Characterization and Clinical Trial of an Innovative High-Speed Lancing Device
Abstract
:1. Introduction
2. Experimental Details
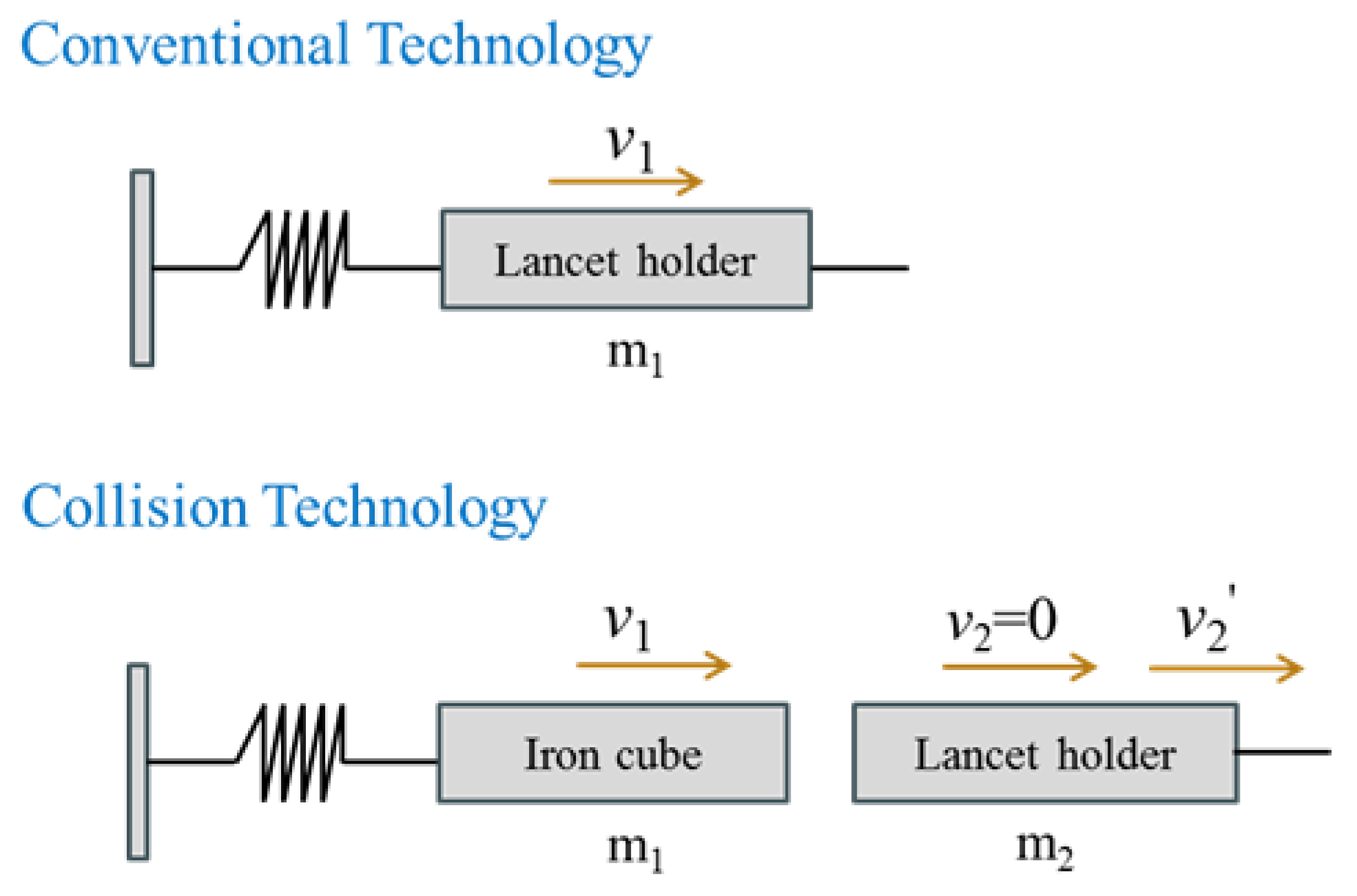
2.1. Lancing Device Principle
- (1)
- If m1 = m2, then
- (2)
- If m1 > m2 and v2 = 0, then
- (3)
- If m1 >> m2 and v2 = 0, then
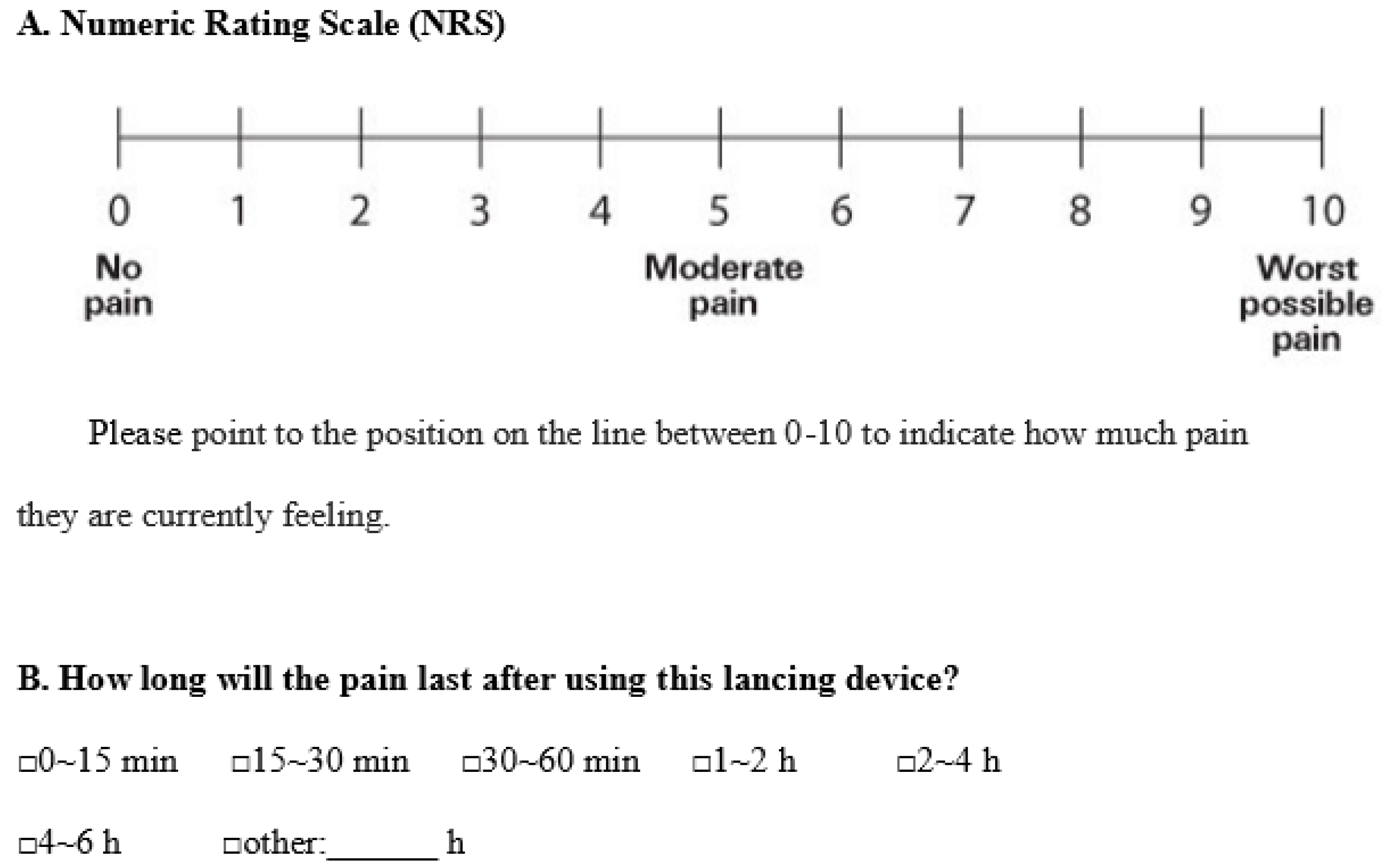
2.2. Clinical Study
3. Results and Discussion
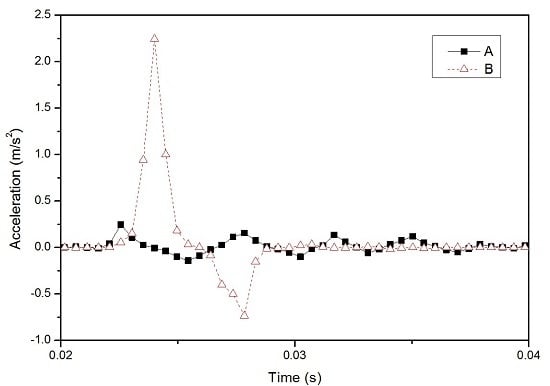
3.1. Characterization of Innovative High-Speed Lancing Device
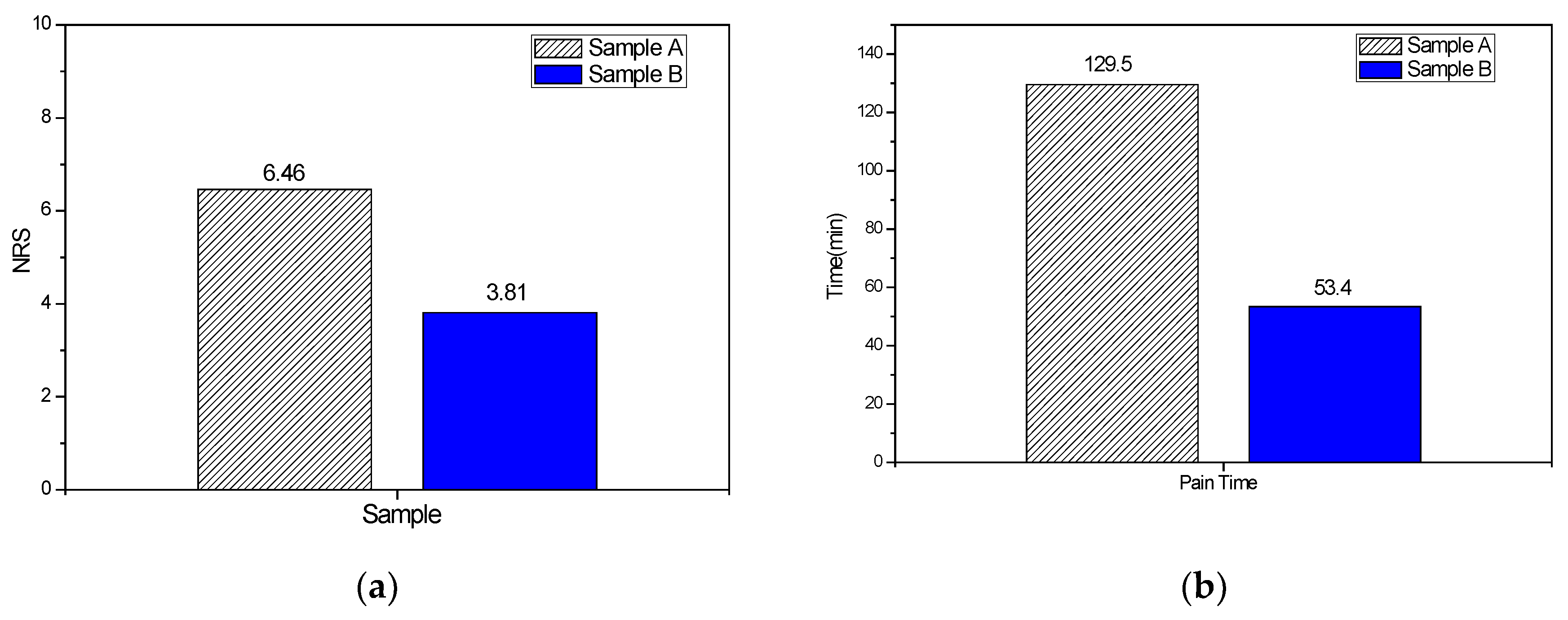
3.2. Results of Clinical Trial
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Laffel, L. Improved accuracy of continuous glucose monitoring systems in pediatric patients with diabetes mellitus: Results from two studies. Diabetes Technol. Ther. 2016, 18, S223–S233. [Google Scholar] [CrossRef] [PubMed]
- Klonoff, D.C. Improving the safety of blood glucose monitoring. J. Diabetes Sci. Technol. 2011, 5, 1307–1311. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, L. Finger pricking and pain: A never ending story. J. Diabet. Sci. Technol. 2008, 2, 919–921. [Google Scholar] [CrossRef]
