Development of an Ionic Liquid-Based Ultrasonic/Microwave-Assisted Simultaneous Distillation and Extraction Method for Separation of Camptothecin, 10-Hydroxycamptothecin, Vincoside-Lactam, and Essential Oils from the Fruits of Camptotheca acuminata Decne
Abstract
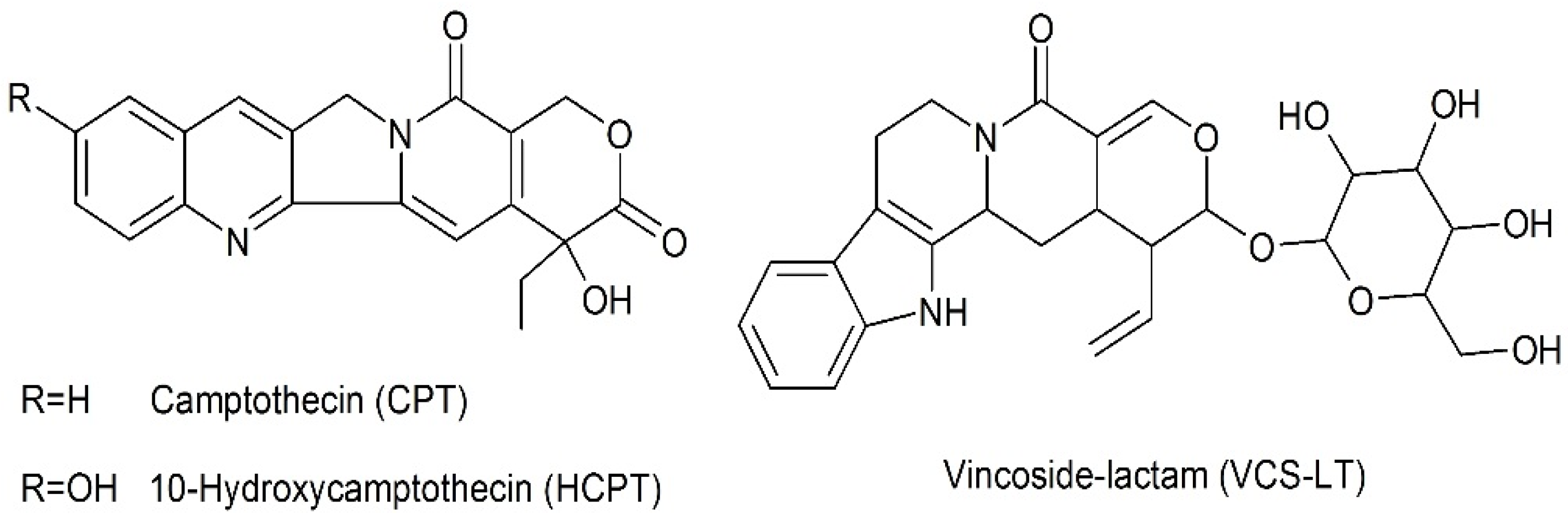
:1. Introduction
2. Experimental
2.1. Plant Materials
2.2. Chemicals
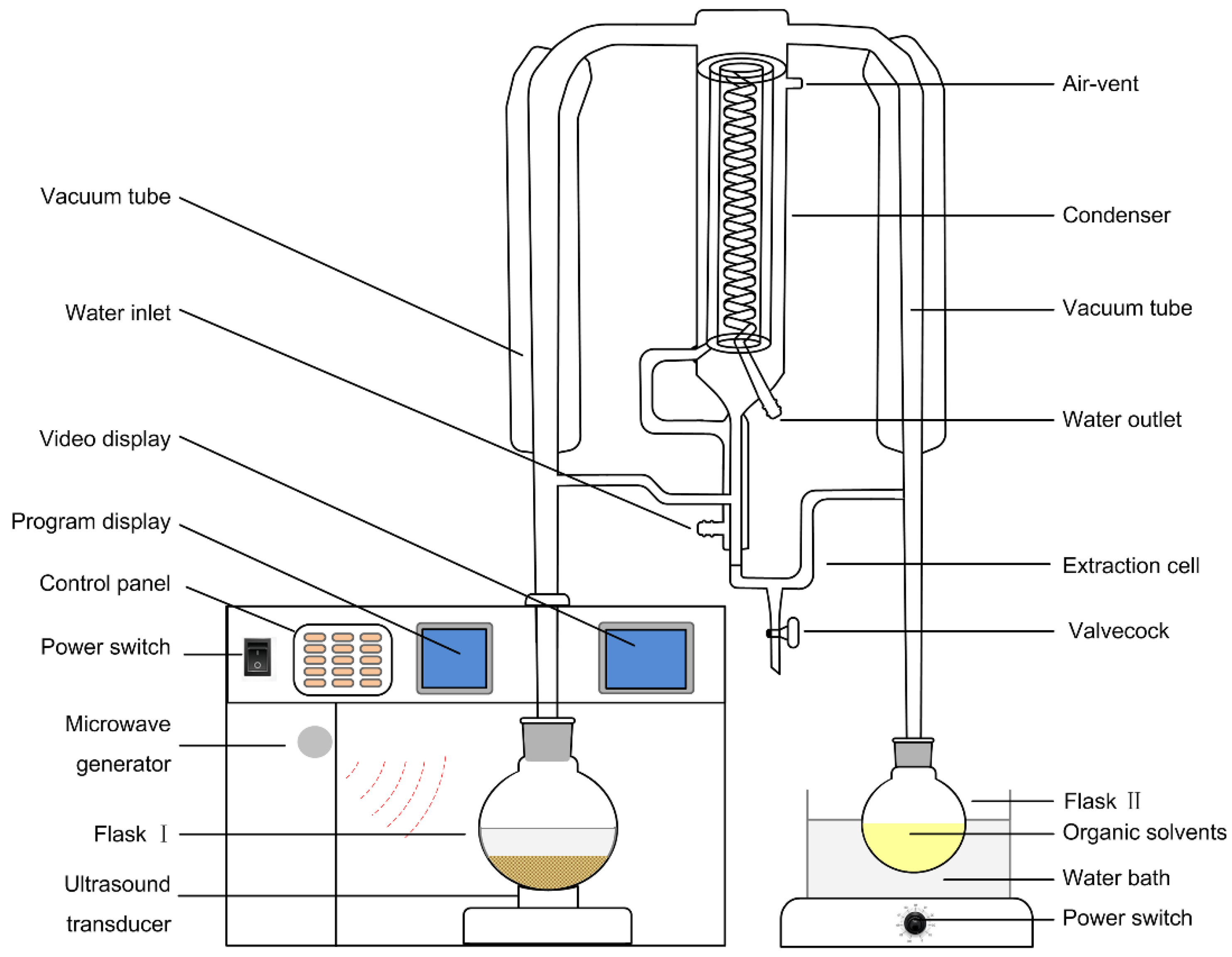
2.3. Apparatus
2.4. Extraction Procedure
2.4.1. Conventional HRE
2.4.2. UAE
2.4.3. Conventional HDE
2.4.4. UMASDE
2.4.5. MASDE
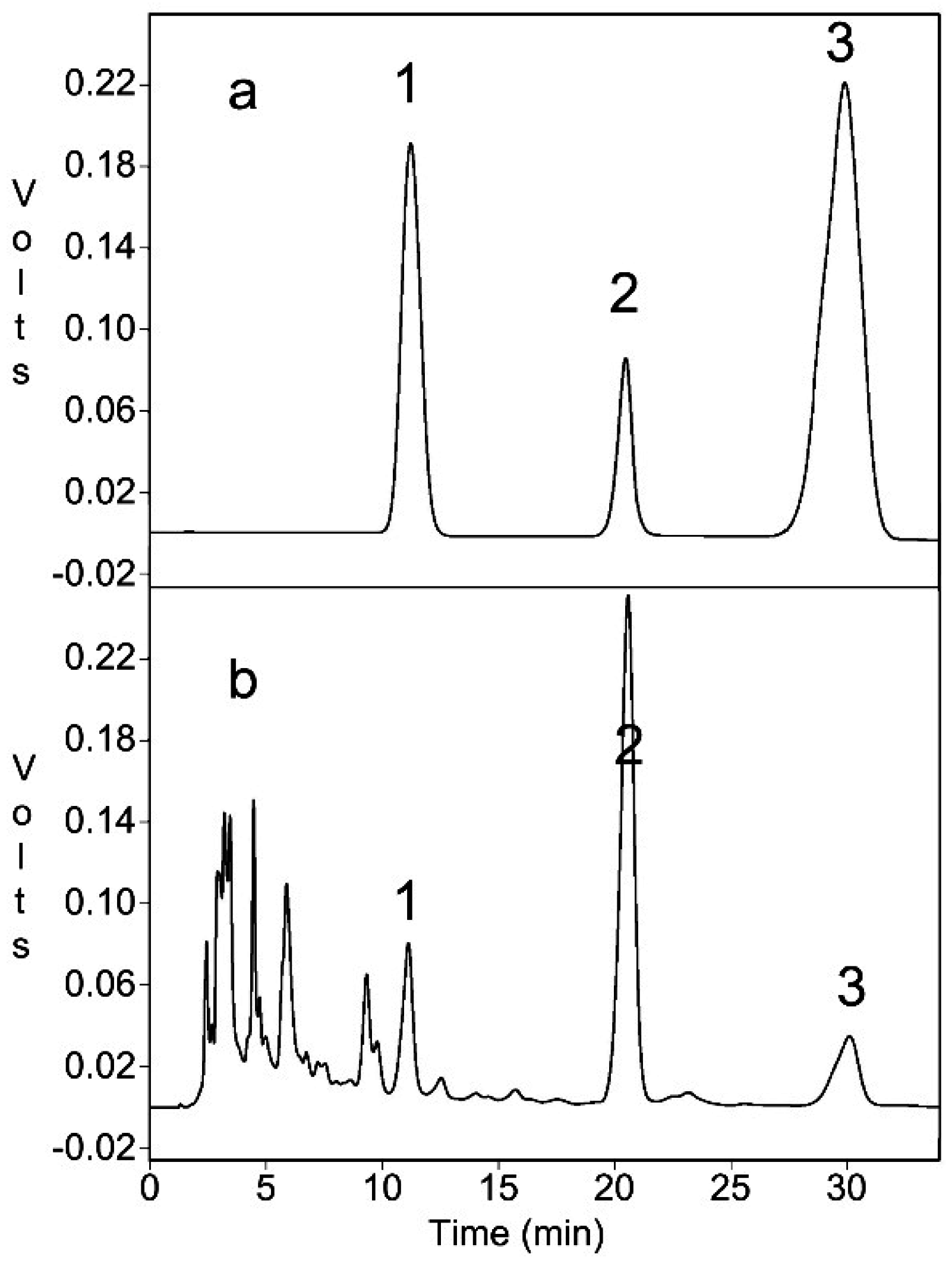
2.5. HPLC Determination of CPT, HCPT, and VCS-LT
2.6. GC-MS Analysis of EOs
2.7. Experimental Design
2.7.1. IL-UMASDE Optimization Using RSM
2.7.2. Statistical Analysis
3. Results and Discussion
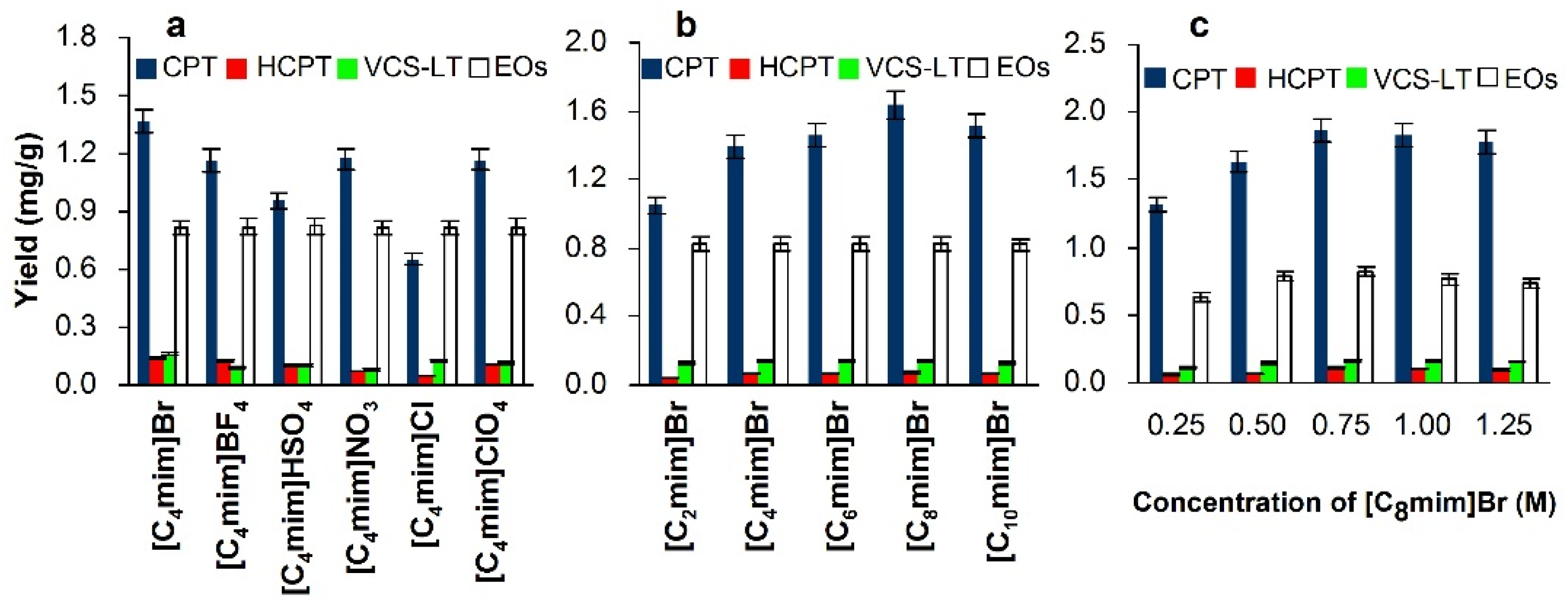
3.1. Choosing an Appropriate IL
3.2. Optimization of IL Concentration
3.3. Optimization of Single Extraction Condition
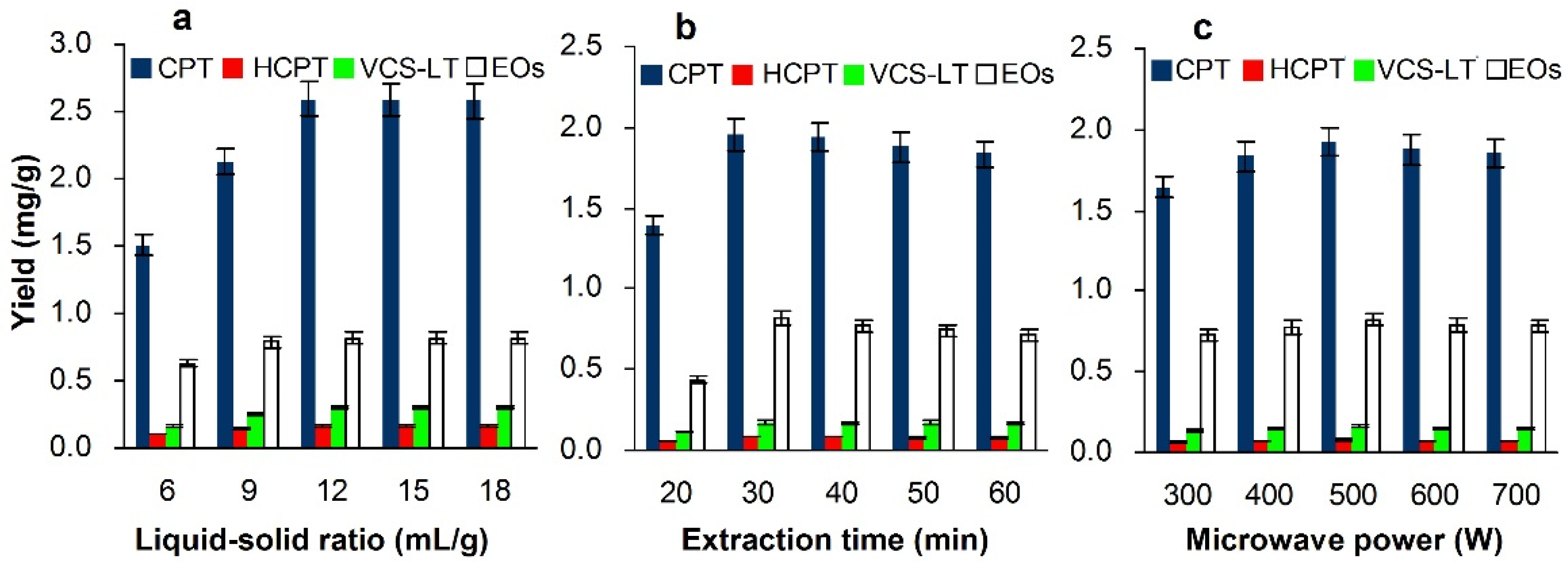
3.3.1. Effect of Liquid–Solid Ratio
3.3.2. Effect of Extraction Time
3.3.3. Effect of Microwave Power
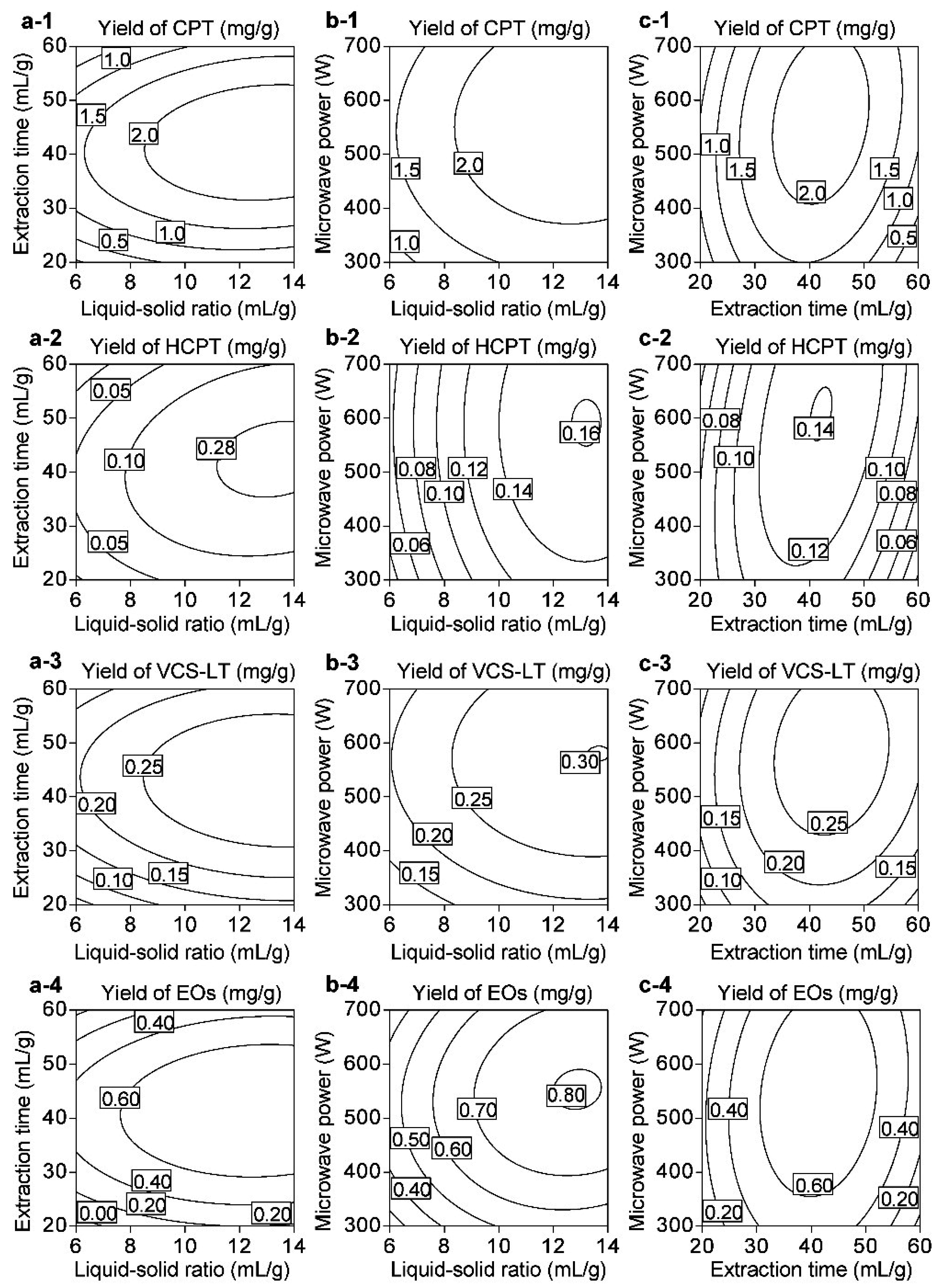
3.4. Optimization of IL-UMASDE
3.4.1. RSM and Model Fit
3.4.2. Validation of the Optimum Extraction Conditions
