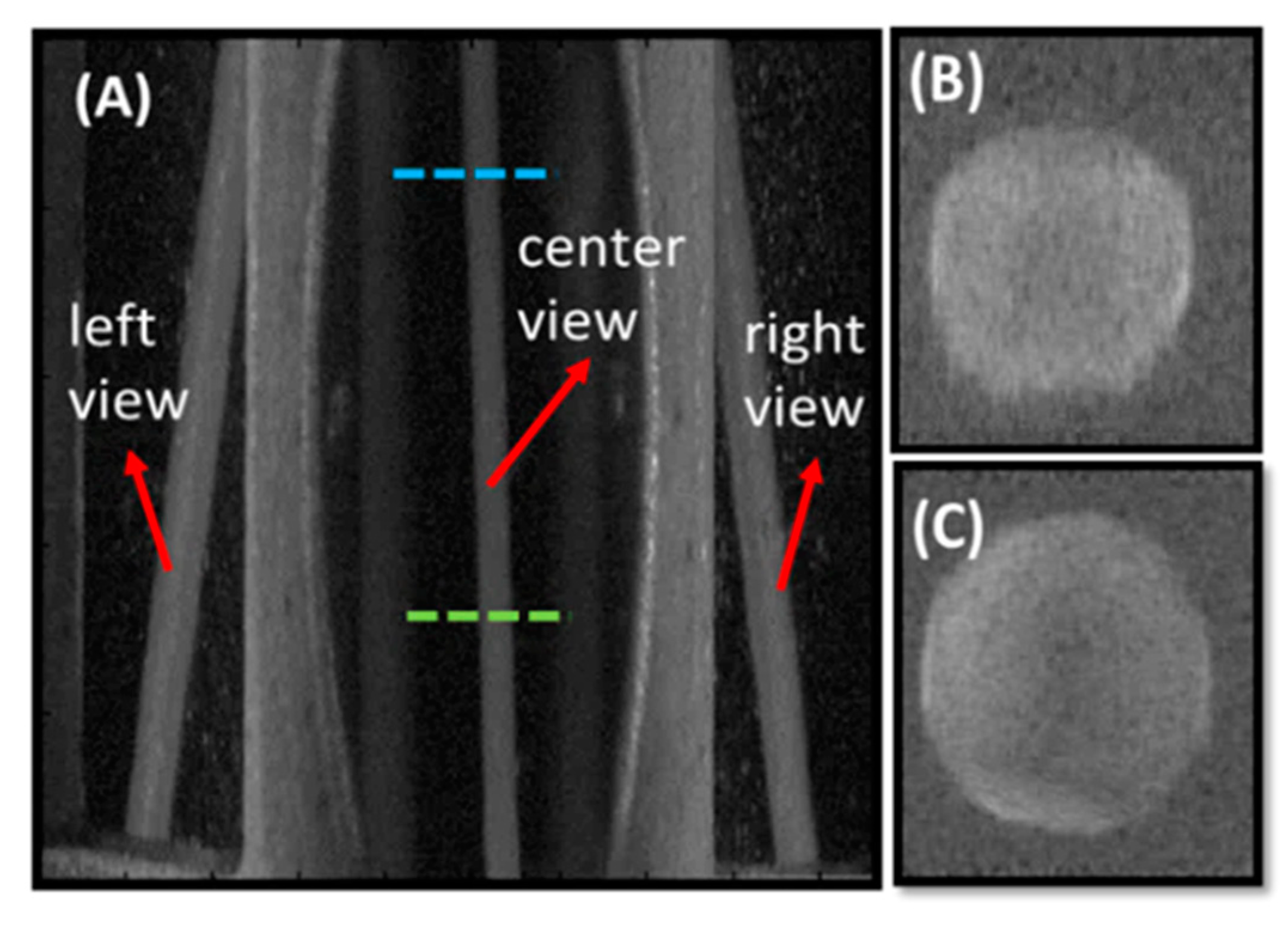
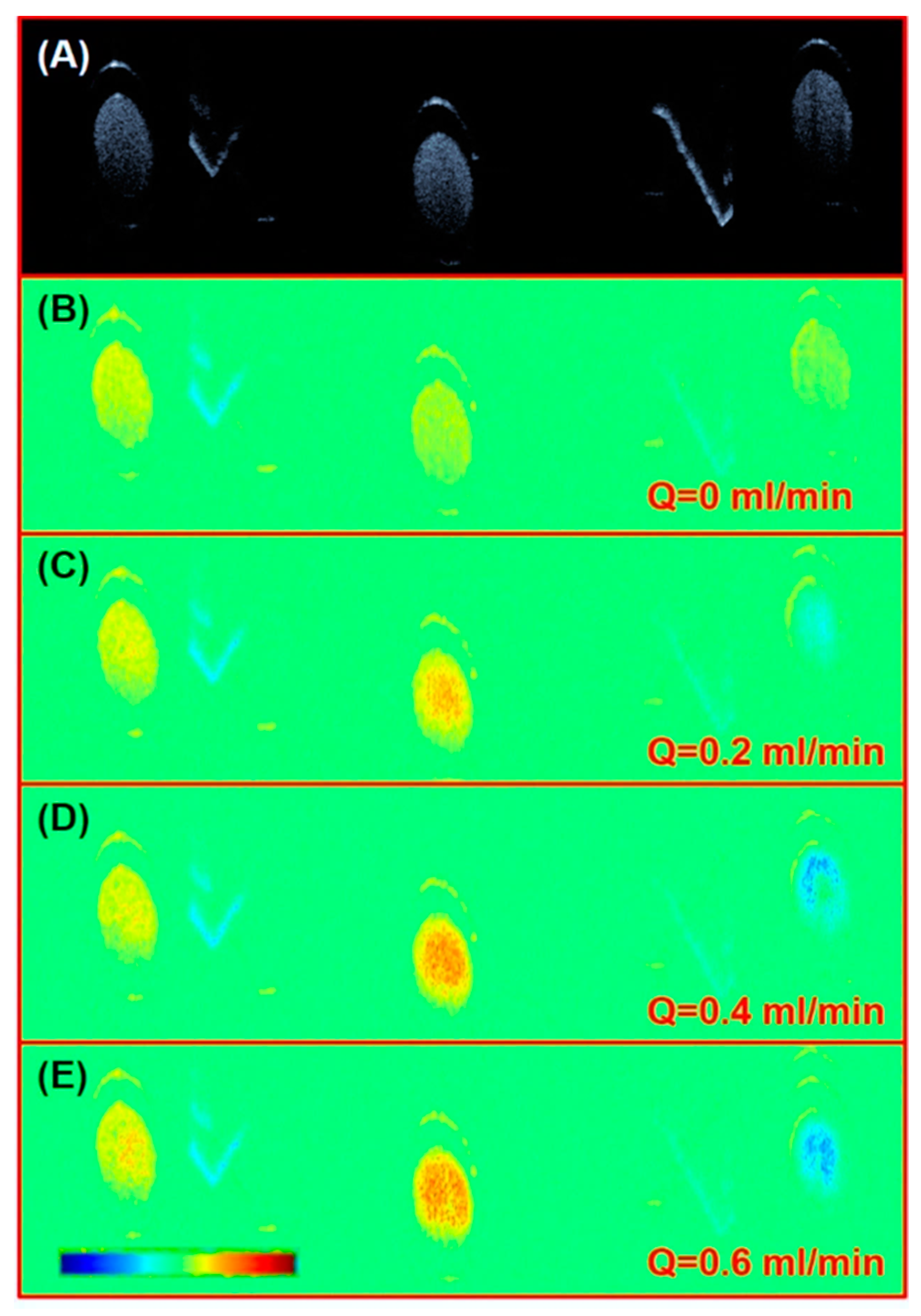
3.2. Rat Femoral Artery Imaging
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rothwell, A. Alexis Carrel: Innovator extraordinaire. J. Perioper. Pract. 2011, 21, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Tong, D.; Zhu, S.; Wu, L.; Mao, Q.; Ibrahim, Z.; Lee, W.P.; Brandacher, G.; Kang, J.U. Evaluation of microvascular anastomosis using real-time, ultra-high-resolution, Fourier domain Doppler optical coherence tomography. Plast. Reconstr. Surg. 2015, 135, 711e–720e. [Google Scholar] [CrossRef] [PubMed]
- Hovius, S.E.; van Adrichem, L.N.; Mulder, H.D.; van Strik, R.; van der Meulen, J.C. The predictive value of the laser Doppler flowmeter for posteroperative microvascular monitoring. Ann. Plast. Surg. 1993, 31, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Swartz, W.M.; Izquierdo, R.; Miller, M.J. Implantable venous Doppler microvascular monitoring: Laboratory investigation and clinical results. Plast. Reconstr. Surg. 1994, 93, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Selber, J.C.; Garvey, P.B.; Clemens, M.W.; Chang, E.I.; Zhang, H.; Hanasono, M.M. A prospective study of Transit-Time flow volume measurement for intraoperative evaluation and optimization of free flaps. Plast. Reconstr. Surg. 2013, 131, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Drexler, W.; Fuijmoto, J.G. Optical Coherence Tomography: Technology and Applications, 1st ed.; Springer: Midtown Manhattan, NY, USA, 2008; pp. 5–51. ISBN 978-3-540-77549-2. [Google Scholar]
