Recent Progress in Synthesis and Functionalization of Multimodal Fluorescent-Magnetic Nanoparticles for Biological Applications
Abstract
:1. Introduction
2. Preparation of Magnetic-Fluorescent Nanocomposites
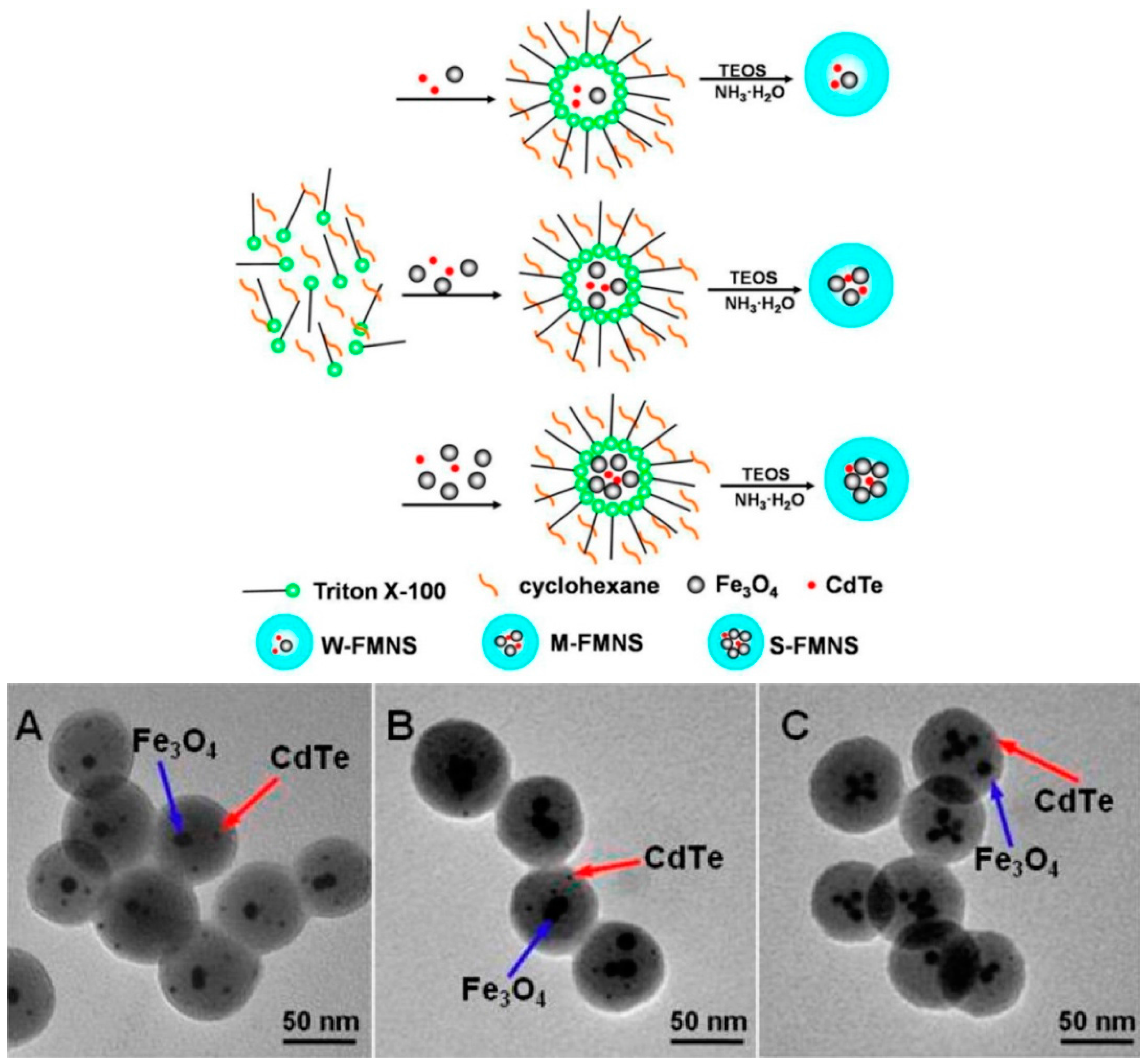
2.1. Fluorescent Silica Coated Magnetic Nanoparticles
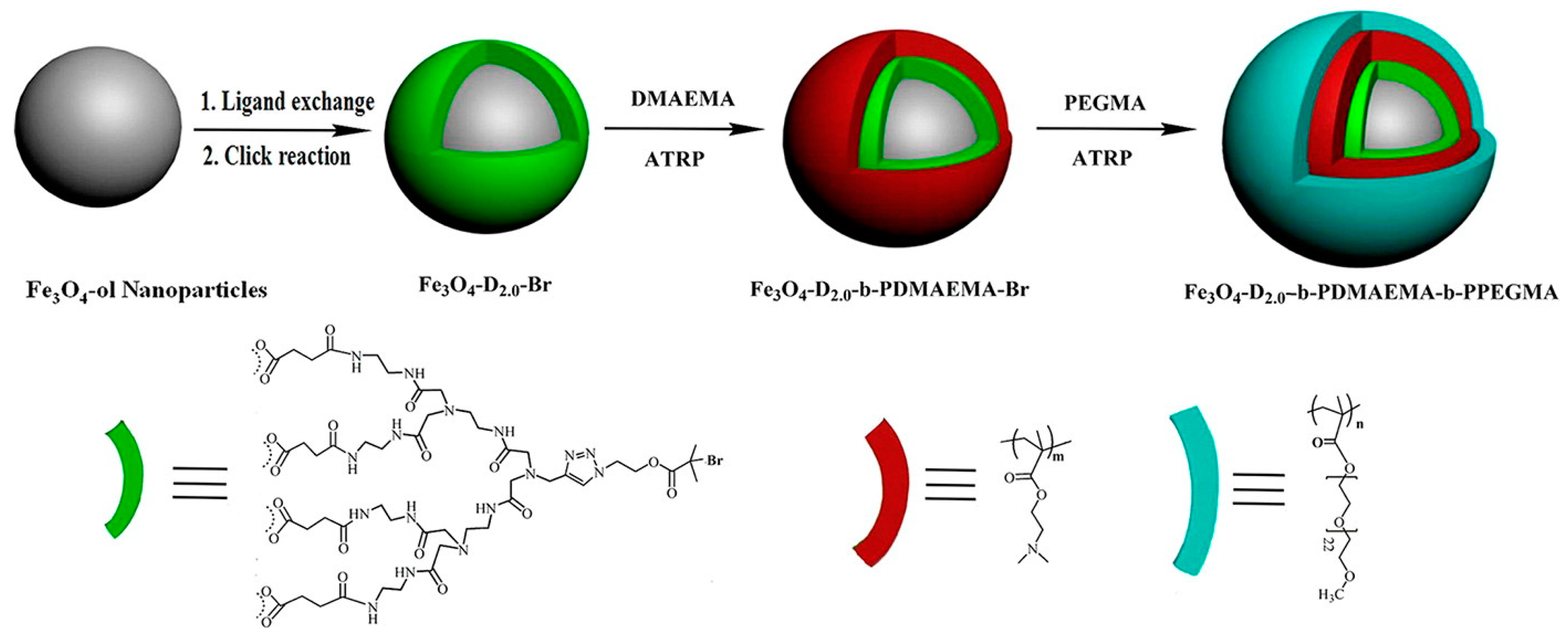
2.2. Fluorescent Polymer Coated Magnetic Nanoparticles
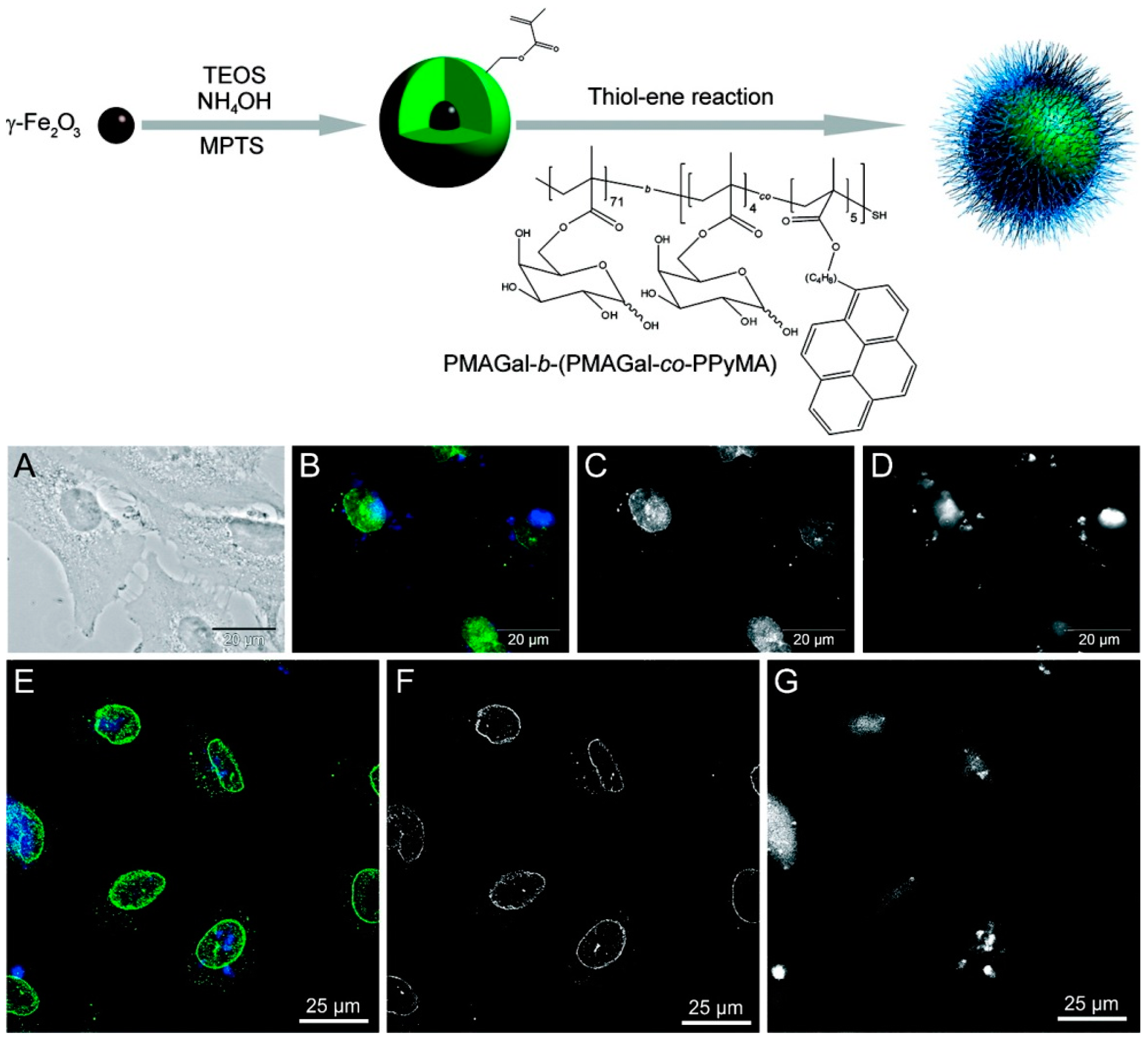
2.2.1. Silica-Polymer Magnetic-Fluorescent Nanocomposites
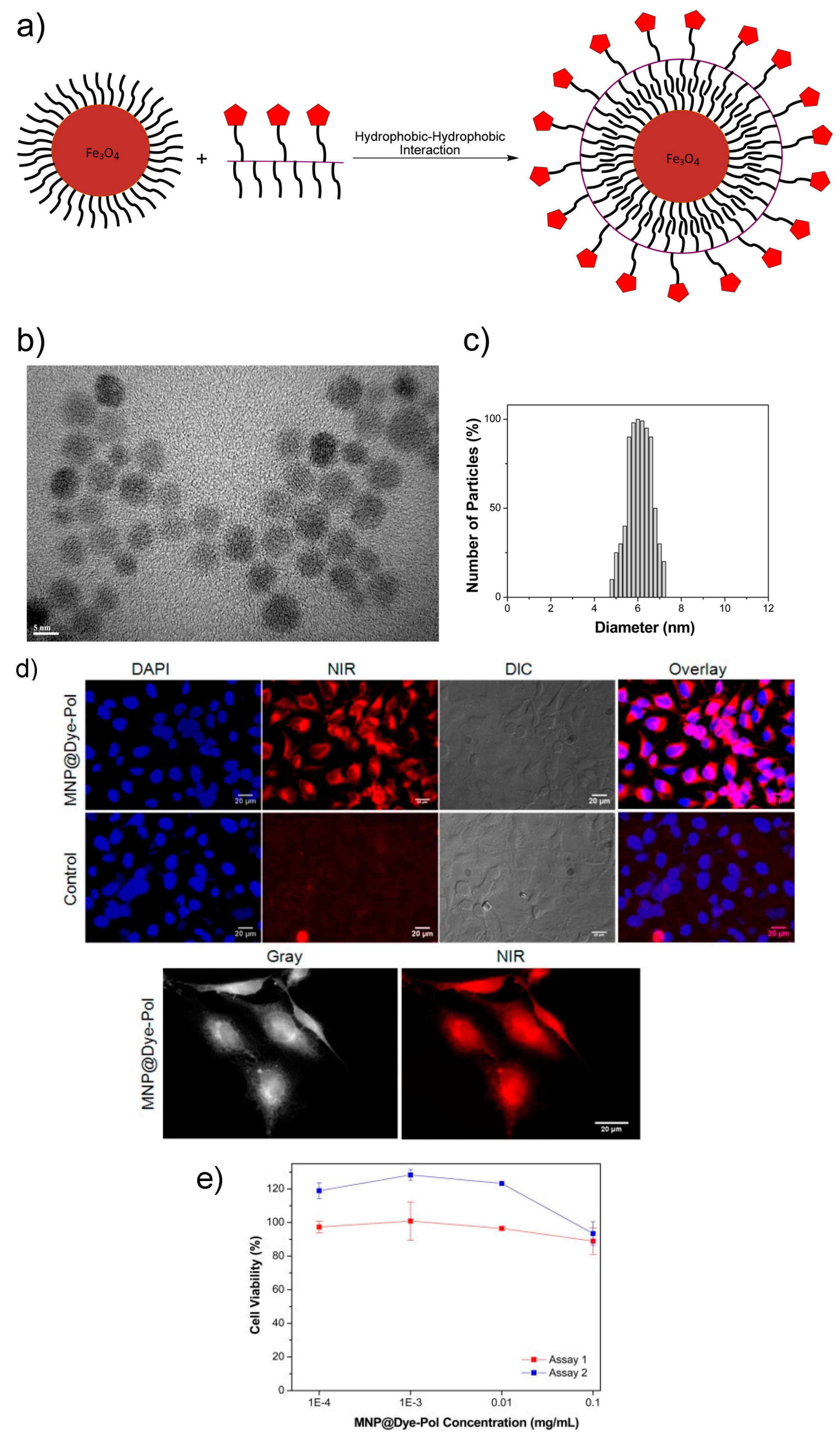
2.2.2. Polymer and Organic Dye Magnetic-Fluorescent Nanocomposites
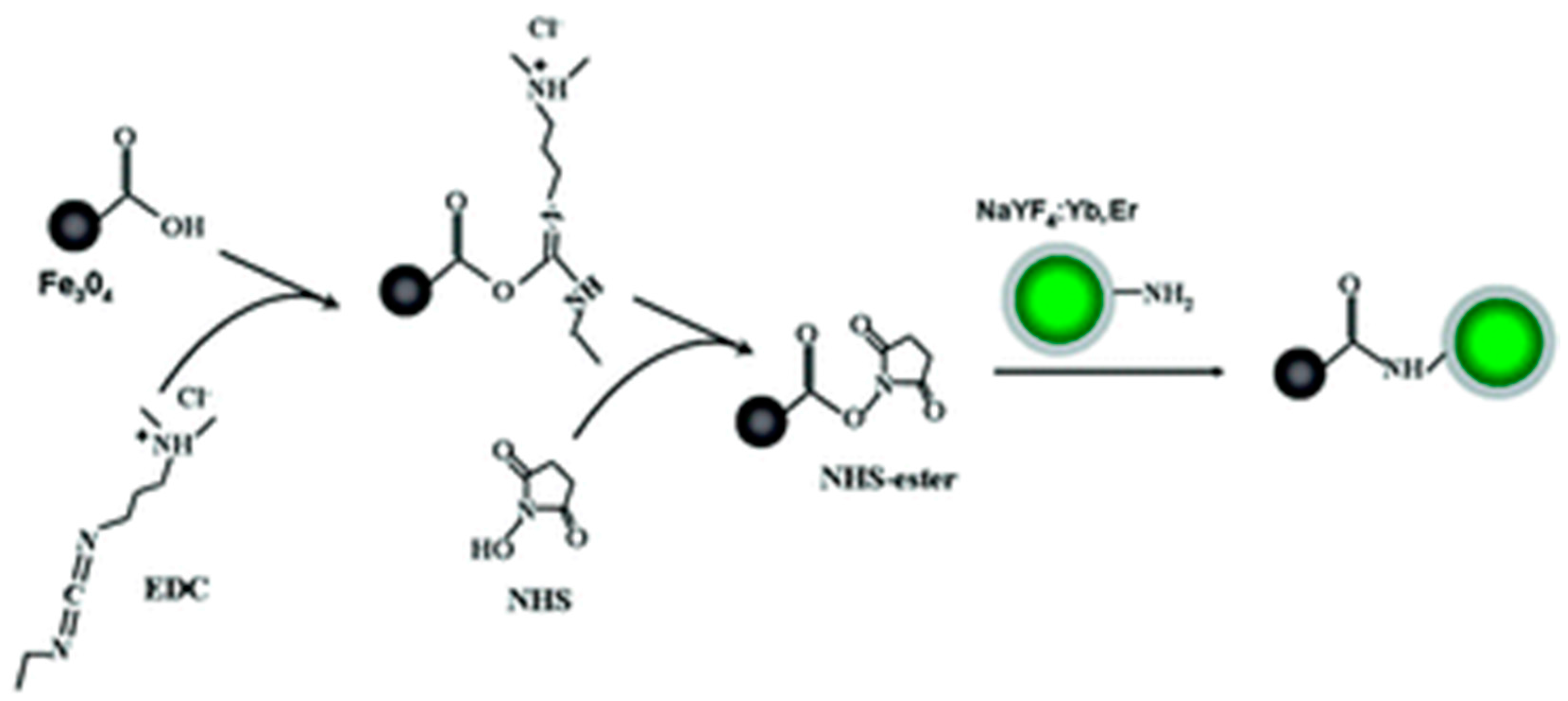
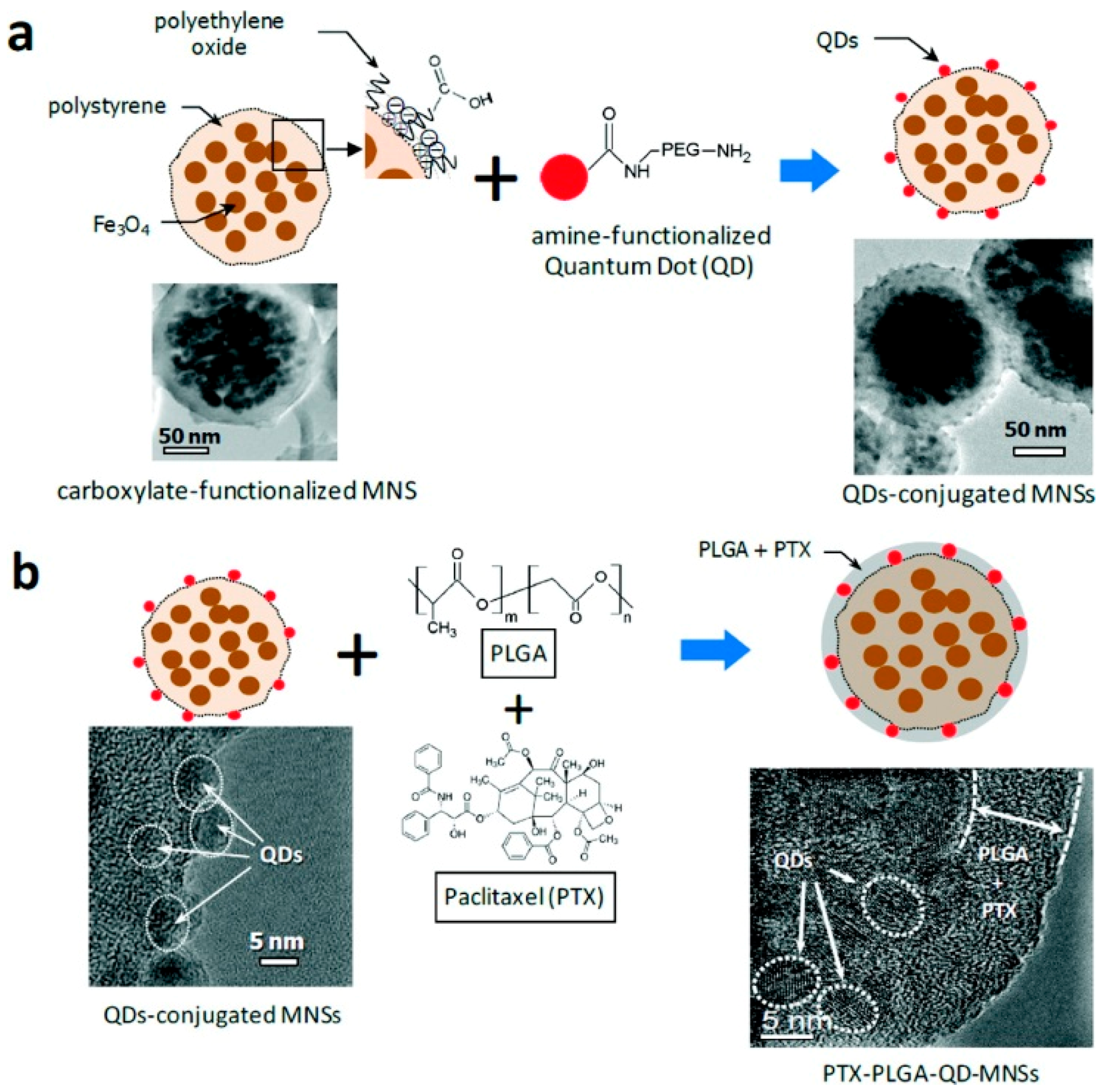
2.2.3. Polymer and QDs-Magnetic-Fluorescent Nanocomposites
3. Applications of Multimodal Fluorescent-Magnetic Nanoparticles
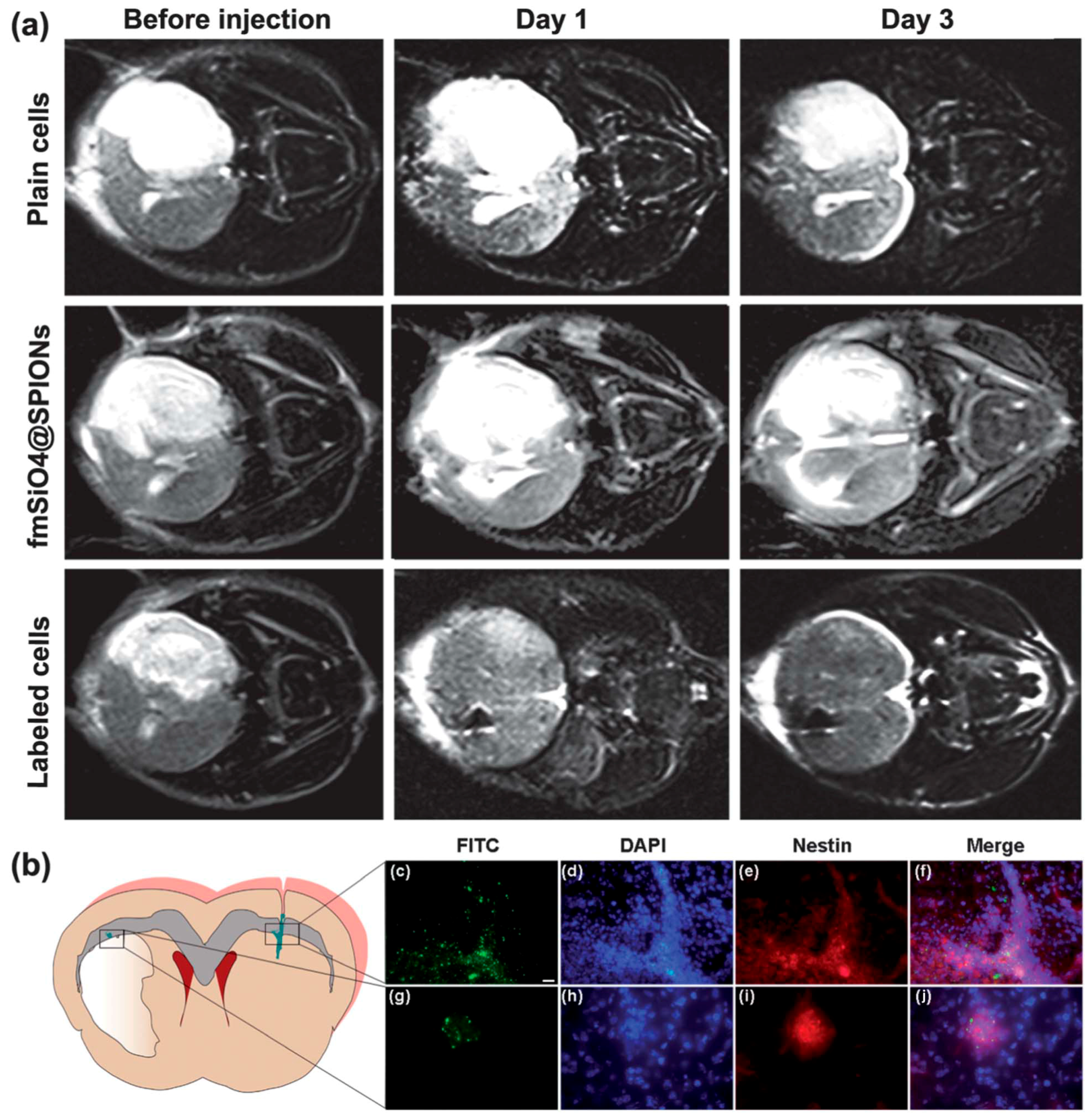
3.1. Multimodal Bioimaging
3.2. Cancer Therapy
3.3. Drug Delivery
4. Conclusions and Future Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hilger, I.; Hergt, R.; Kaiser, W.A. Use of magnetic nanoparticle heating in the treatment of breast cancer. IEE Proc. Nanobiotechnol. 2005, 152, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Zhu, Y.H.; Xu, H.B.; Yang, X.L. Multifunctional Magnetic Nanoparticles: Synthesis, Modification and Biomedical Applications. Prog. Chem. 2011, 23, 2287–2298. [Google Scholar]
- Huang, S.H.; Juang, R.S. Biochemical and biomedical applications of multifunctional magnetic nanoparticles: A review. J. Nanopart. Res. 2011, 13, 4411–4430. [Google Scholar] [CrossRef]
- Schladt, T.D.; Schneider, K.; Schild, H.; Tremel, W. Synthesis and bio-functionalization of magnetic nanoparticles for medical diagnosis and treatment. Dalton Trans. 2011, 40, 6315–6343. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Serpooshan, V.; Laurent, S. Engineered nanoparticles for biomolecular imaging. Nanoscale 2011, 3, 3007–3026. [Google Scholar] [CrossRef] [PubMed]
- Le Trequesser, Q.; Seznec, H.; Delville, M.H. Functionalized nanomaterials: Their use as contrast agents in bioimaging: Mono- and multimodal approaches. Nanotechnol. Rev. 2013, 2, 125–169. [Google Scholar] [CrossRef] [Green Version]
- Yan, K.; Li, P.H.; Zhu, H.E.; Zhou, Y.J.; Ding, J.D.; Shen, J.; Li, Z.; Xu, Z.S.; Chu, P.K. Recent advances in multifunctional magnetic nanoparticles and applications to biomedical diagnosis and treatment. RSC Adv. 2013, 3, 10598–10618. [Google Scholar] [CrossRef]
- Padmanabhan, P.; Kumar, A.; Kumar, S.; Chaudhary, R.K.; Gulyas, B. Nanoparticles in practice for molecular-imaging applications: An overview. Acta Biomater. 2016, 41, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cherukula, K.; Lekshmi, K.M.; Uthaman, S.; Cho, K.; Cho, C.S.; Park, I.K. Multifunctional Inorganic Nanoparticles: Recent Progress in Thermal Therapy and Imaging. Nanomaterials 2016, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.H.; Ding, K.; Kershaw, S.V.; Kempson, I.M.; Rogach, A.L.; Gao, M.Y. Magnetically Engineered Semiconductor Quantum Dots as Multimodal Imaging Probes. Adv. Mater. 2014, 26, 6367–6386. [Google Scholar] [CrossRef] [PubMed]
- Jana, N.R. Design and development of quantum dots and other nanoparticles based cellular imaging probe. Phys. Chem. Chem. Phys. 2011, 13, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Corr, S.A.; Rakovich, Y.P.; Gun’ko, Y.K. Multifunctional magnetic-fluorescent nanocomposites for biomedical applications. Nanosc. Res. Lett. 2008, 3, 87–104. [Google Scholar] [CrossRef]
