2. Materials and Methods
This review is based on publications found through the University of Sydney CrossSearch—ProQuest portal between January and April 2016. The inclusion criteria encompassed publications from 2005 onward with a cranioplasty/craniofacial implant focus, a total patient sample of 10 or greater, provision of a numerical value for total number of implants per material, as well as a numerical value for cases of infection per material. Points of examination included number of patients, materials utilized, fabrication method utilized (where applicable) and infection rate. Only first-time cranioplasty implants are considered in this study. The data from the studies was broken down based on material, leading to the following analysis groups:
* Hand-formed is defined as a cranioplasty implant formed intra-operatively by the surgeon using no specialized tools
** Prefabricated is defined as a cranioplasty implant which has been manufactured independent from and prior-to the surgical procedure
*** Templated is defined as a cranioplasty implant formed intra-operatively by the surgeon using specialized, prefabricated tools.
It must be noted that the combined utilization of materials, size and cause of defect, overlying soft tissue coverage, patient characteristics and complications other than infection (such as extrusion and migration) were not considered as a part of this study. Whilst these are critical issues in examining the effectiveness and survivability of cranioplasty implants from a clinical view-point, the purpose of this study is to examine only infection risk and, as such, these components are excluded. However, they provide points for examination in future reviews.
The search was conducted using the following key words: cranioplasty, craniofacial, implant, material, manufacture, infection, failure, autologous bone, polymethylmethacrylate, PMMA, hand-formed, prefabricated, rapid templated, titanium plate, titanium mesh, hydroxyapatite, polyetheretherketone, polyethylene and calcium phosphate.
Each biomaterial was provided by the authors with an estimated interactive surface form ranking (“ISF”) and a tissue attachment bioactivity ranking (“TAB”), based on material surface and bioactive properties. The ISF ranking is defined as a relative measure of interaction between the implant surface and the bodies tissues and immune system—where a higher ranking represents high interconnected porosity and, as a result, greater access to vascularized tissue ingrowth and the immune system—and the TAB ranking as a relative measure of the ability for a material to support attachment of growing tissue—where a higher ranking represents an increased support for tissue ingrowth. The purpose of these values is to provide a comparison point between implant material, manufacturing method, surface characteristics, bioactivity and infection potential. ISF and TAB rankings have been defined by the authors in
Table 1 and
Table 2.
Each material was provided with a relative risk value in order to provide a degree of comparison. In this review, autologous bone is considered as the gold standard, based on the consideration of full regeneration and integration as a potential patient outcome [
12,
13,
14,
15]. This calculation was taken as follows:
Finally, a one-way ANOVA test was conducted in order to determine if there was any statistical significance between the mean infection rates for each implant material.
3. Results
A total of 58 publications were found to be relevant based on these key words. Based on the search inclusion criteria, 41 of the 58 studies were found to meet the requirements for examination in this review.
All data was extracted based on numerical data found in tables, figures and written body of the included publications, and amalgamated in order to overcome potential bias and provide data which represents an average infection risk for each material. In order to provide a comparison of implantable materials for cranioplasty and craniofacial reconstruction and to provide an evaluation of available data in regards to reconstruction material and potential infection risk, each selected material is examined independently and the results merged into a final comparison table to provide a holistic overview.
3.1. Autologous Bone
In total, 10 of the 41 studies provided data for autologous bone in relation to infection complication risk as per the selection criteria. Implant number varied between 20 and 138 implants. For the purposes of this paper, only autologous bone flaps were considered. Different methods of storage were amalgamated, as a holistic view of autologous bone was desired. This is shown in
Table 3.
Infection rates varied between 5.93% and 25%, with an average infection rate of 10.50%, a standard deviation of 7.03% and a relative risk of 1.
