A Modified Thermal Treatment Method for the Up-Scalable Synthesis of Size-Controlled Nanocrystalline Titania
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis Mechanism
2.2. Thermal Analysis (TGA–DTG)
2.3. Phase Composition Analysis (Fourier Transform Infrared Spectroscopy (FT-IR))
2.4. Elemental Composition Analysis (Energy Dispersive X-ray Spectroscopy (EDX))
2.5. Structural Analysis (X-ray Diffraction (XRD))
2.6. Morphology and Size Distribution
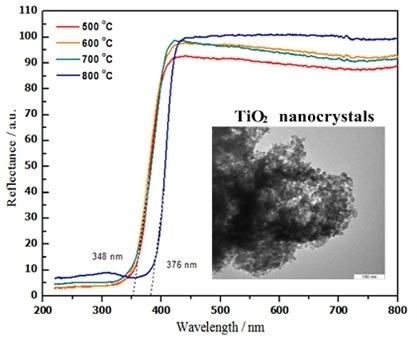
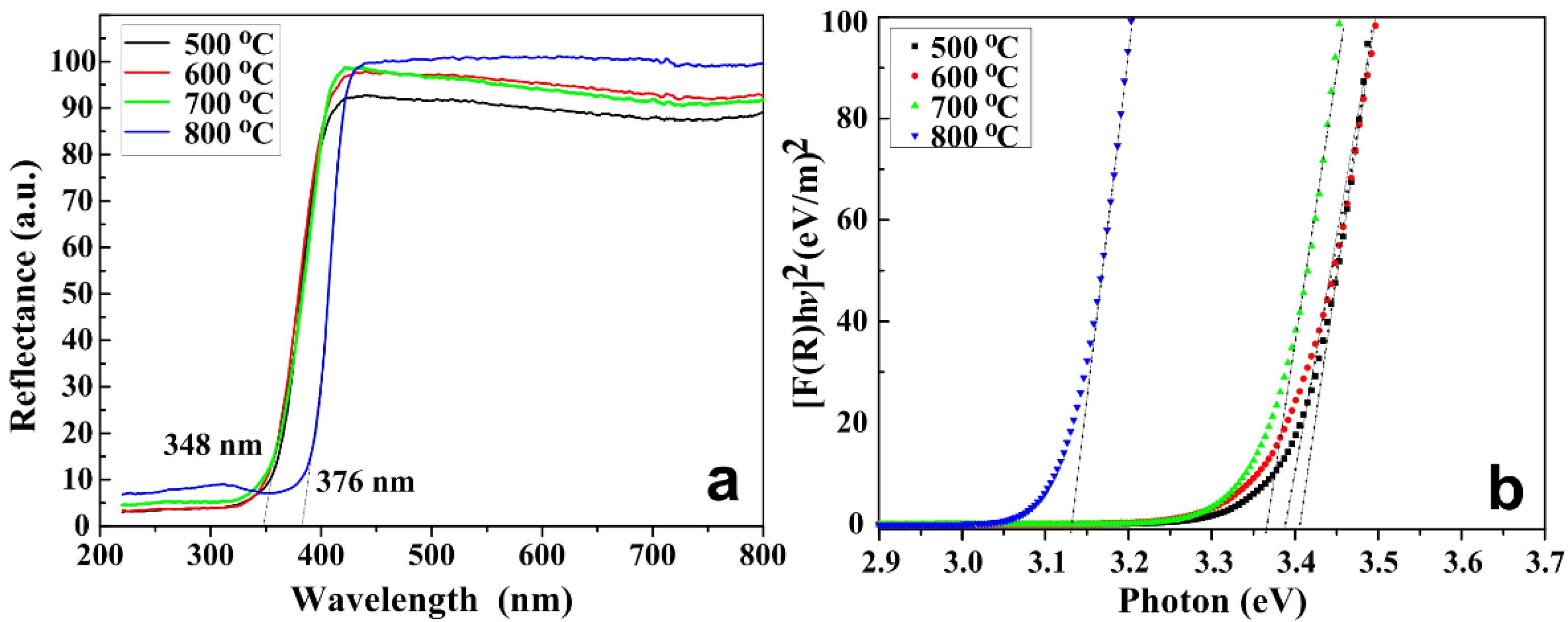
2.7. Optical Properties
3. Materials and Methods
3.1. Materials
3.2. The Synthesis of TiO2 Nanoparticles
3.3. Characterization Techniques
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chowdhury, I.H.; Ghosh, S.; Naskar, M.K. Aqueous-based synthesis of mesoporous TiO2 and Ag–TiO2 nanopowders for efficient photodegradation of methylene blue. Ceram. Int. 2016, 42, 2488–2496. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Liu, S.; Zhang, L. N-doped TiO2 Nanotubes as an Effective Additive to Improve the Catalytic Capability of Methanol Oxidation for Pt/Graphene Nanocomposites. Nanomaterials 2016, 6, 40. [Google Scholar] [CrossRef]
- Kuo, W.K.; Weng, H.P.; Hsu, J.J.; Yu, H.H. Photonic Crystal-Based Sensors for Detecting Alcohol Concentration. Appl. Sci. 2016, 6, 67. [Google Scholar] [CrossRef]