3.5. Comparison of Different Extraction Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yang, L.; Li, X.; Zhao, C.; Li, J.; Zu, Y. Distribution of camptothecin and 10-hydroxycamptothecin in plant of Camptotheca acuminata Decne. Plant Physiol. Commun. 2008, 44, 873–876. [Google Scholar]
- Wall, M.E.; Wani, M.; Cook, C.; Palmer, K.; McPhail, A.; Sim, G. Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from Camptotheca acuminata. J. Am. Chem. Soc. 1966, 88, 3888–3889. [Google Scholar] [CrossRef]
- Hsiang, Y.; Hertzberg, R.; Hecht, S.; Liu, L. Camptothecin induces protein-linked DNA breaks via mammalian dna topoisomerase I. J. Biol. Chem. 1985, 260, 14873–14878. [Google Scholar] [PubMed]
- Palumbo, M.; Sissi, C.; Gatto, B.; Moro, S.; Zagotto, G. Quantitation of camptothecin and related compounds. J. Chromatogr. B Biomed. Sci. Appl. 2001, 764, 121–140. [Google Scholar] [CrossRef]
- Lorence, A.; Nessler, C. Molecules of interest camptothecin, over four decades of surprising findings. Phytochemistry 2004, 65, 2735–2749. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Perez-Soler, R.; Tseng, M. Effect of DNA topoisomerase I inhibitor, 10-hydroxycamptothecin, on the structure and function of nuclei and nuclear matrix in bladder carcinoma MBT-2 cells. Anticancer Res. 1993, 13, 1613–1617. [Google Scholar] [PubMed]