Autologous bone stands as an anomaly when we consider both ISF and TAB values. When we consider its nature, a direct reimplantation of removed host tissue, we note that it has high potential for complete reintegration given the lack of foreign material introduction [
5]; providing the patient with a completely regenerative (in the sense that the host tissue is completely replaced) solution. As such, it can be considered to have a potential IMF and TAB value of 3, representing full integration and regeneration. However, literature highlights resorption of the autologous bone implant as a common issue known to impact the effectiveness of potential vascularization and reintegration [
19,
22], and as a result the autologous bone implant fails to reach the stage when reintegration is possible; shielded from the immune system it presents an ideal scaffold for bacteria and increasing risk of implant failure. As such, the IMF and TAB values, for the purposes of this study, can be considered as 0.
3.2. Polymethylmethacrylate (PMMA)
In total, 19 of the 41 studies provided data for PMMA in relation to infection complication risk as per the selection criteria. This can be further split by manufacture method, examining PMMA (prefabricated), PMMA (hand-formed) and PMMA (templated). This is shown in
Table 4.
3.3. PMMA (Prefabricated)
In total, 8 of the 41 studies provided data for PMMA (prefabricated) in relation to infection complication risk as per the selection criteria. Implant number varied between 3 and 120 implants.
Infection rates varied between 0.00% and 33.33%, with an average infection rate of 6.99%, a standard deviation of 10.67% and a relative risk of 0.66.
3.4. PMMA (Hand-Formed)
In total, 7 of the 41 studies provided data for PMMA (hand-formed) in relation to infection complication risk as per the selection criteria. Implant number varied between 11 and 98 implants.
Infection rates varied between 6.56% and 27.27%, with an average infection rate of 10.98%, a standard deviation of 6.70% and a relative risk of 1.05.
3.5. PMMA (Templated)
In total, 4 of the 41 studies provided data for PMMA (templated) in relation to infection complication risk as per the selection criteria. Implant number varied between 16 and 31 implants.
Infection rates varied between 3.70% and 9.68%, with an average infection rate of 6.86%, a standard deviation of 2.14% and a relative risk of 0.65.
PMMA implant manufacture produces surface textures ranging from smooth to coarse and can have varying degrees of porosity from 0% to 40% [
32]. As such, the ISF ranking assigned to PMMA is 1. PMMA is a bioinert material and is shown to have very little natural support for attachment and ingrowth of tissue [
32], however small holes may be bored into the implant to provide pathways for tissue growth [
24]. Whilst these holes can allow some tissue connection through the implant, they are not considered to be an extensive and integral component of the PMMA implant. When we consider, in conjunction, that PMMA does not actively provide any native support for tissue ingrowth, the TAB value is considered as 0.
3.6. Titanium
In total, 10 of the 41 studies provided data for titanium in relation to infection complication risk as per the selection criteria. This can be further split examining titanium (mesh) and titanium (plate). This is shown in
Table 5.
3.7. Titanium (Mesh)
In total, 4 of the 41 studies provided data for titanium (mesh) in relation to infection complication risk as per the selection criteria. Implant number varied between 12 and 218 implants.
Infection rates varied between 2.60% and 16.67%, with an average infection rate of 7.71%, a standard deviation of 5.21% and a relative risk of 0.73.
3.8. Titanium (Plate)
In total, 6 of the 41 studies provided data for titanium (plate) in relation to infection complication risk as per the selection criteria. Implant number varied between 26 and 174 implants.
Infection rates varied between 0.00% and 14.17%, with an average infection rate of 8.31%, a standard deviation of 4.58% and a relative risk of 0.79.
Titanium is a versatile metal and, in the field of cranioplasty, finds usage in a number of different structural forms. Titanium is considered a bioactive metal, with strong potential of osseointegration given appropriate porosity and surface texture [
41,
42]. Titanium plate, however, has no porosity and does not provide a surface adequate for tissue ingrowth; it is given both an ISF ranking and a TAB ranking of 0. Titanium mesh, however, does provide a greater degree of integration potential [
43], and is given an ISF ranking of 1. Like titanium plate, the smooth nature of the mesh itself does not promote ingrowth into the material, but rather into the surrounding space. As such, a TAB ranking of 0 is provided to titanium mesh.
3.9. Hydroxyapatite (HA)
In total, 5 of the 41 studies provided data for HA in relation to infection complication risk as per the selection criteria. Implant number varied between 25 and 1608 implants. This is shown in
Table 6.
