1. Introduction
The preparation of Sichuan Paocai (fermented Chinese cabbage) follows a number of steps: fresh vegetables are washed and sealed in a jar for anaerobic fermentation in salt water [
1]. Salt water plays a very important role for the exudation of vegetable juice and the soluble components are metabolized by lactic acid bacteria (saccharides and nitrogen substance). Acidic substances are generated and flavor components are metabolized, causing the unique crispy taste of Paocai [
2]. An abundance of natural lactic acid bacteria can be found in Paocai, which play a central role in the formation of both flavor and quality [
3]. Several lactic acid bacteria are also used as probiotics with a number of excellent health benefits for humans, including the prevention of constipation, colitis, liver injury, and diabetes [
4,
5,
6,
7]. The difference in the types of lactic acid bacteria is caused by many factors, such as areas, climate, and the method of preparation. To better utilize these microorganisms, methods for isolation and identification should be further developed in order to accumulate these abundant bacterial resources for the development of probiotics. The microorganisms in Sichuan Paocai include
Lactobacillus plantarum,
Lactobacillus casei,
Saccharomyces cerevisiae, and
Lactobacillus acidophilus,
Brevibacterium spec. [
8,
9,
10]. Several Paocai variations are common in the East Asian region. The lactic acid bacteria in Paocai are good leavening agents and they can be used to ferment food, as well as to prepare functional foods due to their bioactivity. The microorganisms that were used in this study (lactic acid bacteria) were isolated and identified from Sichuan Paocai.
With the exception of probiotics, the intestinal tract contains several harmful bacterial species. Under normal conditions, these are at a state of equilibrium [
11]. The probiotics in the intestinal tract participate in the digestion, preventing dyspepsia and digestive tract dysfunction [
12]. Mediated by the lactic acid metabolism, lactic acid bacteria can effectively inhibit both the growth and reproduction of harmful bacteria in the gastrointestinal tract and maintain the intestinal ecological balance and normal function. Imbalance within the intestinal lactic acid bacteria is related to chronic diarrhea, constipation, abdominal distension, and dyspepsia [
13]. Lactic acid bacteria cannot only activate phagocytosis of macrophages, but they also play an important role in intestinal colonization. Lactic acid bacteria stimulate peritoneal macrophages, induce interferon, promote cell division, generate antibodies, promote cellular immunity, improve both the non-specific and specific immune response, and improve the body's ability to restore tissue damage and malfunction [
14,
15]. Constipation is a problematic medical condition that manifests as a difficulty to defecate and dryness of faeces [
16]. Constipation leads to slow intestinal peristalsis and an increase of harmful bacteria, which cause additional intestinal tract diseases [
17]. Lactic acid bacteria have been used to remedy constipation, because they can generate organic acids within the intestinal tract, repair and promote the intestinal function, reduce the pH in the enteric cavity, regulate neuromuscular activity, improve the peristalsis function of the intestinal tract, and promote both digestion and absorption. Moreover, they can effectively inhibit the proliferation of putrefying bacteria in the intestinal tract and improve the intestinal environment, softening the faeces, thus facilitating defecation [
18].
Constipation is more of a bad state of life, intestinal regulation by probiotics can effectively prevent constipation, but there are not many probiotics with a better constipation prevention effect, from the traditional fermented food to find more effective strains of bacteria is the focus of current research. In this study, activated carbon influenced the normal physiology of the small intestine, leading to constipation in mice. An inhibitory effect of LP-CQPC10 on constipation was observed, and the mechanism of this effect was investigated with experiments of molecular biology, which provided a theoretical basis for the application of this bacterial strain.