- Zhang, X.; Qing, C.; Xu, B. Apoptosis induction and cell cycle perturbation in human hepatoma Hep G2 cells by 10-hydroxycamptothecin. Anticancer Drugs 1999, 10, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qing, C.; Xu, B. Changes in the protein spectrum of mitochondria isolated from hydroxycamptothecin-treated hepatoma cells. Anticancer Drugs 2007, 18, 1045–1052. [Google Scholar]
- Yin, F.; Hu, L. Two DNA topoisomerase I inhibitors from Camptotheca acuminata Decne. (Nyssaceae). Chin. J. Nat. Med. 2005, 3, 21–24. [Google Scholar]
- Yuan, D.; Ma, B.; Wu, C.; Yang, J.; Zhang, L.; Liu, S.; Wu, L.; Kano, Y. Alkaloids from the leaves of Uncaria rhynchophylla and their inhibitory activity on NO production in lipopolysaccharide-activated microglia. J. Nat. Prod. 2008, 71, 1271–1274. [Google Scholar] [CrossRef] [PubMed]
- Burt, A. Essential oils: Their antibacterial properties and potential applications in foods: A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Milhau, G.; Valentin, A.; Benoit, F.; Mallie, M.; Bastide, J.; Pelissier, Y.; Bessiere, J. In vitro antimicrobial activity of eight essential oils. J. Essent. Oil Res. 1997, 9, 329–333. [Google Scholar] [CrossRef]