Infection rates varied between 0.00% and 2.05%, with an average infection rate of 2.04%, a standard deviation of 1.04% and a relative risk of 0.19.
HA cranioplasty implants are praised for their regenerative and integrative potential, having a high degree of tissue ingrowth and vascularization [
46,
47,
48,
49,
50,
51,
52,
53]. Given these properties, and the materials highly porous nature it is given an ISF rank of 3 and TAB rank of 2.
3.10. Polyetheretherketone (PEEK)
In total, 8 of the 41 studies provided data for PEEK in relation to infection complication risk as per the selection criteria. Implant number varied between 2 and 66 implants. This is shown in
Table 7.
Infection rates varied between 0.00% and 14.29%, with an average infection rate of 7.89%, a standard deviation of 5.16% and a relative risk of 0.75.
For cranioplasty applications, PEEK tends to be manufactured with a smooth surface. In addition, its hydrophobic properties are such that concerns exist in regards to the materials anatomical integration potential [
53,
58,
59], as such, both the ISF and TAB ranking can be considered to be 0.
3.11. Polyethylene (PE)
In total, 5 of the 41 studies provided data for PE in relation to infection complication risk as per the selection criteria. Implant number varied between 7 and 69 implants. This is shown in
Table 8.
Infection rates varied between 0.00% and 14.29%, with an average infection rate of 5.93%, a standard deviation of 5.33% and a relative risk of 0.57.
One of most common form of PE used in cranioplasty applications is a porous mesh form [
43,
60,
61]. The implant itself is a highly porous structure and is given an ISF ranking of 3. However, as a bioinert material PE does not actively promote tissue ingrowth or regeneration [
62], although it can take a more bioactive role upon coating with bioactive materials [
63]. However, given its natural characteristics, PE is provided with a TAB ranking of 0.
3.12. Calcium Phosphate
In total, 3 of the 41 studies provided data for calcium phosphate in relation to infection complication risk as per the selection criteria. Implant number varied between 16 and 92 implants. This is shown in
Table 9.
Infection rates varied between 0.00% and 18.75%, with an average infection rate of 6.70%, a standard deviation of 8.04% and a relative risk of 0.64.
Much like HA, calcium phosphate cranioplasty implants are considered to have strong regenerative and reintegration properties. Given these properties, and the materials relatively porous nature [