4. Discussion
Constipation can influence normal life and long-term constipation will induce other diseases, eventually severely threatening health [
4]. Better colonization of probiotics in the intestines will play a better role in probiotics; Probiotics need to enter more intestines, which required good anti gastric acid and bile salt, the survival rates in pH 3.0 artificial gastric juice and 0.3% bile salt could preliminarily determine whether lactic acid bacteria have probiotic potential [
6]. In this study, LP-CQPC10 had the better in vitro anti gastric acid and bile salt than commercial used LB. LP-CQPC10 is a kind of strain that might have potential for probiotics. The level of harmful microorganisms increases in response to constipation and the intestinal wall tissue is injured. When this happens, peristalsis will be negatively influenced. Slow intestinal tract peristalsis is one of the causes of constipation [
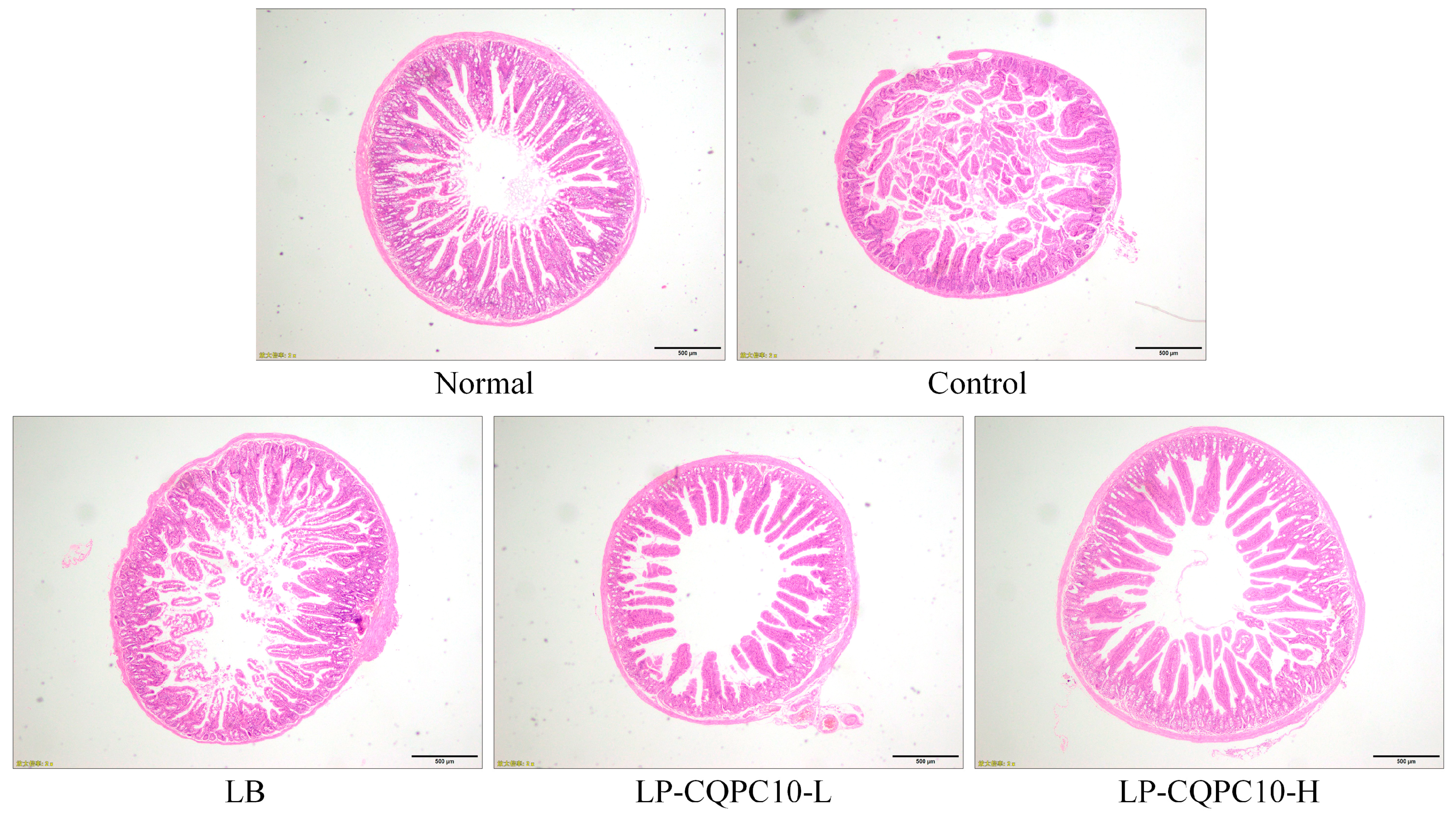
6]. Thus, the integrity of intestinal villi is very important for the evaluation of constipation, which can be preliminarily judged via pathological observation [
17]. In this study, LF-CQPC10 was preliminarily shown to have an inhibitory effect on constipation.