- Koole, R.; Mulder, W.J.M.; van Schooneveld, M.M.; Strijkers, G.J.; Meijerink, A.; Nicolay, K. Magnetic quantum dots for multimodal imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 475–491. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.H.; Gu, H.W.; Xu, B. Multifunctional Magnetic Nanoparticles: Design, Synthesis, and Biomedical Applications. Acc. Chem. Res. 2009, 42, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.L.; Sadat, M.E.; Dunn, A.W.; Mast, D.B. Photo-fluorescent and magnetic properties of iron oxide nanoparticles for biomedical applications. Nanoscale 2015, 7, 8209–8232. [Google Scholar] [CrossRef] [PubMed]
- Acharya, A. Luminescent Magnetic Quantum Dots for In Vitro/In Vivo Imaging and Applications in Therapeutics. J. Nanosci. Nanotechnol. 2013, 13, 3753–3768. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Karmakar, A.; Wang, D.; Mahmood, M.W.; Watanabe, F.; Zhang, Y.; Fejleh, A.; Fejleh, P.; Li, Z.; Kannarpady, G. Multifunctional Fe3O4 cored magnetic-quantum dot fluorescent nanocomposites for RF nanohyperthermia of cancer cells. J. Phys. Chem. C 2010, 114, 5020–5026. [Google Scholar] [CrossRef]
- Lim, E.-K.; Yang, J.; Dinney, C.P.N.; Suh, J.-S.; Huh, Y.-M.; Haam, S. Self-assembled fluorescent magnetic nanoprobes for multimode-biomedical imaging. Biomaterials 2010, 31, 9310–9319. [Google Scholar] [CrossRef] [PubMed]
- Chekina, N.; Horak, D.; Jendelova, P.; Trchova, M.; Benes, M.J.; Hruby, M.; Herynek, V.; Turnovcova, K.; Sykova, E. Fluorescent magnetic nanoparticles for biomedical applications. J. Mater. Chem. 2011, 21, 7630–7639. [Google Scholar] [CrossRef]
- Wang, F.; Chen, X.; Zhao, Z.; Tang, S.; Huang, X.; Lin, C.; Cai, C.; Zheng, N. Synthesis of magnetic, fluorescent and mesoporous core-shell-structured nanoparticles for imaging, targeting and photodynamic therapy. J. Mater. Chem. 2011, 21, 11244–11252. [Google Scholar] [CrossRef]
- Jiaqi, W.; Xiangxi, M.; Enzhong, L.; Kezheng, C. Incorporation of magnetite nanoparticle clusters in fluorescent silica nanoparticles for high-performance brain tumor delineation. Nanotechnology 2010, 21, 235104. [Google Scholar] [CrossRef]
- Badruddoza, A.Z.M.; Rahman, M.T.; Ghosh, S.; Hossain, M.Z.; Shi, J.; Hidajat, K.; Uddin, M.S. β-Cyclodextrin conjugated magnetic, fluorescent silica core–shell nanoparticles for biomedical applications. Carbohydr. Polym. 2013, 95, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Torkpur-Biglarianzadeh, M.; Salami-Kalajahi, M. Multilayer fluorescent magnetic nanoparticles with dual thermoresponsive and pH-sensitive polymeric nanolayers as anti-cancer drug carriers. RSC Adv. 2015, 5, 29653–29662. [Google Scholar] [CrossRef]
- Li, L.; Liu, C.; Zhang, L.; Wang, T.; Yu, H.; Wang, C.; Su, Z. Multifunctional magnetic–fluorescent eccentric-(concentric-Fe3O4@SiO2)@ polyacrylic acid core–shell nanocomposites for cell imaging and pH-responsive drug delivery. Nanoscale 2013, 5, 2249–2253. [Google Scholar] [CrossRef] [PubMed]