66,
67] it is given and ISF rank of 2 and TAB rank of 2.
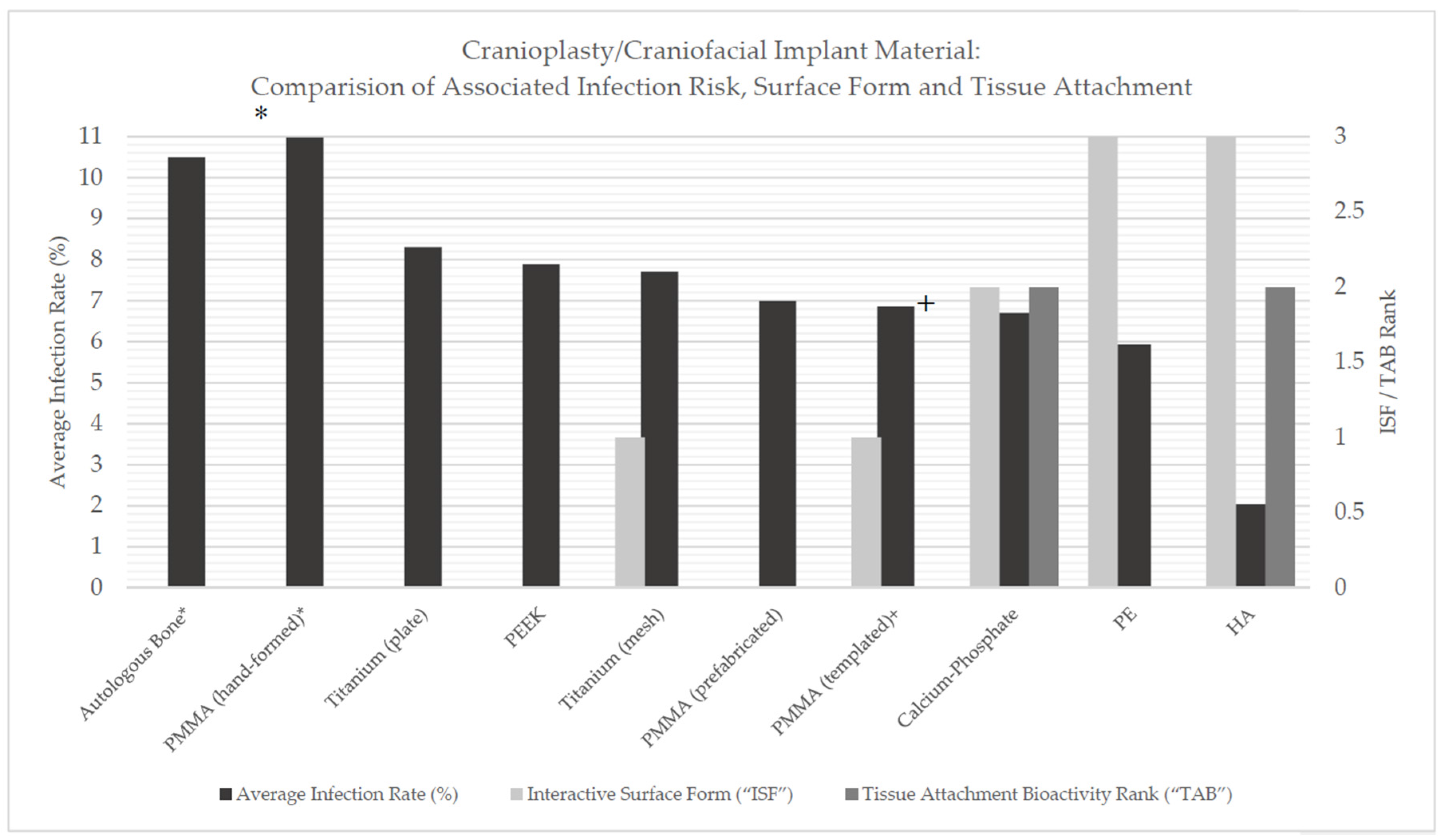
Table 10 and
Figure 1 provide a source of comparison between material, manufacturing method (where applicable), infection rate, estimated ISF and TAB values and relative risk. This table highlights variation between materials in regards to total infection risk, however, depending on the materials compared, this value may be insignificant.
Table 11 highlights the results of the one-way ANOVA test conducted.
There were not found to be statistically significant differences between all implant material infection rate means as ascertained by one-way ANOVA (p = 0.12).
4. Discussion
When we consider a comparison of the infection rate of different biomaterials, it is fundamental to recognize differences in material characteristics: material properties, biocompatibility, bioactivity etc.
Autologous bone is considered as having the potential to provide an immediate patient specific implant, providing a repair solution with no manufacturing requirements. Key advantages of use lie in high immunological compatibility [
36], as the implant is comprised of the patient’s own tissue, as well as superior anatomical cosmesis, given it matches the injury profile directly [
29]. Often, direct re-implantation of the autologous bone flap is not feasible and storage (either cryogenic or subcutaneous) is required to ensure the flap is preserved until re-implantation [
21,
68,
69]. Whilst functional, these methods may not provide adequate storage, with infection and resorption potential rendering the autologous flap unusable [
19,
21,
70].
PMMA is a medically accepted thermoplastic with high compatibility. It finds common use as a grout-like-material or for lost bone remodeling [
24,
25,
28,
71,
72]. Whilst PMMA is a biocompatible material, the raw methyl methacrylate component is a potential irritant [
73]. The curing process for PMMA is exothermic (between 70 and 120 °C). This has significant implications for in vitro use, as such temperatures can lead to death of surrounding tissue through thermal necrosis [
74,
75,
76]. In addition, there is evidence which suggests that the curing process of PMMA can lead to cardiopulmonary complications (hypotension) [
77]. However, these properties are tied directly to the shaping and curing of PMMA within the body (hand-formation) and can be minimized effectively through pre-surgical fabrication (prefabrication) or intraoperative molding external to the patient (templating).