Observation of the faeces status is the most direct method. Constipation can lead to a decrease of the defecation amount, and the faeces are dry [
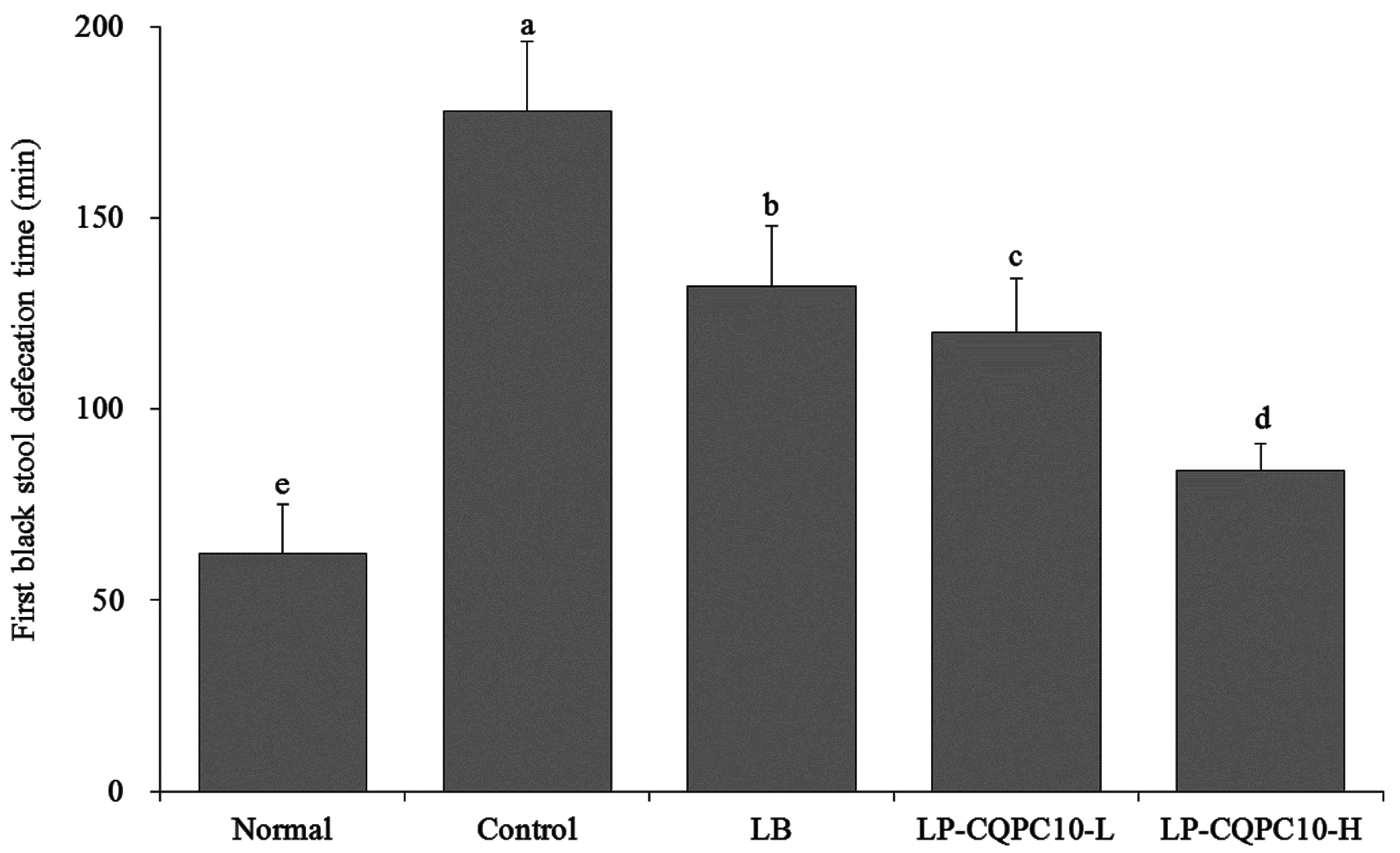
4]. In this study, we found that LF-CQPC10 could significantly remit these effects. The effectiveness was superior to that of the commonly used LB. Furthermore, the defecation time of the first tarry stool was used to evaluate the severity of the resulting constipation. Peristalsis was slowed down and the detention time in the intestinal tract elongated [
17]. In this study, the time in the control group was longer than in other groups, and the time in the LF-CQPC10 group was significantly decreased, thus showing constipation remission.