- Darokar, M.; Mathur, A.; Dwivedi, S.; Bhalla, R.; Khanuja, S.; Kumar, S. Detection of antibacterial activity in the floral petals of some higher plants. Curr. Sci. 1998, 75, 187–189. [Google Scholar]
- Yu, T.; Wang, Y.; Yin, L.; Zu, Y. Study on the chemical composition of volatile oil from seeds of Camptotheca acuminate. Bull. Bot. Res. 1999, 19, 179–182. [Google Scholar]
- Yu, M.; Wang, B.; Zhang, Y. The extraction, separation and purification of alkaloidsin the natural medicine. J. Chem. Pharm. Res. 2014, 6, 338–345. [Google Scholar]
- Sharma, S.; Kumar, A.; Namdeo, A. Pharmacognostical and phytochemical analysis of Nothapodytes nimmoniana stem. Int. J. Pharm. Sci. 2012, 4, 455–459. [Google Scholar]
- Shi, W.; Zu, Y.; Zhao, C.; Yang, L. Homogenated extraction of camptothecin from Camptotheca acuminata Decne. seed and leaves. J. For. Res. 2009, 20, 168–170. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, Y.; Liu, D.; Liu, Z. Extraction and composition of three naturally occurring anti-cancer alkaloids in Camptotheca acuminata seed and leaf extracts. Phytomedicine 2007, 14, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Fulzele, D.; Satdive, K. Comparison of techniques for the extraction of the anti-cancer drug camptothecin from Nothapodytes foetida. J. Chromatogr. 2005, 1063, 9–13. [Google Scholar] [CrossRef]