- Heinemann, L.; Boecker, D. Lancing: Quo vadis? J. Diabetes Sci. Technol. 2011, 5, 996–981. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, R.K.; Tai, B.L.; McLaughlin, P.W.; Shih, A.J. Optimal needle design for minimal insertion force and bevel length. Med. Eng. Phys. 2014, 36, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tai, B.L.; Chen, B.L.; Shih, A.J. The needle with lancet point: Geometry for needle tip grinding and tissue insertion force. J. Manuf. Sci. Eng. 2013. [Google Scholar] [CrossRef]
- Flora, B.A.; Ruggiero, J.E. Multiple Tip Lancet. 1771112 A2, 11 April 2007. [Google Scholar]
- Fruhstorfer, H.; Schmelzeisen-Redeker, G.; Weiss, T. Capillary blood sampling: Relation between lancet diameter, lancing pain and blood volume. Eur. J. Pain 1999, 3, 283–286. [Google Scholar] [CrossRef]
- Arendt-Nielsen, L.; Egekvist, H.; Bjerring, P. Pain following controlled cutaneous insertion of needles with different diameters. Somatosens. Motor Res. 2006, 23, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Christopher, R. Lancing Device. 20080082117 A1, 3 April 2008. [Google Scholar]
- Kuhr, H.J.; Forster, R. Device for Withdrawing Blood for Diagnostic Applications. 7322998 B2, 29 January 2008. [Google Scholar]
- Robert, U. Lancing Device with Dampener. 1810616 B1, 2 December 2009. [Google Scholar]
- Prais, E.; Sams, R.S.; Yao, S. Lancing Device. 20140276221 A1, 18 September 2014. [Google Scholar]
- Ruan, T.; Hoover, S. Lancing Device. 20140107689 A1, 17 April 2014. [Google Scholar]
- Lai, S.K.; Yeo, C.K. Lancing Device for Minimizing Pain. 20100145377 A1, 10 June 2010. [Google Scholar]
- Kocher, S.; Tshiang, T.J.K.; Koubek, R. Comparison of lancing devices for self-monitoring of blood glucose regarding lancing pain. J. Diabetes Sci. Technol. 2009, 3, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, B.H.; Shemain, A.; Pynes, M.K.; Wallace, D.A.; Pineau, M. Evaluating the OneTouch® Delica™: A Low-pain lancing system for self-monitoring of blood glucose. Postgrad. Med. 2011, 123, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Huskisson, E.C. Measurement of pain. Lancet 1974, 2, 1127–1131. [Google Scholar] [CrossRef]
- Liao, Y.M. Validation of the Chinese Version Behavioral Pain Scale. Master Thesis, Taipei Medical University, Taipei, Taiwan, April 2007. [Google Scholar]