Titanium is well established in the field of cranial and craniofacial medicine. This is primarily due to its biological inertness, favorable strength-to-weight ratio and favorable cosmetic and functional outcomes [
36,
38,
39,
78]. Another key advantage of titanium is its natural osseointegration factor, which promotes active bone growth into the implant [
41,
42]. Studies have shown that titanium implants for cranial and maxillofacial applications have a lower survival rate (higher complication rate) than alterative materials such as PMMA [
27]. Despite its lower Young’s modulus than alterative metals, the value for titanium is higher than that of natural bone, leading to a lessened, but still present, risk of stress shielding [
79]. Titanium implants will often require pre-fabrication [
39,
78], leading to an increase lead time and cost. In addition, intraoperative alteration remains difficult [
80].
Hydroxyapatite (HA) is a natural inorganic mineral compound found naturally in human teeth and bone tissue and, as such, represents a prime candidate for orthopedic applications, acting as both a filer for replacing lost bone [
46,
47,
48,
49,
50,
51,
52,
53] and as a coating for implantable structures to promote bone ingrowth [
52,
53]. HA is not capable of withstanding high loads and, as such, is not as suited for larger defects when compared to other implantable materials [
4,
5]. HA remains relatively costly when compared to other non-metal implant materials [
3]. It must be noted that HA has numerous advantages when combined with alternative materials, as the benefits of its bioactive, pro-osseointegration properties are accompanied by a more rigid and stable structure [
49,
52], however, this is not a consideration in this review.
Polyetheretherketone (PEEK) is a biocompatible linear thermoplastic which is finding increased acceptance in the medical industry as an alternative to metal components. The core driving factor to the development of PEEK as a biomaterial was the need to balance strength and rigidity: to develop an implant system which met the structural strength requirements of the body, whilst minimizing rigidity in an attempt to reduce stress shielding risk [
33,
81]. Traditionally PEEK found use in spinal treatment applications, as well as cranial and maxillofacial applications [
54,
56,
82]. It must be noted that PEEK is a hydrophobic material. As such, it does not actively bond to tissue, creating potential issues in regards to anatomical integration as bone cells will not bond to the implants [
59]. Whilst there are a number of coatings which can alleviate these concerns [
53,
58], un-coated PEEK remains at a disadvantage in regards to achieving stable fixation in a short timeframe.
Polyethylene is a thermoplastic material which finds use in numerous areas of the medical industry. This is due to its variability and adaptability, as its method of manufacture and formation can give it various physical properties tailoring it for a broad range of applications, from medical packaging to implants [
83]. It can be manufactured as a porous structure, commonly used in cranioplasty applications, allowing for the creation of semi-rigid structures which are both strong and flexible and allow for alteration with minimal effort [
60,
84,
85]. It must be noted that for certain applications such porous structures this material may not have the mechanical properties to act in load-bearing structures [
86,
87].
Calcium phosphate bone cements are finding acceptance as methods of cranial reconstruction given their strong biocompatibility and osteoconductive nature [
66]. In addition, ready availability and ease of use present calcium phosphate cements as appealing alternatives to current reconstruction materials [
67].
Based on the findings from the systematic review, the following can be inferred from the results:
● Implant material/manufacturing method has an effect on infection risk.
Whilst the results of the one-way ANOVA (p = 0.12) show no statistical significance when analyzing all implant materials as a whole, analysis of means highlights variation between individual implant materials and manufacturing methods; which, dependent on the materials compared directly, can be considered significant.
Further literature analysis highlights the following factors as prime sources of increased infection and complication risk for cranial and craniofacial implants.
Latency Period between Tissue Removal and Implant [
1,
8]
- ○
The literature suggests that a necessary latency period, from initial flap removal to implantation of the desired implant, may lead to a lower infection risk.
Previous Multiple Operations/Recent Operation [
1,
9]
- ○
The literature suggests that a higher number of operations can lead to a higher risk of infection. Additionally, short timeframes between surgeries are considered to further increase infection risk.