It has been reported that the neurotransmitter level will change in some patients with constipation (such as MTL, Gas, ET, SS, Ach, SP, and VIP). MTL has been used to evaluate gastrointestinal tract peristalsis and it has been widely considered to promote the mobility of the gastrointestinal tract, while decreased release will slow peristalsis [
22]. Gas is an important gastrointestinal hormone, which has been shown to promote gastric secretion, improve peristalsis, accelerate gastric emptying, and promote pyloric sphincter relaxation [
23]. Currently, AchE has been considered as one of two neurotransmitters that play a very important role in the motility of the intestinal tract. AchE promotes peristalsis by binding to the receptor [
24]. SP is an excitatory transmitter in gastrointestinal motor neurons. It greatly promotes the shrinkage of the smooth muscle in the digestive tract, stimulates water and electrolyte secretion in the small intestine and colonic mucosa, and promotes peristalsis [
25]. The obtained results indicated that MTL, Gas, AchE, and SP levels in the model group were significantly lower than in the normal group; however, the neurotransmitter levels significantly increased in the LP-CQPC10 group. This suggests that the decrease in these levels was related to the constipation. The increase in the levels by LP-CQPC10 indicated that it could remit the constipation. ET is a multi-functional peptide that plays a very important role in cardiovascular and intestinal tract function. SS can inhibit the release of gastrointestinal hormones, slow down gastric emptying, and reduce smooth muscle contraction, which might cause constipation [
26]. VIP is an inhibitory neurotransmitter that stimulates peristalsis and promotes gastrointestinal motility [
27]. The SS level was highest in the model group, while it was significantly decreased in the LP-CQPC10 group (
p < 0.05), suggesting that LP-CQPC10 could have a preventive effect on constipation.
In the metabolism, superoxide anions (O
2−) and oxygen radicals participate in physiological reactions in the body. Imbalance will greatly increase O
2− and oxygen radicals, which will disorder the metabolism [