- Ebrahiminezhad, A.; Ghasemi, Y.; Rasoul-Amini, S.; Barar, J.; Davaran, S. Preparation of novel magnetic fluorescent nanoparticles using amino acids. Colloids Surf. B Biointerfaces 2013, 102, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-S.; Yoon, T.-J.; Jang, E.-S.; Hong, K.S.; Lee, S.Y.; Kim, O.R.; Park, C.; Kim, Y.-J.; Yi, G.-C.; Chang, K. Cetuximab-conjugated magneto-fluorescent silica nanoparticles for in vivo colon cancer targeting and imaging. Cancer Lett. 2010, 299, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; He, B.; Shen, J.; Li, J.; Yang, W.; Yin, M. Multifunctional magnetic and fluorescent core–shell nanoparticles for bioimaging. Nanoscale 2015, 7, 1606–1609. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zang, Y.; Sun, M.; Wang, H.; Zhu, X.; Xu, S.; Yang, Q.; Li, Y.; Shan, Y. Synthesis of magnetic and fluorescent multifunctional hollow silica nanocomposites for live cell imaging. J. Colloid Interface Sci. 2010, 350, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Han, W.; Li, J.; Jiang, Y.; Cheng, D.; Song, Y.; Zhang, P.; Tan, W. Magnetic-encoded fluorescent multifunctional nanospheres for simultaneous multicomponent analysis. Anal. Chem. 2014, 86, 9434–9442. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zou, X.; Zhao, J.X.; Li, Y.; Su, X. Graphene oxide-based magnetic fluorescent hybrids for drug delivery and cellular imaging. Colloids Surf. B Biointerfaces 2013, 112, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ruan, J.; Qian, Q.; Song, H.; Bao, C.; Zhang, X.; Kong, Y.; Zhang, C.; Hu, G.; Ni, J. BRCAA1 monoclonal antibody conjugated fluorescent magnetic nanoparticles for in vivo targeted magnetofluorescent imaging of gastric cancer. J. Nanobiotechnol. 2011, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Koc, K.; Karakus, B.; Rajar, K.; Alveroglu, E. Synthesis and characterization of ZnS@Fe3O4 fluorescent-magnetic bifunctional nanospheres. Superlattices Microstruct. 2017, 110, 198–204. [Google Scholar] [CrossRef]
- Yin, N.; Wu, P.; Liang, G.; Cheng, W. A multifunctional mesoporous Fe3O4/SiO2/CdTe magnetic-fluorescent composite nanoprobe. Appl. Phys. A 2016, 122, 243. [Google Scholar] [CrossRef]
- Gui, R.; Wang, Y.; Sun, J. Encapsulating magnetic and fluorescent mesoporous silica into thermosensitive chitosan microspheres for cell imaging and controlled drug release in vitro. Colloids Surf. B Biointerfaces 2014, 113, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Borgohain, C.; Senapati, K.K.; Mishra, D.; Sarma, K.C.; Phukan, P. A new CoFe2O4–Cr2O3–SiO2 fluorescent magnetic nanocomposite. Nanoscale 2010, 2, 2250–2256. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Rout, S.R.; Das, R.K.; Nayak, S.; Ghosh, S.K. Highly hydrophilic luminescent magnetic mesoporous carbon nanospheres for controlled release of anticancer drug and multimodal imaging. Langmuir 2016, 32, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, F.; Tang, F.; Ma, N.; Li, L. Preparation of hybrid fluorescent–magnetic nanoparticles for application to cellular imaging by self-assembly. Colloids Surf. A Physicochem. Eng. Asp. 2011, 392, 103–109. [Google Scholar] [CrossRef]
- Pfaff, A.; Schallon, A.; Ruhland, T.M.; Majewski, A.P.; Schmalz, H.; Freitag, R.; Müller, A.H. Magnetic and fluorescent glycopolymer hybrid nanoparticles for intranuclear optical imaging. Biomacromolecules 2011, 12, 3805–3811. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Jiang, M.; Li, N.; Gu, H.; Xu, Q.; Ge, J.; Xia, X.; Lu, J. Modification of magnetic silica/iron oxide nanocomposites with fluorescent polymethacrylic acid for cancer targeting and drug delivery. J. Mater. Chem. 2010, 20, 6422–6429. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, T.; Li, L.; Wang, C.; Su, Z.; Li, J. Multifunctional fluorescent-magnetic polyethyleneimine functionalized Fe3O4–mesoporous silica yolk–shell nanocapsules for siRNA delivery. Chem. Commun. 2012, 48, 8706–8708. [Google Scholar] [CrossRef] [PubMed]