- Liu, M.; Chen, Z.; Zabihian, B.; Sinz, C.; Zhang, E.; Beard, P.C.; Ginner, L.; Hoover, E.; Minneman, M.P.; Leitgeb, R.A.; et al. Combined multi-modal photoacoustic tomography, optical coherence tomography (OCT) and OCT angiography system with an articulated probe for in vivo human skin structure and vasculature imaging. Biomed. Opt. Express 2016, 7, 3390–3402. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.P.; Merkle, C.W.; Cooke, D.F.; Zhang, T.; Radhakrishnn, H.; Krubitzer, L.; Srinivasan, V.J. Noninvasive, in vivo imaging of subcortical mouse brain regions with 1.7 μm optical coherence tomography. Opt. Lett. 2015, 40, 4911–4914. [Google Scholar] [CrossRef] [PubMed]
- Bhaduri, B.; Nolan, R.M.; Shelton, R.L.; Pilutti, L.A.; Motl, R.W.; Moss, H.E.; Pula, J.H.; Boppart, S.A. Detection of retinal blood vessel changes in multiple sclerosis with optical coherence tomography. Biomed. Opt. Express 2016, 7, 2321–2330. [Google Scholar] [CrossRef] [PubMed]
- Ginner, L.; Schmoll, T.; Kumar, A.; Salas, M.; Pricoupenko, N.; Wurster, L.M.; Leitgeb, R.A. Holographic line field en-face OCT with digital adaptive optics in the retina in vivo. Biomed. Opt. Express 2018, 9, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.C.; Traverso, G.; Lee, H.C.; Ahsen, O.O.; Wang, Z.; Potsaid, B.; Giacomelli, M.; Jayaraman, V.; Barman, R.; Cable, A.; et al. Ultrahigh speed en face OTC capsule for endoscopic imaging. Biomed. Opt. Express 2015, 6, 1146–1163. [Google Scholar] [CrossRef] [PubMed]
- Felberer, F.; Rechenmacher, M.; Haindl, R.; Baumann, B.; Hitzenberger, C.K.; Pircher, M. Imaging of retinal vasculature using adaptive optics SLO/OCT. Biomed. Opt. Express 2015, 6, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Yabushita, H.; Bouma, B.E.; Houser, S.L.; Aretz, H.T.; Jang, I.K.; Schlendorf, K.H.; Kauffman, C.R.; Shishkov, M.; Kang, D.H.; Halpern, E.F.; et