28]. It has been reported that the Cu/Zn-SOD activity in patients with constipation is lower than in normal people. The likely reason is that long-term retention and stimulation of hard faeces causes inflammation in the intestinal tract [
29]. Mn-SOD can reduce the activity under inflammation [
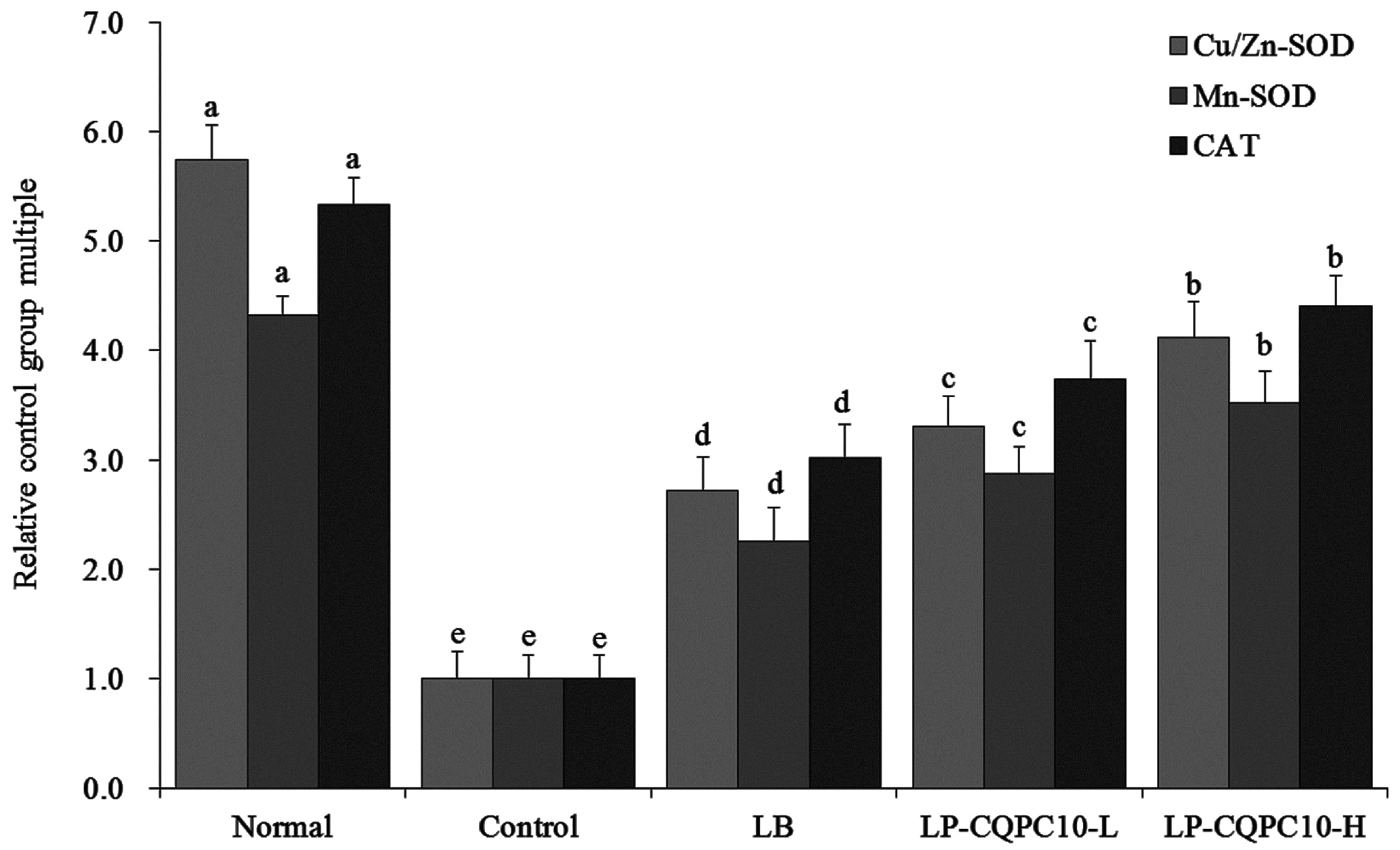
30]. SOD can transform harmful superoxide radicals into hydrogen peroxide. Although hydrogen peroxide is still harmful to the body, CAT can degrade it into water. SOD and CAT will form into an oxidation resistant chain to remit the damage to the intestinal tract that is caused by constipation [
31]. The results of our study indicate that LP-CQPC10 could effectively remit the reduced activity of Cu/Zn-SOD, Mn-SOD, and CAT caused by constipation.
Cajal cells (ICC) are a type of special mesenchymal cells. Colon ICC amount, morphological change, and abnormality of the cellular network structure will slow the peristalsis rate, further leading to slow transit constipation [
32]. C-Kit is one of the specific markers for ICC, and SCF is the natural ligand of the C-kit receptor [
33]. It has been reported that ICC density in the small intestine of patients with constipation is decreased, indicating that reduced ICC is related to the down-regulation of the c-Kit gene in the sigmoid colon, and reduced c-kit protein and mRNA expression [
34]. The results showed that the mRNA expression of both c-Kit and SCF in the LP-CQPC10 group was significantly increased (
p < 0.05), suggesting that LP-CQPC10 could increase the ICC amount and thus remit constipation.
TRPV1 is closely related to defecation and activation of TRPV1 could trigger the release of neurotransmitters, thus leading to intestinal motility dysfunction. The increase in TRPV1 expression is an important manifestation of intestinal injury and the damage that is caused by gastrointestinal tract diseases can increase TRPV1 expression in patients with constipation [
35]. GDNF regulates ganglion cells and aids the repair of the damaged intestinal tract, while also preventing constipation. Constipation is related to the intestinal nervous system, leading to muscular tension and weak gastrointestinal motility [
36]. Regulation of TRPV1 and GDNF expression is one of the important mechanisms to remit constipation. LP-CQPC10 also exerts such an effect of constipation remission.