- Mi, C.; Zhang, J.; Gao, H.; Wu, X.; Wang, M.; Wu, Y.; Di, Y.; Xu, Z.; Mao, C.; Xu, S. Multifunctional nanocomposites of superparamagnetic (Fe3O4) and NIR-responsive rare earth-doped up-conversion fluorescent (NaYF4: Yb, Er) nanoparticles and their applications in biolabeling and fluorescent imaging of cancer cells. Nanoscale 2010, 2, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Gai, S.; Yang, P.; Li, C.; Wang, W.; Dai, Y.; Niu, N.; Lin, J. Synthesis of Magnetic, Up-Conversion Luminescent, and Mesoporous Core–Shell-Structured Nanocomposites as Drug Carriers. Adv. Funct. Mater. 2010, 20, 1166–1172. [Google Scholar] [CrossRef]
- Lehmann, A.D.; Parak, W.J.; Zhang, F.; Ali, Z.; Röcker, C.; Nienhaus, G.U.; Gehr, P.; Rothen-Rutishauser, B. Fluorescent–Magnetic Hybrid Nanoparticles Induce a Dose-Dependent Increase in Proinflammatory Response in Lung Cells in vitro Correlated with Intracellular Localization. Small 2010, 6, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Cheng, L.; Wang, C.; Ma, X.; Li, Y.; Liu, Z. Polymer encapsulated upconversion nanoparticle/iron oxide nanocomposites for multimodal imaging and magnetic targeted drug delivery. Biomaterials 2011, 32, 9364–9373. [Google Scholar] [CrossRef] [PubMed]
- Howes, P.; Green, M.; Bowers, A.; Parker, D.; Varma, G.; Kallumadil, M.; Hughes, M.; Warley, A.; Brain, A.; Botnar, R. Magnetic Conjugated Polymer Nanoparticles as Bimodal Imaging Agents. J. Am. Chem. Soc. 2010, 132, 9833–9842. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wu, X.; Cai, X.; Lin, S.; Xie, M.; Zhu, X.; Yan, D. Functionalization of Magnetic Nanoparticles with Dendritic–Linear–Brush-Like Triblock Copolymers and Their Drug Release Properties. Langmuir 2012, 28, 11929–11938. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Abe, E.; Takahashi, M.; Anzai, J.-I. Loading and release of fluorescent dye from layer-by-layer film-coated magnetic particles in response to hydrogen peroxide. J. Colloid Interface Sci. 2014, 432, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Ding, D.; Huo, D.; Pu, K.Y.; Thao, N.N.P.; Hu, Y.; Li, Z.; Liu, B. Conjugated Polymer Based Nanoparticles as Dual-Modal Probes for Targeted In Vivo Fluorescence and Magnetic Resonance Imaging. Adv. Funct. Mater. 2012, 22, 3107–3115. [Google Scholar] [CrossRef]
- Wang, L.; Neoh, K.-G.; Kang, E.-T.; Shuter, B.; Wang, S.-C. Biodegradable magnetic-fluorescent magnetite/poly(dl-lactic acid-co-α,β-malic acid) composite nanoparticles for stem cell labeling. Biomaterials 2010, 31, 3502–3511. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, W.; Du, H.; Guo, F.; Wang, H.; Chang, J.; Gong, X.; Zhang, B. Multifunctional reduction-responsive SPIO&DOX-loaded PEGylated polymeric lipid vesicles for magnetic resonance imaging-guided drug delivery. Nanotechnology 2016, 27, 165101. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Shang, Y.; Wang, W.; Zhou, Y.; Li, P.; Yan, K.; Wu, S.; Yeung, K.W.; Xu, Z.; Xu, H. Fluorescent Magnetic Fe3O4/Rare Earth Colloidal Nanoparticles for Dual-Modality Imaging. Small 2013, 9, 2991–3000. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Li, H.; Li, P.; Zhu, H.; Shen, J.; Yi, C.; Wu, S.; Yeung, K.W.K.; Xu, Z.; Xu, H.; et al. Self-assembled magnetic fluorescent polymeric micelles for magnetic resonance and optical imaging. Biomaterials 2014, 35, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Perillo, E.; Hervé-Aubert, K.; Allard-Vannier, E.; Falanga, A.; Galdiero, S.; Chourpa, I. Synthesis and in vitro evaluation of fluorescent and magnetic nanoparticles functionalized with a cell penetrating peptide for cancer theranosis. J. Colloid Interface Sci. 2017, 499, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Chen, K.J.; Noh, S.H.; Garcia, M.A.; Wang, H.; Lin, W.Y.; Jeong, H.; Kong, B.J.; Stout, D.B.; Cheon, J. On-demand drug release system for in vivo cancer treatment through self-assembled magnetic nanoparticles. Angew. Chem. 2013, 125, 4480–4484. [Google Scholar] [CrossRef]
- Hu, J.; Xie, M.; Wen, C.-Y.; Zhang, Z.-L.; Xie, H.-Y.; Liu, A.-A.; Chen, Y.-Y.; Zhou, S.-M.; Pang, D.-W. A multicomponent recognition and separation system established via fluorescent, magnetic, dualencoded multifunctional bioprobes. Biomaterials 2011, 32, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Ortgies, D.H.; de la Cueva, L.; del Rosal, B.; Sanz-Rodríguez, F.; Fernández, N.; Iglesias-de la Cruz, M.C.; Salas, G.; Cabrera, D.; Teran, F.J.; Jaque, D.; et al. In Vivo Deep Tissue Fluorescence and Magnetic Imaging Employing Hybrid Nanostructures. ACS Appl. Mater. Interfaces 2016, 8, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Lou, L.; Yu, K.; Zhang, Z.; Li, B.; Zhu, J.; Wang, Y.; Huang, R.; Zhu, Z. Functionalized magnetic-fluorescent hybrid nanoparticles for cell labelling. Nanoscale 2011, 3, 2315–2323. [Google Scholar] [CrossRef] [PubMed]
- Koktysh, D.; Bright, V.; Pham, W. Fluorescent magnetic hybrid nanoprobe for multimodal bioimaging. Nanotechnology 2011, 22, 275606. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Hu, J.; Wen, C.-Y.; Zhang, Z.-L.; Xie, H.-Y.; Pang, D.-W. Fluorescent–magnetic dual-encoded nanospheres: A promising tool for fast-simultaneous-addressable high-throughput analysis. Nanotechnology 2011, 23, 035602. [Google Scholar] [CrossRef] [PubMed]
- Vargas, J.M.; McBride, A.A.; Plumley, J.B.; Fichou, Y.; Memon, T.A.; Shah, V.; Cook, N.C.; Akins, B.A.; Rivera, A.C.; Smolyakov, G.A. Synthesis and characterization of core/shell Fe3O4/ZnSe fluorescent magnetic nanoparticles. J. Appl. Phys. 2011, 109, 07B536. [Google Scholar] [CrossRef] [PubMed]
- Ruan, G.; Vieira, G.; Henighan, T.; Chen, A.; Thakur, D.; Sooryakumar, R.; Winter, J.O. Simultaneous Magnetic Manipulation and Fluorescent Tracking of Multiple Individual Hybrid Nanostructures. Nano Lett. 2010, 10, 2220–2224. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-S.; Dong, Z.; Pauletti, G.M.; Zhang, J.; Xu, H.; Gu, H.; Wang, L.; Ewing, R.C.; Huth, C.; Wang, F.; et al. Fluorescent, Superparamagnetic Nanospheres for Drug Storage, Targeting, and Imaging: A Multifunctional Nanocarrier System for Cancer Diagnosis and Treatment. ACS Nano 2010, 4, 5398–5404. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhang, H.; Zhai, C.; Du, N.; Sun, C.; Xue, J.; Yang, D.; Huang, H.; Zhang, B.; Xie, Q.; et al. Carbon nanotube-based magnetic-fluorescent nanohybrids as highly efficient contrast agents for multimodal cellular imaging. J. Mater. Chem. 2010, 20, 9895–9902. [Google Scholar] [CrossRef]