- Ikeda, M. Public health problems of organic solvents. Toxicol. Lett. 1992, 64–65, 191–201. [Google Scholar] [CrossRef]
- Naushad, M.; Alothman, Z.; Khan, A.; Ali, M. Effect of ionic liquid on activity, stability, and structure of enzymes: A review. Int. J. Boil. Macromol. 2012, 51, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Passos, H.; Freire, M.; Coutinho, J. Ionic liquid solutions as extractive solvents for value-added compounds from biomass. Green Chem. 2014, 16, 4786–4815. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wang, S.; Yang, L.; Zu, Y.; Yang, F.; Zhang, L.; Zhang, Z. Ionic liquid-aqueous solution ultrasonic-assisted extraction of camptothecin and 10-hydroxycamptothecin from Camptotheca acuminata samara. Chem. Eng. Process. 2012, 57–58, 59–64. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Li, Y.; Chi, R. Optimization of ionic liquid-based microwave-assisted extraction of isoflavones from Radix puerariae by response surface methodology. Sep. Purif. Technol. 2014, 129, 71–79. [Google Scholar] [CrossRef]
- Liu, Z.; Jia, J.; Chen, F.; Yang, F.; Zu, Y.; Yang, L. Development of an ionic liquid-based microwave-assisted method for the extraction and determination of taxifolin in different parts of Larix gmelinii. Molecules 2014, 19, 19471–19490. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lu, Z.; Zhao, C.; Yang, L.; Fu, Y.; Shi, K.; He, X.; Li, Z.; Zu, Y. Ionic liquid-based ultrasound/microwave-assisted extraction of 1,4-benzoxazin-3-one and 6-methoxy-benzoxazolin-2-one from maize (Zea mays L.) seedlings. J. Sep. Sci. 2015, 38, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Kong, W.; Yang, X.; Xie, L.; Wen, J.; Yang, M. GC–MS combined with chemometric techniques for the X. Quality control and original discrimination of Curcumae longae rhizome: Analysis of essential oils. J. Sep. Sci. 2014, 37, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sun, Z.; Huang, C.; Lin, S.; He, D. The comparation of chemical component analysis about volatile oil from Cinnamomum cassia bark, Cinnamomum cassia Presl and Cinnamomum cassia leaf by gas chromatography–mass spectrometry. Food Res. Dev. 2010, 31, 144–147. [Google Scholar]
- Cassel, E.; Vargas, R.; Martinez, N.; Lorenzo, D.; Dellacassa, E. Steam distillation modeling for essential oil extraction process. Ind. Crops Prod. 2009, 29, 171–176. [Google Scholar] [CrossRef]