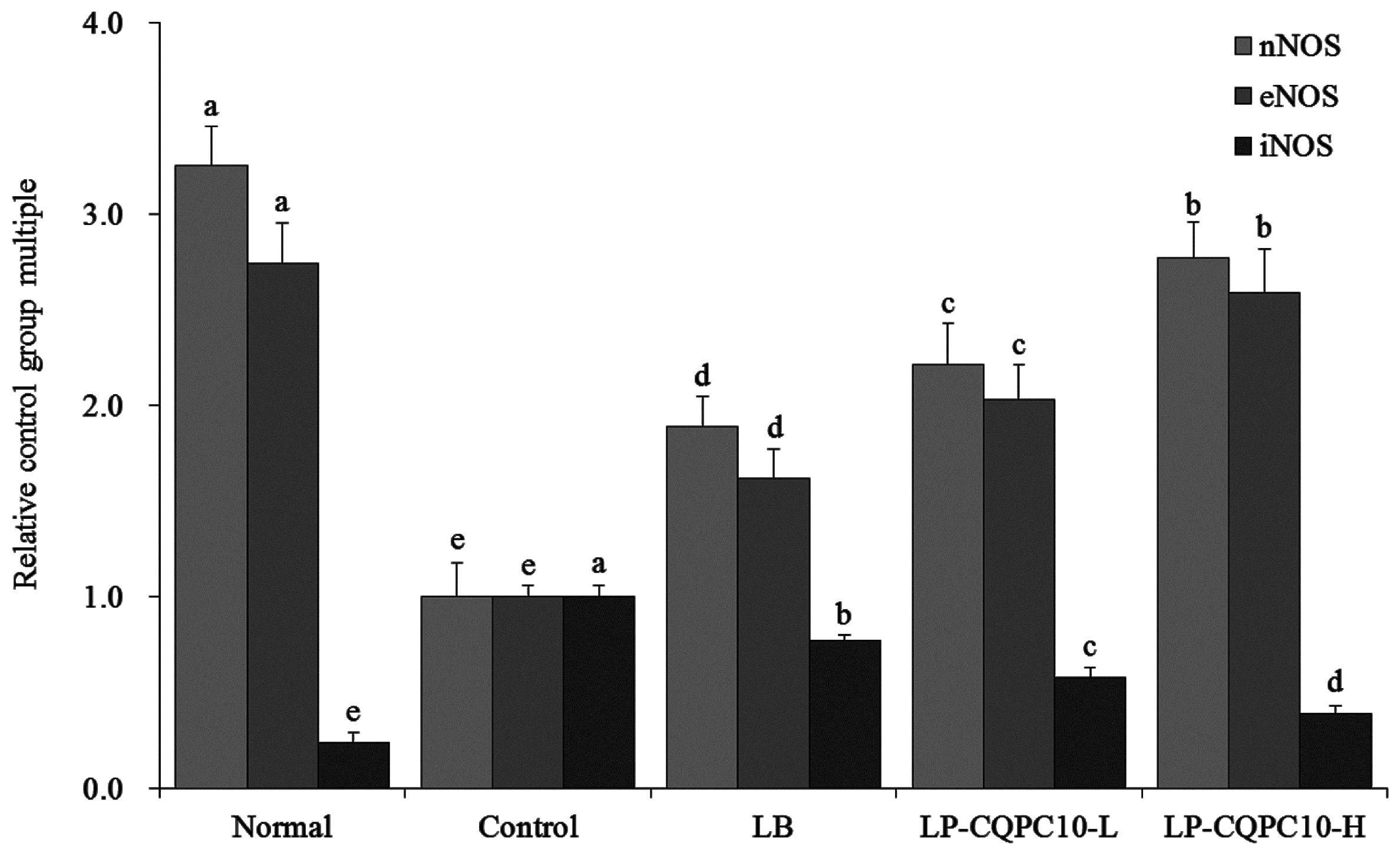
NOS participates in the regulation of gastrointestinal motility. An increase in NOS will lead to an increase in the NO content, influencing intestinal function and leading to constipation [
37]. Continuous increase of NO can cause a more severe colonic motility disorder [
38]. Endothelial dysfunction can cause constipation and the decrease in NO bioavailability is an important factor for this dysfunction [
39]. NO is synthesized via NOS catalysis. Three subtypes for NOS have been described, including NOS1 (nNOS), NOS2 (iNOS), and NOS3 (eNOS) [
40]. Under normal physiological conditions, NO in vascular endothelial cells mainly originates from eNOS, whose main effect is the regulation of the normal physiological function [
41]. The expression of nNOS is greatly decreased in the small intestine of animals with constipation [
42]. iNOS is not expressed under the resting-state, while a high level of iNOS and NO will be generated when the body is injured or under other pathological conditions [
43]. A decrease in NO content by controlling NOS is a feasible method to control constipation [
42]. Here, we found that LP-CQPC10 could significantly up-regulate the expression of eNOS and nNOS, down-regulate expression of iNOS, and remit constipation.