- Liu, L.; Xiao, L.; Zhu, H.-Y. Preparation and characterization of CS–Fe3O4@ZnS:Mn magnetic-fluorescent nanoparticles in aqueous media. Chem. Phys. Lett. 2012, 539, 112–117. [Google Scholar] [CrossRef]
- Walia, S.; Sharma, S.; Markand Kulurkar, P.; Patial, V.; Acharya, A. A bimodal molecular imaging probe based on chitosan encapsulated magneto-fluorescent nanocomposite offers biocompatibility, visualization of specific cancer cells in vitro and lung tissues in vivo. Int. J. Pharm. 2016, 498, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Song, Y.; Liu, C.; Li, X.; Zhou, G.; Fan, Y. Magnetic and fluorescent bifunctional chitosan microspheres embedding with fluorescent-labeling drug as a drug delivery system. Mater. Lett. 2014, 114, 132–135. [Google Scholar] [CrossRef]
- Jha, D.K.; Saikia, K.; Chakrabarti, S.; Bhattacharya, K.; Varadarajan, K.S.; Patel, A.B.; Goyary, D.; Chattopadhyay, P.; Deb, P. Direct one-pot synthesis of glutathione capped hydrophilic FePt-CdS nanoprobe for efficient bimodal imaging application. Mater. Sci. Eng. C 2017, 72, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yin, H.; Shen, S.; Sun, K.; Liu, F. Chitosan-based magnetic/fluorescent nanocomposites for cell labelling and controlled drug release. New J. Chem. 2017, 41, 1736–1743. [Google Scholar] [CrossRef]
- McCarthy, J.E.; Prina-Mello, A.; Rakovich, T.; Volkov, Y.; Gun’ko, Y.K. Fabrication and characterization of multimodal magnetic—Fluorescent polystyrene nanowires as selective cell imaging probes. J. Mater. Chem. 2011, 21, 14219–14225. [Google Scholar] [CrossRef]
- Hwang, D.W.; Song, I.C.; Lee, D.S.; Kim, S. Smart magnetic fluorescent nanoparticle imaging probes to monitor microRNAs. Small 2010, 6, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Magro, M.; Sinigaglia, G.; Nodari, L.; Tucek, J.; Polakova, K.; Marusak, Z.; Cardillo, S.; Salviulo, G.; Russo, U.; Stevanato, R.; et al. Charge binding of rhodamine derivative to OH− stabilized nanomaghemite: Universal nanocarrier for construction of magnetofluorescent biosensors. Acta Biomater. 2012, 8, 2068–2076. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Jiang, R.; Fan, Q.; Zhang, L.; Zhang, H.; Yang, M.; Ma, Y.; Wang, L.; Huang, W. Fluorescent-magnetic poly(poly(ethyleneglycol)monomethacrylate)-grafted Fe3O4 nanoparticles from post-atom-transfer-radical-polymerization modification: Synthesis, characterization, cellular uptake and imaging. J. Mater. Chem. 2012, 22, 6965–6973. [Google Scholar] [CrossRef]
- Huang, P.; Li, Z.; Lin, J.; Yang, D.; Gao, G.; Xu, C.; Bao, L.; Zhang, C.; Wang, K.; Song, H.; et al. Photosensitizer-conjugated magnetic nanoparticles for in vivo simultaneous magnetofluorescent imaging and targeting therapy. Biomaterials 2011, 32, 3447–3458. [Google Scholar] [CrossRef] [PubMed]
- Wate, P.S.; Banerjee, S.S.; Jalota-Badhwar, A.; Mascarenhas, R.R.; Zope, K.R.; Khandare, J.; Misra, R.D.K. Cellular imaging using biocompatible dendrimer-functionalized graphene oxide-based fluorescent probe anchored with magnetic nanoparticles. Nanotechnology 2012, 23, 415101. [Google Scholar] [CrossRef] [PubMed]