| Variables (Age) | Number (n) | % |
|---|---|---|
| Below 20 | 10 | 10% |
| 21–30 | 30 | 30% |
| 31–40 | 23 | 23% |
| 41–50 | 24 | 24% |
| 51–60 | 10 | 10% |
| Above 61 | 3 | 3% |
| Variables | Pain levelM ± SE | 95% CI |
|---|---|---|
| NRS | ||
| Sample A | 6.46 ± 0.22 | 6.01–6.90 |
| Sample B | 3.81 ± 0.27 | 3.24–4.35 |
| Pain duration time | ||
| Sample A | 129.5 ± 15 | 83.9–160 |
| Sample B | 53.4 ± 7.67 | 37.9–68.8 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, H.; Yeh, Y.-J.; Lee, R.; Lee, C.-C.; Shyu, J.-H. Characterization and Clinical Trial of an Innovative High-Speed Lancing Device. Appl. Sci. 2016, 6, 111. https://doi.org/10.3390/app6040111
Chang H, Yeh Y-J, Lee R, Lee C-C, Shyu J-H. Characterization and Clinical Trial of an Innovative High-Speed Lancing Device. Applied Sciences. 2016; 6(4):111. https://doi.org/10.3390/app6040111
Chicago/Turabian StyleChang, Ho, Yao-Jen Yeh, Rahnfong Lee, Chia-Chun Lee, and Jenq-Huey Shyu. 2016. "Characterization and Clinical Trial of an Innovative High-Speed Lancing Device" Applied Sciences 6, no. 4: 111. https://doi.org/10.3390/app6040111