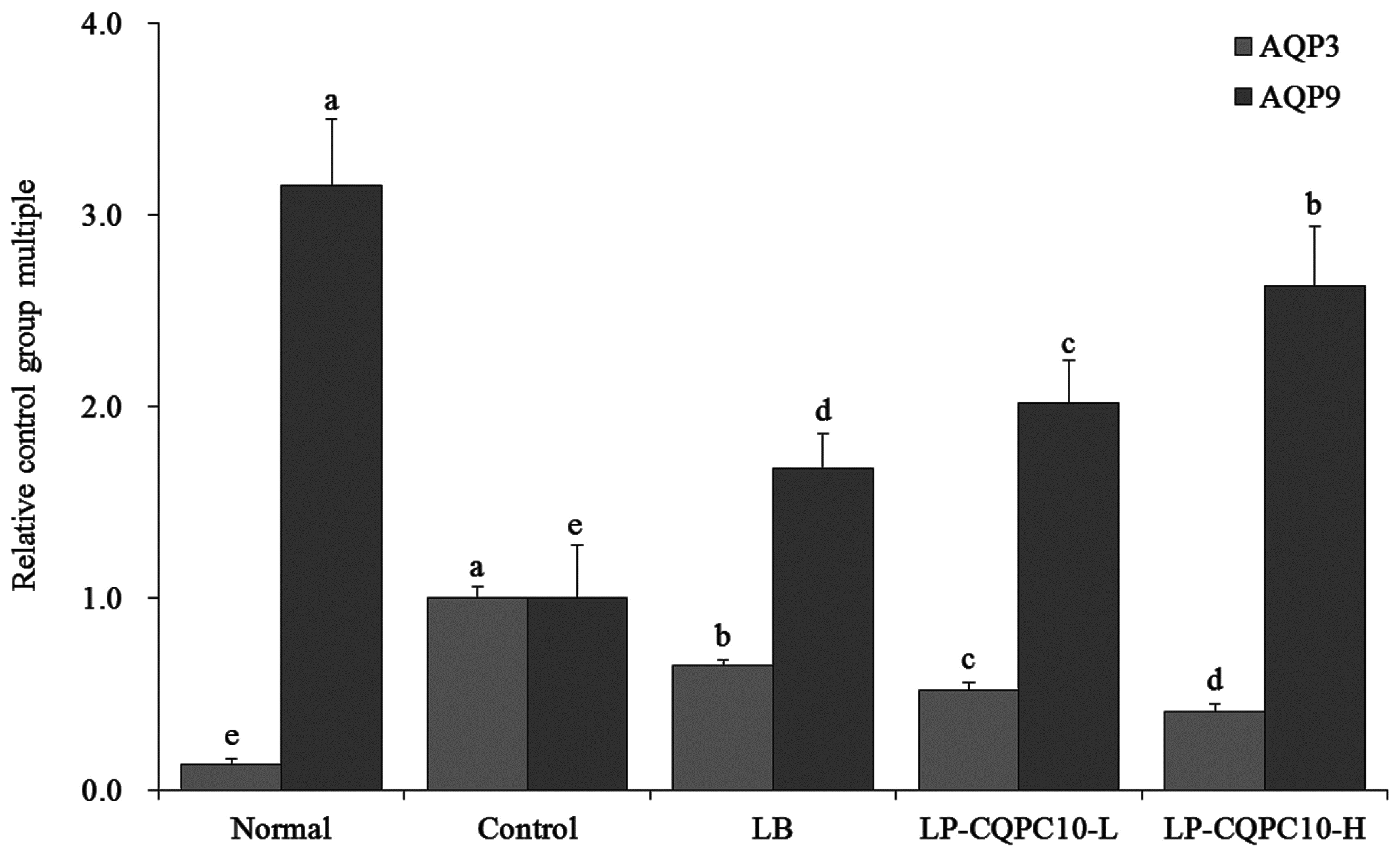
Aquaporin (AQP) has been reported to specifically transport water and some AQPs could participate in constipation by influencing the over-absorption of water in the colon and/or reduce the secretion of intestinal juice [
44]. AQP3 in constipation rats has been reported to be significantly increased when compared to normal mice, and AQP3 participated in the water absorption of the colon in the enteric cavity. This suggested that the overexpression of AQP3 aggravated the water adsorption of colonic mucosa, leading to constipation [
45,
46]. AQP9 in the intestinal tract of constipated rats was significantly reduced. AQP9 participated in the secretion of colonic mucus, protected the mucous membrane, and promoted defecation. This suggested that AQP9 was expressed at a low level and the secretion of goblet cell mucus was reduced, leading to constipation [
46]. LP-CQPC10 could remit the influence of constipation on AQPs, reduce the expression of AQP3, and increase the expression of AQP9.