- Yang, L.; Luo, S.; Cai, Q.; Yao, S. A review on TiO2 nanotube arrays: Fabrication, properties, and sensing applications. Chin. Sci. Bull. 2009, 55, 331–338. [Google Scholar] [CrossRef]
- Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Mor, G.K.; Varghese, O.K.; Paulose, M.; Shankar, K.; Grimes, C.A. A review on highly ordered, vertically oriented TiO2 nanotube arrays: Fabrication, material properties, and solar energy applications. Sol. Energy Mater. Sol. Cells 2006, 90, 2011–2075. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T.L.; Yates, J.T. Surface science studies of the photoactivation of TiO2 new photochemical processes. Chem. Rev. 2006, 106, 4428–4453. [Google Scholar] [CrossRef] [PubMed]
- Linsebigler, A.L.; Lu, G.; Yates, J.T., Jr. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Wisitsoraat, A.; Tuantranont, A.; Comini, E.; Sberveglieri, G.; Wlodarski, W. Characterization of n-type and p-type semiconductor gas sensors based on NiOx doped TiO2 thin films. Thin Solid Films 2009, 517, 2775–2780. [Google Scholar] [CrossRef]
- Wunderlich, W.; Oekermann, T.; Miao, L.; Hue, N.T.; Tanemura, S.; Tanemura, M. Electronic properties of nano-porous TiO2- and ZnO thin films- comparison of simulations and experiments. J. Ceram. Process. Res. 2004, 5, 343–354. [Google Scholar]