al. Characterization of human atherosclerosis by optical coherence tomography. Circulation 2002, 106, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Milner, T.E.; Srinivas, S.; Wang, X.; Malekafzali, A.; van Gemert, M.J.; Nelson, J.S. Noninvasive imaging of in vivo blood flow velocity using optical Doppler tomography. Opt. Lett. 1997, 22, 1119–1121. [Google Scholar] [CrossRef] [PubMed]
- Byers, R.A.; Fisher, M.; Brown, N.J.; Tozer, G.M.; Matcher, S.J. Vascular patterning of subcutaneous mouse fibrosarcomas expressing individual VEGF isoforms can be differentiated using angiographic optical coherence tomography. Biomed. Opt. Express 2017, 8, 4551–4567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakash, A.; Hewko, M.; Sowa, M.; Sherif, S. Texture based segmentation method to detect atherosclerotic plaque from optical tomography images. In Proceedings of the European Conference on Biomedical Optics, Munich, Germany, 12–16 May 2013. SPIE 8802. [Google Scholar] [CrossRef]
- Wicks, R.T.; Huang, Y.; Zhang, K.; Zhao, M.; Tyler, B.M.; Suk, I.; Hwang, L.; Ruzevick, J.; Jallo, G.; Brem, H.; et al. Extravascular Optical Coherence Tomography. Stroke 2014, 45, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ibrahim, Z.; Tong, D.; Zhu, S.; Mao, Q.; Pang, J.; Lee, W.P.A.; Brandacher, G.; Kang, J.U. Microvascular anastomosis guidance and evaluation using real-time three-dimensional Fourier-domain Doppler optical coherence tomography. J. Biomed. Opt. 2013, 18, 111404. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Furtmüller, G.J.; Tong, D.; Zhu, S.; Lee, W.P.; Brandacher, G.; Kang, J.U. MEMS-Based Handheld Fourier Domain Doppler Optical Coherence Tomography for Intraoperative Microvascular Anastomosis Imaging. PLoS ONE. 2014, 9, e114215. [Google Scholar] [CrossRef] [PubMed]