- Yen, S.K.; Jańczewski, D.; Lakshmi, J.L.; Dolmanan, S.B.; Tripathy, S.; Ho, V.H.B.; Vijayaragavan, V.; Hariharan, A.; Padmanabhan, P.; Bhakoo, K.K.; et al. Design and Synthesis of Polymer-Functionalized NIR Fluorescent Dyes–Magnetic Nanoparticles for Bioimaging. ACS Nano 2013, 7, 6796–6805. [Google Scholar] [CrossRef] [PubMed]
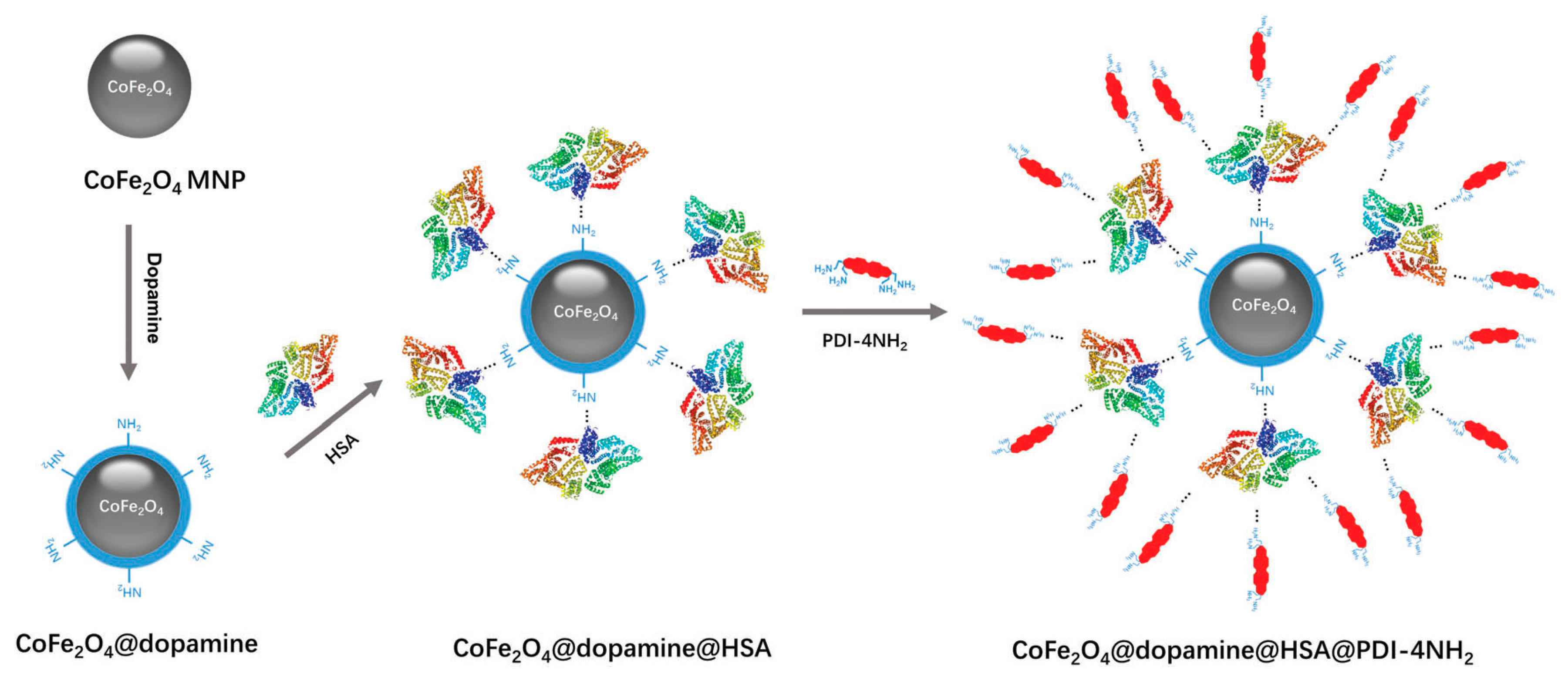
- Yao, Q.; Zheng, Y.; Cheng, W.; Chen, M.; Shen, J.; Yin, M. Difunctional fluorescent HSA modified CoFe2O4 magnetic nanoparticles for cell imaging. J. Mater. Chem. B 2016, 4, 6344–6349. [Google Scholar] [CrossRef]
- Xi, P.; Cheng, K.; Sun, X.; Zeng, Z.; Sun, S. Fluorescent magnetic nanoparticles based on a ruthenium complex and Fe3O4. J. Mater. Chem. 2011, 21, 11464–11467. [Google Scholar] [CrossRef]
- Xi, P.; Cheng, K.; Sun, X.; Zeng, Z.; Sun, S. Magnetic Fe3O4 nanoparticles coupled with a fluorescent Eu complex for dual imaging applications. Chem. Commun. 2012, 48, 2952–2954. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zou, M.; Chen, K. Novel Fe3O4@YPO4: Re (Re= Tb, Eu) multifunctional magnetic–fluorescent hybrid spheres for biomedical applications. Chem. Commun. 2010, 46, 5100–5102. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Tao, J.; Wang, W.; Zhou, Y.; Li, P.; Li, Z.; Yan, K.; Wu, S.; Yeung, K.W.K.; Xu, Z.; et al. Magnetic, fluorescent, and thermo-responsive Fe3O4/rare earth incorporated poly(St-NIPAM) core–shell colloidal nanoparticles in multimodal optical/magnetic resonance imaging probes. Biomaterials 2013, 34, 2296–2306. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Xia, T.; Gong, Y.; Wang, X.; Liu, R.-Q.; Zhang, Q.-Y.; Yi, C.-F.; Xu, Z.-S.; Guo, D.-Z. Emulsifier-free emulsion polymerized poly (MMA-HEMA-Eu (AA) 3Phen)/Fe3O4 magnetic fluorescent bifunctional nanospheres for magnetic resonance and optical imaging. Chin. J. Polym. Sci. 2016, 34, 135–146. [Google Scholar] [CrossRef]
- Kusrini, E.; Prassanti, R.; Nurjaya, D.M.; Gunawan, C. Multifunctional Microsphere Formulation of Fluorescent Magnetic Properties for Drug Delivery System; AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2017. [Google Scholar]
- Carretta, P.; Lascialfari, A. NMR-MRI, μSR and Mössbauer Spectroscopies in Molecular Magnets; Springer-Verlag Mailand: Milan, Italy, 2007. [Google Scholar]
- Catherine, C.B.; Adam, S.G.C. Functionalisation of magnetic nanoparticles for applications in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R198. [Google Scholar] [CrossRef]
- Mi, P.; Kokuryo, D.; Cabral, H.; Kumagai, M.; Nomoto, T.; Aoki, I.; Terada, Y.; Kishimura, A.; Nishiyama, N.; Kataoka, K. Hydrothermally synthesized PEGylated calcium phosphate nanoparticles incorporating Gd-DTPA for contrast enhanced MRI diagnosis of solid tumors. J. Control. Release 2014, 174, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Casula, M.F.; Floris, P.; Innocenti, C.; Lascialfari, A.; Marinone, M.; Corti, M.; Sperling, R.A.; Parak, W.J.; Sangregorio, C. Magnetic resonance imaging contrast agents based on iron oxide superparamagnetic ferrofluids. Chem. Mater. 2010, 22, 1739–1748. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Tang, Y.; Jiao, Z.; Xie, C.; Zhang, H.; Gu, P.; Wei, X.; Yang, G.-Y.; Gu, H.; et al. High MRI performance fluorescent mesoporous silica-coated magnetic nanoparticles for tracking neural progenitor cells in an ischemic mouse model. Nanoscale 2013, 5, 4506–4516. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Chen, K.; Huang, J.; Lee, S.; Wang, J.; Gao, J.; Li, X.; Chen, X. PET/NIRF/MRI triple functional iron oxide nanoparticles. Biomaterials 2010, 31, 3016–3022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yin, T.; Gao, G.; Shapter, J.G.; Lai, W.E.; Huang, P.; Qi, W.; Song, J.; Cui, D.X. Multifunctional Core@Shell Magnetic Nanoprobes for Enhancing Targeted Magnetic Resonance Imaging and Fluorescent Labeling in Vitro and in Vivo. ACS Appl. Mater. Interfaces 2017, 9, 17777–17785. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, R.; Medal, R.; Shorey, W.D.; Hanselman, R.C.; Parrott, J.C.; Taylor, C.B. Selective inductive heating of lymph nodes. Ann. Surg. 1957, 146, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Kim, J.; Lee, J.Y.; Matsuda, S.; Hideshima, S.; Mori, Y.; Osaka, T.; Na, K. Stimuli-responsive magnetic nanoparticles for tumor-targeted bimodal imaging and photodynamic/hyperthermia combination therapy. Nanoscale 2016, 8, 11625–11634. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.K.; Diagaradjane, P.; Krishnan, S. Nanoparticle-mediated hyperthermia in cancer therapy. Ther. Deliv. 2011, 2, 1001–1014. [Google Scholar] [CrossRef] [PubMed]
- Brodin, N.P.; Guha, C.; Tome, W.A. Photodynamic Therapy and Its Role in Combined Modality Anticancer Treatment. Technol. Cancer Res. Treat. 2015, 14, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.H.; Sheng, Z.H.; Gao, G.H.; Siu, F.M.; Liu, C.B.; Wan, Q.; Gong, P.; Zheng, H.R.; Ma, Y.F.; Cai, L.T. Activatable albumin-photosensitizer nanoassemblies for triple-modal imaging and thermal-modulated photodynamic therapy of cancer. Biomaterials 2016, 93, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Hirschberg, H.; Sun, C.H.; Tromberg, B.J.; Yeh, A.T.; Madsen, S.J. Enhanced cytotoxic effects of 5-aminolevulinic acid-mediated photodynamic therapy by concurrent hyperthermia in glioma spheroids. J. Neuro-Oncol. 2004, 70, 289–299. [Google Scholar] [CrossRef]
- De Paula, L.B.; Primo, F.L.; Pinto, M.R.; Morais, P.C.; Tedesco, A.C. Evaluation of a chloroaluminium phthalocyanine-loaded magnetic nanoemulsion as a drug delivery device to treat glioblastoma using hyperthermia and photodynamic therapy. RSC Adv. 2017, 7, 9115–9122. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, P.E. Vascular Targeting Agents as Cancer Therapeutics. Am. Assoc. Cancer Res. 2004, 10, 415–427. [Google Scholar] [CrossRef]
- Knezevic, N.Z.; Lin, V.S.Y. A magnetic mesoporous silica nanoparticle-based drug delivery system for photosensitive cooperative treatment of cancer with a mesopore-capping agent and mesopore-loaded drug. Nanoscale 2013, 5, 1544–1551. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano García, R.; Stafford, S.; Gun’ko, Y.K. Recent Progress in Synthesis and Functionalization of Multimodal Fluorescent-Magnetic Nanoparticles for Biological Applications. Appl. Sci. 2018, 8, 172. https://doi.org/10.3390/app8020172
Serrano García R, Stafford S, Gun’ko YK. Recent Progress in Synthesis and Functionalization of Multimodal Fluorescent-Magnetic Nanoparticles for Biological Applications. Applied Sciences. 2018; 8(2):172. https://doi.org/10.3390/app8020172
Chicago/Turabian StyleSerrano García, Raquel, Shelley Stafford, and Yurii K. Gun’ko. 2018. "Recent Progress in Synthesis and Functionalization of Multimodal Fluorescent-Magnetic Nanoparticles for Biological Applications" Applied Sciences 8, no. 2: 172. https://doi.org/10.3390/app8020172