- Praveen, P.; Viruthagiri, G.; Mugundan, S.; Shanmugam, N. Structural, optical and morphological analyses of pristine titanium di-oxide nanoparticles–Synthesized via sol–gel route. Spectrochim. Acta. A 2014, 117, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Nolan, N.T.; Seery, M.K.; Pillai, S.C. Spectroscopic Investigation of the Anatase-to-Rutile Transformation of Sol−Gel-Synthesized TiO2 Photocatalysts. J. Phys. Chem. C 2009, 113, 16151–16157. [Google Scholar] [CrossRef]
- Hu, Y.; Tsai, H.-L.; Huang, C.-L. Effect of brookite phase on the anatase–rutile transition in titania nanoparticles. J. Eur. Ceram. Soc. 2003, 23, 691–696. [Google Scholar] [CrossRef]
- Zhang, Q.; Gao, L. Preparation of oxide nanocrystals with tunable morphologies by the moderate hydrothermal method: Insights from rutile TiO2. Langmuir 2003, 19, 967–971. [Google Scholar] [CrossRef]
- Vu, T.H.T.; Au, H.T.; Tran, L.T.; Nguyen, T.M.T.; Tran, T.T.T.; Pham, M.T.; Do, M.H.; Nguyen, D.L. Synthesis of titanium dioxide nanotubes via one-step dynamic hydrothermal process. J. Mater. Sci. 2014, 49, 5617–5625. [Google Scholar] [CrossRef]
- Wu, J.-J.; Yu, C.-C. Aligned TiO2 nanorods and nanowalls. J. Phys. Chem. B 2004, 108, 3377–3379. [Google Scholar] [CrossRef]