- Malmström, M. Multi-Angle Oblique Optical Coherence Tomography; Lund University: Lund, Sweden, 2008. [Google Scholar]
- Wu, C.; Le, H.; Ran, S.; Singh, M.; Larina, I.V.; Mayerich, D.; Dickinson, M.E.; Larin, K.V. Comparison and combination of rotational imaging optical coherence tomography and selective plane illumination microscopy for embryonic study. Biomed. Opt. Express 2017, 8, 4629–4639. [Google Scholar] [CrossRef] [PubMed]
- Song, S.Z.; Xu, J.J.; Wang, R.K. Long-range and wide field of view optical coherence tomography for in vivo 3D imaging of large volume object based on akinetic programmable swept source. Biomed. Opt. Express 2016, 7, 4734–4748. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, S.; Jiang, Y.; Zhang, K.; Wu, C.; Ai, D.; Yang, J.; Wang, Y.; Huang, Y. Cooperative Three-View Imaging Optical Coherence Tomography for Intraoperative Vascular Evaluation. Appl. Sci. 2018, 8, 1551. https://doi.org/10.3390/app8091551
Peng S, Jiang Y, Zhang K, Wu C, Ai D, Yang J, Wang Y, Huang Y. Cooperative Three-View Imaging Optical Coherence Tomography for Intraoperative Vascular Evaluation. Applied Sciences. 2018; 8(9):1551. https://doi.org/10.3390/app8091551
Chicago/Turabian StylePeng, Shizhao, Yuanzhen Jiang, Kailin Zhang, Chuanchao Wu, Danni Ai, Jian Yang, Yongtian Wang, and Yong Huang. 2018. "Cooperative Three-View Imaging Optical Coherence Tomography for Intraoperative Vascular Evaluation" Applied Sciences 8, no. 9: 1551. https://doi.org/10.3390/app8091551