Increased Surgical Time [
6,
10,
11]
- ○
The literature suggests that increased surgical time dramatically increases the risk of infection. Longer surgical times (greater than 4 h) were associated with a higher risk of infection.
Additional factors, including gender, pre-existing infection, surgical errors, surgical complexity and poor outcomes, were also considered to potentially lead to an increase in infection potential. Infection risk can be further impacted through factors such as implant and defect size, fixation position and fixation stability. However, like the inherent infection resistance of implant materials, these factors were considered to have a lesser impact on potential risk than the aforementioned prime sources.
A reflection to be made from this examination is that implant material does provide an effect on potential infection risk, as greater levels of surface interaction and active support of tissue ingrowth can be considered as indicators of greater infection resistance. This can be seen as occurring in part due to the capacity of increased vascularization and access for the body’s natural system to access the implant and assist in either preventing and/or combating possible sources of infection. Such characteristics are due to the physical structures of the implants and, as a result, it can be said that the method of production can, through alteration of these physical properties, assist in minimizing implant infection risk. It is to be noted that interconnected porosity is fundamental in tissue regeneration as this allows propagation of appropriate cells (osteoblasts) through the implant and the eventual formation of appropriate vascularization. Whilst the size of the pores may vary, it is generally accepted that a pore size of approximately 100 µm is required to regenerate bone tissue [
88]. As such, a trend can be seen where those implant materials with higher ISF and TAB rankings trend towards a lower infection risk; however, the true validity of this remains a point for future study.
Building on this, manufacturing methods can further minimize infection risk through influences of prime sources of infection risk. By removing the implant contouring and/or alteration requirements from cranioplasty/craniofacial repair, the processes become simplified, providing better outcomes with fewer surgical requirements. Using the example of PMMA implants for cranioplasty and craniofacial reconstruction, it can be observed that both the prefabricated and templated methods of implant production are considered to minimize potential surgical timeframes, whilst also reducing variance in surgical time between surgeries. This is reflected particularly in relation to the templating method by Kim et al. 2012, with cranioplasty surgery taking 184.36 ± 26.07 min for PMMA (templated), as opposed to previously recorded values of 285 ± 128 min for alternative fabrication methods [
11]. The ability to either prefabricate patient specific implants, or template these intra-operatively external to the patient, provided clear benefits by removing the risk of thermal necrosis or hypotension associated with the curing process of PMMA [
74,
75,
76,
77]. In addition, the nature of geometry generation becomes controlled through accepted physical design methodologies and computer-aided design (CAD), allowing for better form, fit and function [
26,
89,
90]; which in turn leads to less chance of abnormal biomechanics and fit-based complications, without the need to shape or alter the implant by hand, as well as minimizing the complexity of the surgical procedure. This example can be considered in the case of easily alterable materials (PMMA, porous polyethylene, titanium mesh, etc.) which can be readily shaped using commonly available surgical tools [
11,
25,
84,
85,
91] and adapted to meet anatomical requirements which may not be visible or known prior to surgery. However, for certain applications, such as titanium plate, this is not appropriate as intraoperative alteration of the implant is near impossible without specialized tools.
A fundamental consideration not discussed within the body of this review is the relationship between implant graft material and patient context. Different injury profiles and patient lifestyles require different outcomes, which may provide a degree of preference to certain reconstructive materials. Features such as size, shape, cause, complexity and functional outcomes can drastically impact the pool of applicable implant materials and the potential associated fabrication and implantation cost. However, this topic is beyond the scope of this review.
It is key to note that each study analyzed within this review portrayed varying results for infection rate for each material examined, with standard deviations within each material category ranging from 1.04 to 10.67. In addition, each study examined a different number of patients and overall sample sizes for each material category varied significantly, ranging from 2 to 1608. As such, this raises the point that clinical studies may not present enough data individually in order to provide significant evidence of a material or methods utility over potential alternatives. This does not mean that clinical studies are ineffective, but rather aims to emphasize that collaboratively clinical studies can increase our understanding of how materials science impacts patient outcomes to a greater extent.