- Wu, J.-M.; Shih, H.C.; Wu, W.-T.; Tseng, Y.-K.; Chen, I.-C. Thermal evaporation growth and the luminescence property of TiO2 nanowires. J. Cryst. Growth 2005, 281, 384–390. [Google Scholar] [CrossRef]
- Wu, J.-M.; Shih, H.C.; Wu, W.-T. Electron field emission from single crystalline TiO2 nanowires prepared by thermal evaporation. Chem. Phys. Lett. 2005, 413, 490–494. [Google Scholar] [CrossRef]
- Acayanka, E.; Djowe, A.T.; Laminsi, S.; Tchoumkwe, C.; Nzali, S.; Mbouopda, A.P.; Ndifon, P.; Gaigneaux, E.M. Plasma-assisted synthesis of TiO2 nanorods by gliding arc discharge processing at atmospheric pressure for photocatalytic applications. Plasma Chem. Plasma Process. 2013, 33, 725–735. [Google Scholar] [CrossRef]
- Gressel-Michel, E.; Chaumont, D.; Stuerga, D. From a microwave flash-synthesized TiO2 colloidal suspension to TiO2 thin films. J. Colloid Interface Sci. 2005, 285, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-S.; Lee, M.S.; Park, S.S.; Lee, G.-D. Synthesis of nanosized TiO2/SiO2 particles in the microemulsion and their photocatalytic activity on the decomposition of p-nitrophenol. Catal. Today 2003, 87, 99–105. [Google Scholar] [CrossRef]
- Laranjo, M.T.; Ricardi, N.C.; Arenas, L.T.; Benvenutti, E.V.; de Oliveira, M.C.; Santos, M.J.L.; Costa, T.M.H. TiO2 and TiO2/SiO2 nanoparticles obtained by sol–gel method and applied on dye sensitized solar cells. J. Sol.-Gel Sci. Technol. 2014, 72, 273–281. [Google Scholar] [CrossRef]
- Bakar, S.A.; Soltani, N.; Yunus, W.M.M.; Saion, E.; Bahrami, A. Structural and paramagnetic behavior of spinel NiCr2O4 nanoparticles synthesized by thermal treatment method: Effect of calcination temperature. Solid State. Commun. 2014, 192, 15–19. [Google Scholar] [CrossRef]
- Naseri, M.G.; Saion, E.B.; Ahangar, H.A.; Shaari, A.H. Fabrication, characterization, and magnetic properties of copper ferrite nanoparticles prepared by a simple, thermal-treatment method. Mater. Res. Bull. 2013, 48, 1439–1446. [Google Scholar] [CrossRef]
- Giri, N.; Natarajan, R.; Gunasekaran, S.; Shreemathi, S. 13C-NMR and FTIR spectroscopic study of blend behavior of PVP and nano silver particles. Arch. Appl. Sci. Res. 2011, 3, 624–630. [Google Scholar]
- Soltani, N.; Dehzangi, A.; Kharazmi, A.; Saion, E.; Yunus, W.M.M.; Majlis, B.Y.; Zare, M.R.; Gharibshahi, E.; Khalilzadeh, N. Structural, optical and electrical properties of ZnS nanoparticles affecting by organic coating. Chalcogenide Lett. 2014, 11, 79–90. [Google Scholar]
- Goodarz Naseri, M.; Saion, E.B.; Kamali, A. An overview on nanocrystalline ZnFe2O4, MnFe2O4, and CoFe2O4 synthesized by a thermal treatment method. ISRN Nanotechnol. 2012, 2012. [Google Scholar] [CrossRef]
- Ba-Abbad, M.M.; Kadhum, A.A.H.; Mohamad, A.B.; Takriff, M.S.; Sopian, K. Synthesis and catalytic activity of TiO2 nanoparticles for photochemical oxidation of concentrated chlorophenols under direct solar radiation. Int. J. Electrochem. Sci. 2012, 7, 4871–4888. [Google Scholar]
- Thangavelu, K.; Annamalai, R.; Arulnandhi, D. Preparation and characterization of nanosized TiO2 powder by sol–gel precipitation route. Int. J. Emerg. Technol. Adv. Eng. 2013, 3, 636–639. [Google Scholar]
- White, L.; Koo, Y.; Yun, Y.; Sankar, J. TiO2 deposition on AZ31 magnesium alloy using plasma electrolytic oxidation. J. Nanomater. 2013, 2013, 280–289. [Google Scholar] [CrossRef]
- Ohtani, B.; Prieto-Mahaney, O.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photochem. Photobiol. A 2010, 216, 179–182. [Google Scholar] [CrossRef]
- Gene, S.A.; Saion, E.; Shaari, A.H.; Kamaruddin, M.A.; Al-Hada, N.M.; Kharazmi, A. Structural, optical, and magnetic characterization of spinel zinc chromite nanocrystallines synthesised by thermal treatment method. J. Nanomater. 2014, 2014, 177–186. [Google Scholar] [CrossRef]
- Kominami, H.; Kohno, M.; Takada, Y.; Inoue, M.; Inui, T.; Kera, Y. Hydrolysis of titanium alkoxide in organic solvent at high temperatures: A new synthetic method for nanosized, thermally stable titanium(IV) oxide. Ind. Eng. Chem. Res. 1999, 38, 3925–3931. [Google Scholar] [CrossRef]
- Yu, J.G.; Yu, H.G.; Cheng, B.; Zhao, X.J.; Yu, J.C.; Ho, W.K. The effect of calcination temperature on the surface microstructure and photocatalytic activity of TiO2 thin films prepared by liquid phase deposition. J. Phys. Chem. B 2003, 107, 13871–13879. [Google Scholar] [CrossRef]
- Ananth, S.; Arumanayagam, T.; Vivek, P.; Murugakoothan, P. Direct synthesis of natural dye mixed titanium dioxide nano particles by sol–gel method for dye sensitized solar cell applications. Opt. J. Light Electron Opt. 2014, 125, 495–498. [Google Scholar] [CrossRef]
- Torrent, J.; Barrón, V. Diffuse reflectance spectroscopy of iron oxides. In Encyclopedia of Surface and Colloid Science; Taylor and Francis: New York, NY, USA, 2006; pp. 1438–1446. [Google Scholar]
- Lee, P.J.; Saion, E.; Al-Hada, N.M.; Soltani, N. A Simple Up-Scalable Thermal Treatment Method for Synthesis of ZnO Nanoparticles. Metals 2015, 5, 2383–2392. [Google Scholar] [CrossRef]
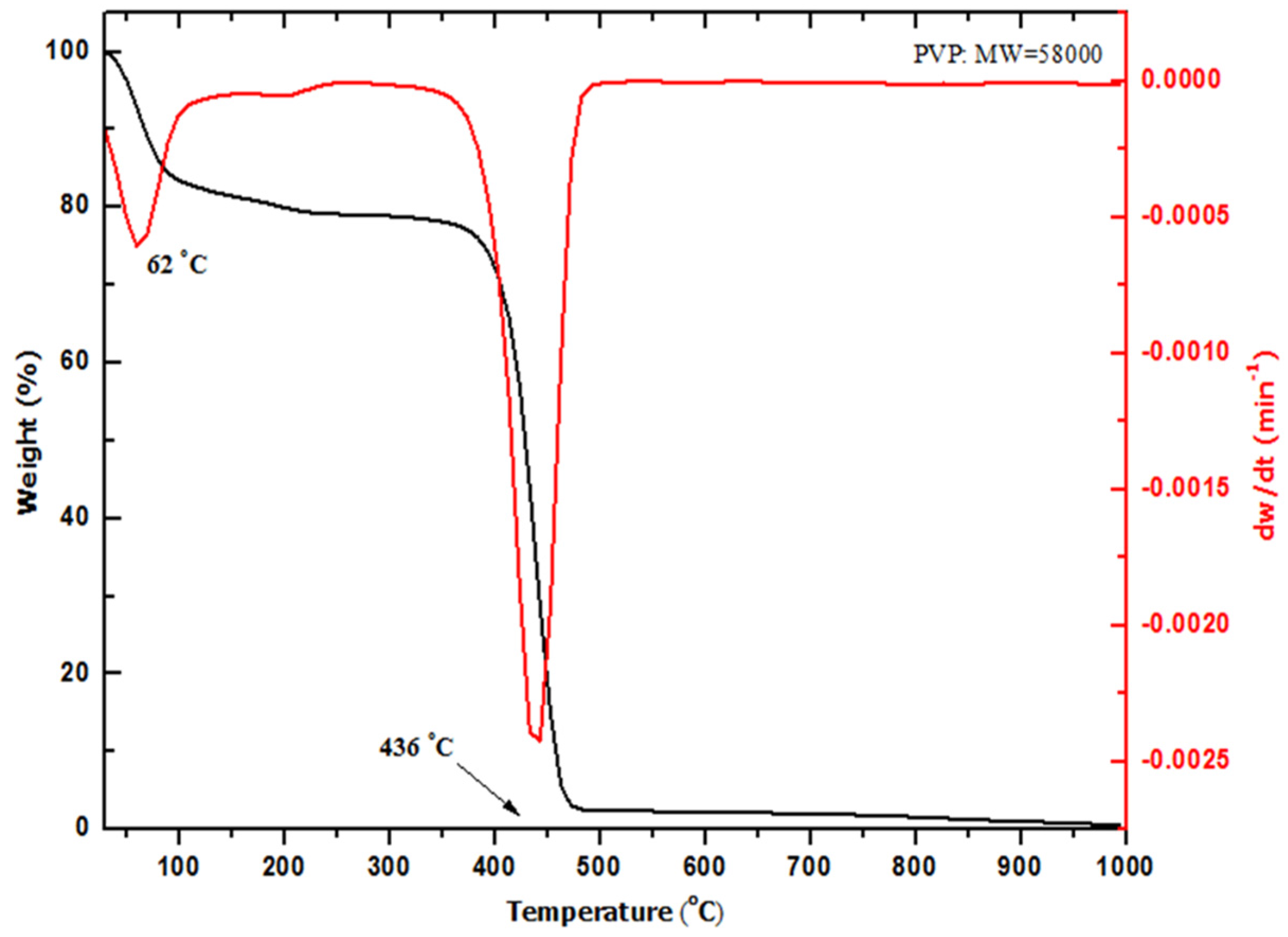
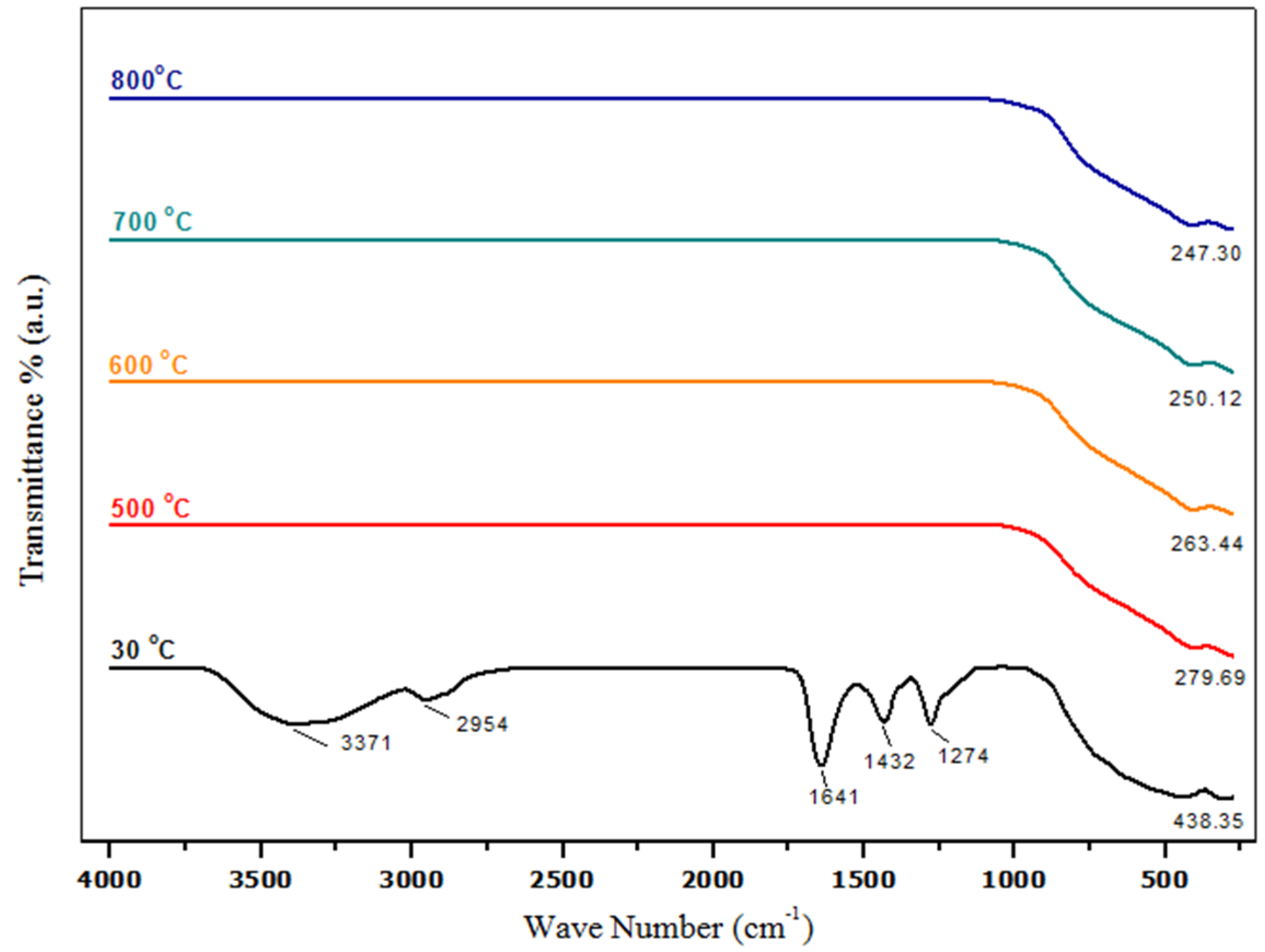
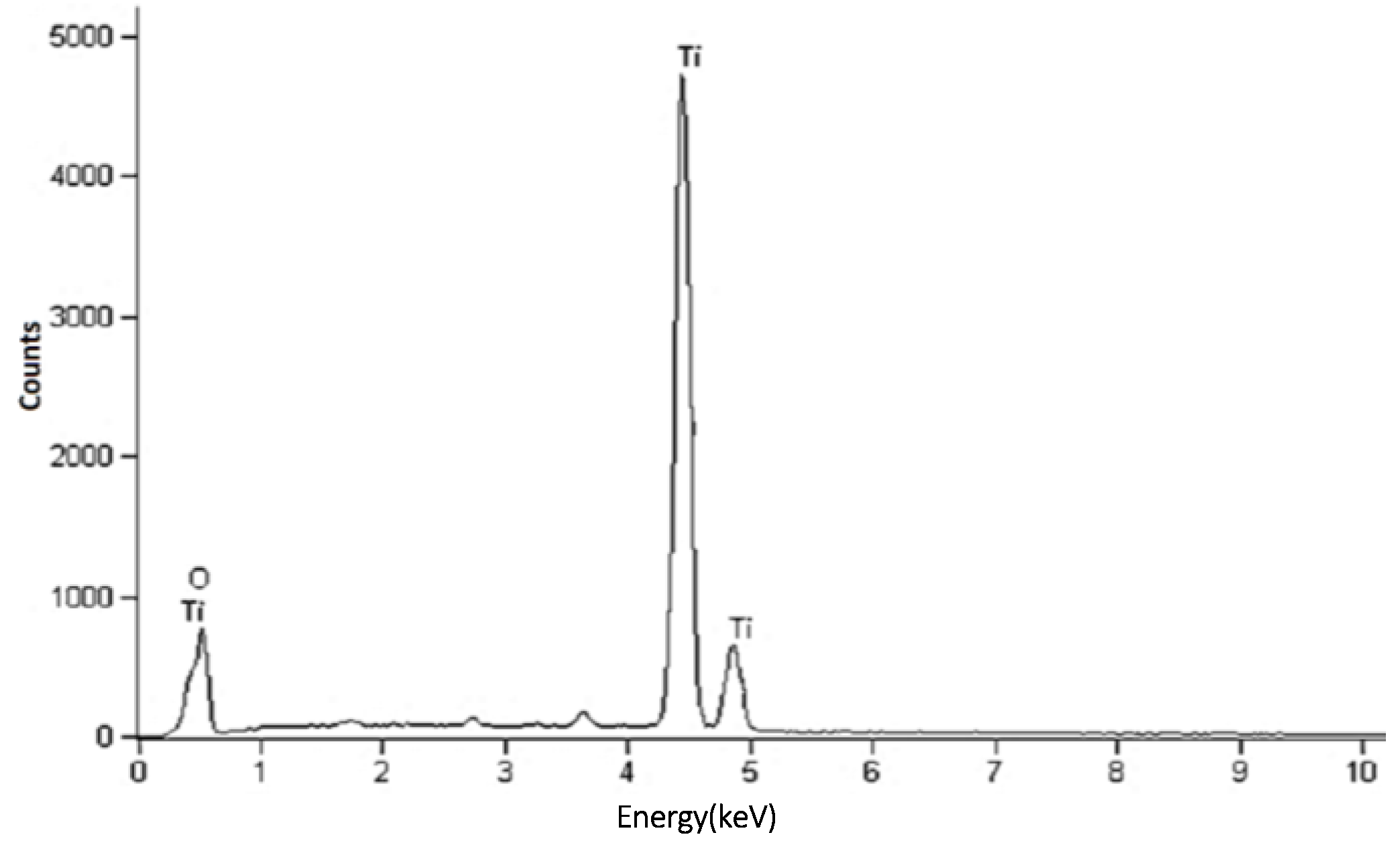
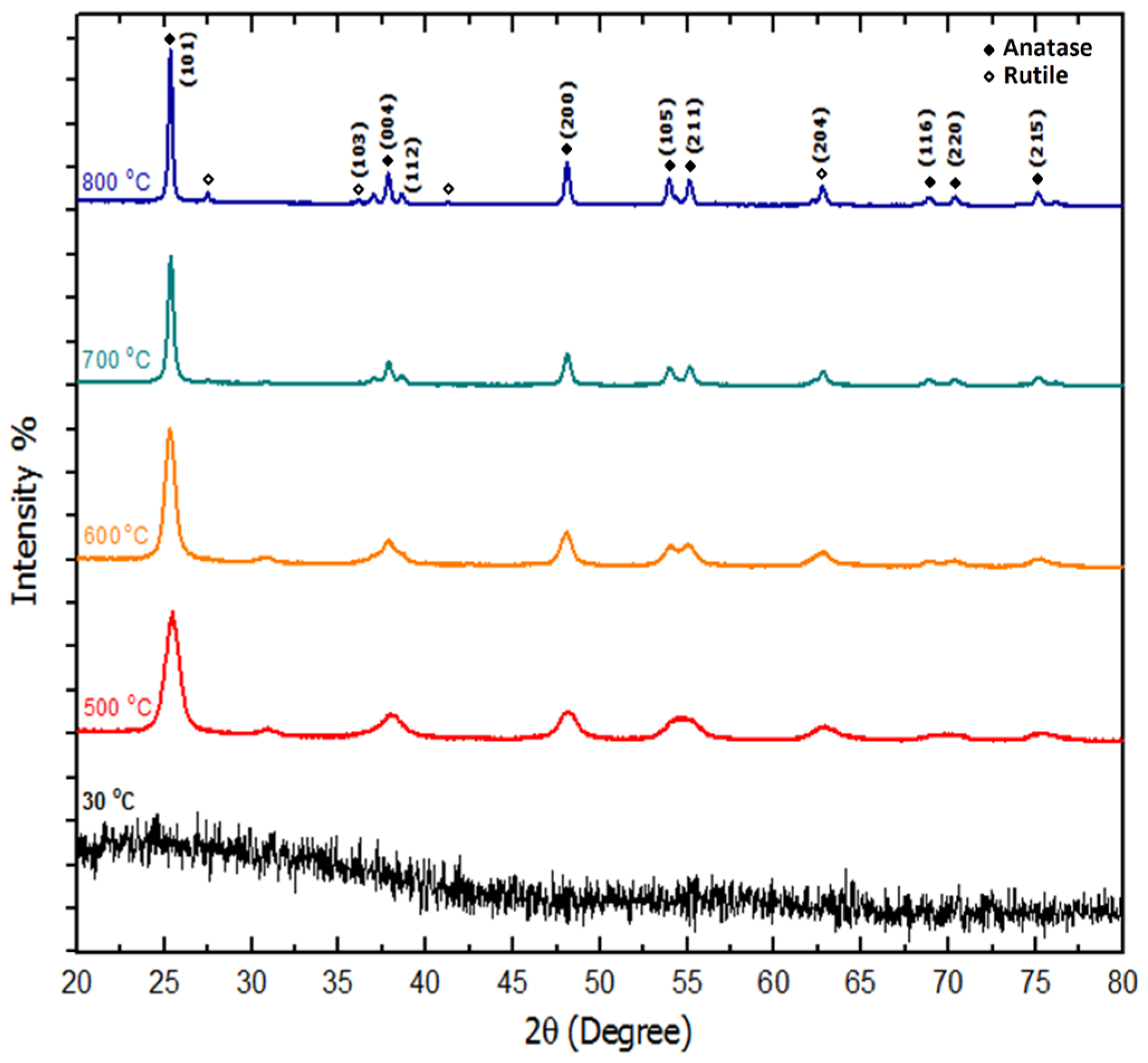
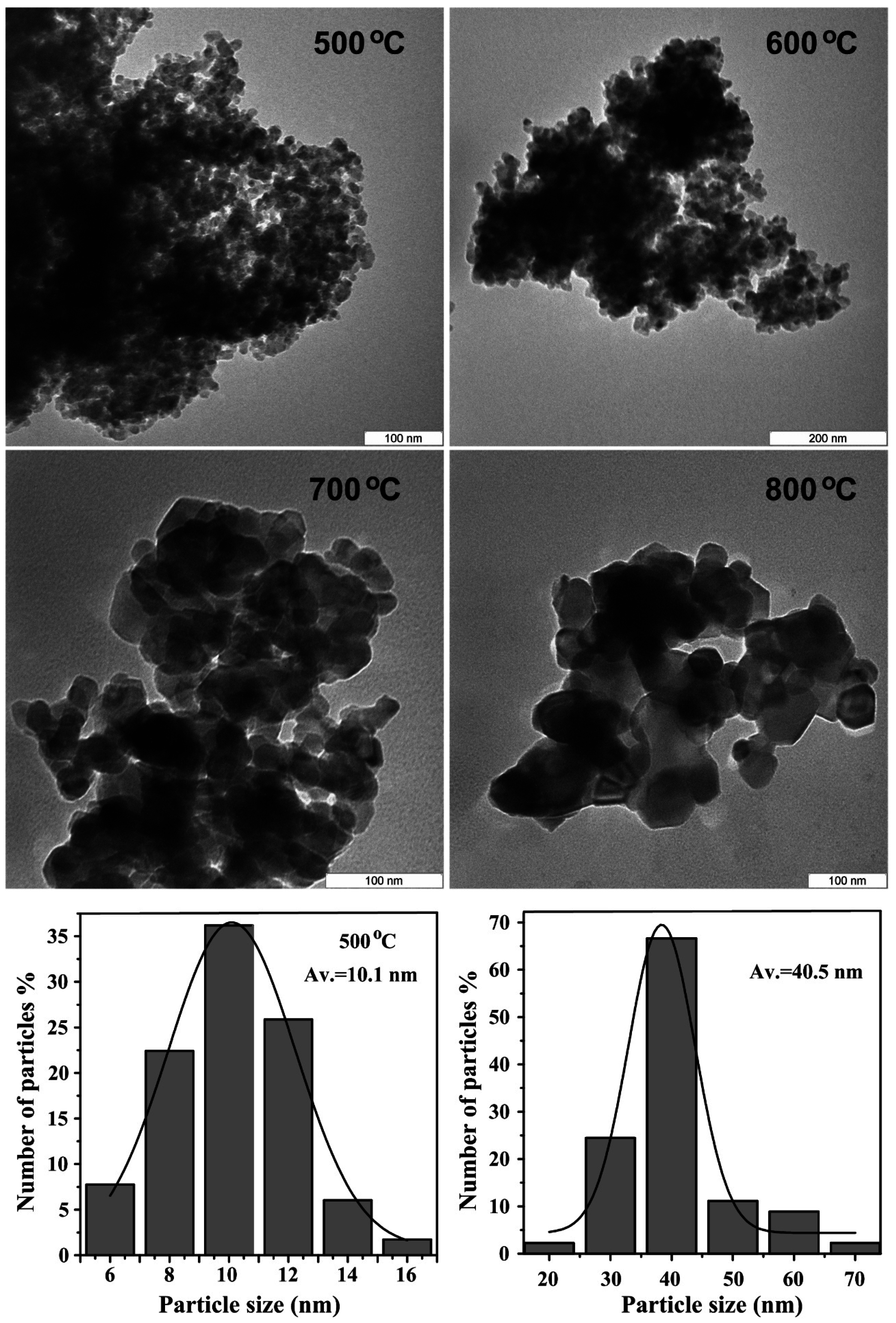
| Temp. (°C) | 2θ° | Crystallite Size from XRD (±0.3 nm) | Particle Size from TEM (±0.1 nm) |
|---|---|---|---|
| 500 | 25.436 | 8.4 | 10.1 |
| 600 | 25.352 | 28.4 | 31.3 |
| 700 | 25.300 | 36.7 | 38.0 |
| 800 | 25.241 | 42.9 | 40.5 |
| Temperature (°C) | Particle Size (±0.1 nm) | Eg (±0.01 eV) |
|---|---|---|
| 500 | 10.1 | 3.40 |
| 600 | 31.3 | 3.38 |
| 700 | 38.0 | 3.36 |
| 800 | 40.5 | 3.12 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keiteb, A.S.; Saion, E.; Zakaria, A.; Soltani, N.; Abdullahi, N. A Modified Thermal Treatment Method for the Up-Scalable Synthesis of Size-Controlled Nanocrystalline Titania. Appl. Sci. 2016, 6, 295. https://doi.org/10.3390/app6100295
Keiteb AS, Saion E, Zakaria A, Soltani N, Abdullahi N. A Modified Thermal Treatment Method for the Up-Scalable Synthesis of Size-Controlled Nanocrystalline Titania. Applied Sciences. 2016; 6(10):295. https://doi.org/10.3390/app6100295
Chicago/Turabian StyleKeiteb, Aysar Sabah, Elias Saion, Azmi Zakaria, Nayereh Soltani, and Nura Abdullahi. 2016. "A Modified Thermal Treatment Method for the Up-Scalable Synthesis of Size-Controlled Nanocrystalline Titania" Applied Sciences 6, no. 10: 295. https://doi.org/10.3390/app6100295
APA StyleKeiteb, A. S., Saion, E., Zakaria, A., Soltani, N., & Abdullahi, N. (2016). A Modified Thermal Treatment Method for the Up-Scalable Synthesis of Size-Controlled Nanocrystalline Titania. Applied Sciences, 6(10), 295. https://doi.org/10.3390/app6100295