- Okoh, O.; Sadimenko, A.; Afolayan, A. Comparative evaluation of the antibacterial activities of the essential oils of Rosmarinus officinalis L. obtained by hydrodistillation and solvent free microwave extraction methods. Food Chem. 2010, 120, 308–312. [Google Scholar] [CrossRef]
- Patra, J.; Kim, S.; Baek, K. Antioxidant and free radical-scavenging potential of essential oil from Enteromorpha linza L. prepared by microwave-assisted hydrodistillation. J. Food Biochem. 2015, 39, 80–90. [Google Scholar] [CrossRef]
- Benkaci-Ali, F.; Akloul, R.; Boukenouche, A.; Pauw, E. Chemical composition of the essential oil of Nigella sativa seeds extracted by microwave steam distillation. J. Essent. Oil Bear. Plants 2013, 16, 781–794. [Google Scholar] [CrossRef]
- Gavrila, L.; Gavrila, A.; Ivopol, M.; Ivopol, G.; Popescu, M.; Mircioaga, N. Microwave assisted extraction of essential oils from enzymatically pretreated lavender (Lavandula angustifolia Miller). Cent. Eur. J. Chem. 2014, 112, 829–836. [Google Scholar]
- Kraujalyte, V.; Leitner, E.; Venskutonis, P. Characterization of Aronia melanocarpa volatiles by headspace-solid-phase microextraction (HS-SPME) simultaneous distillation/extraction (SDE) and gas chromatography-olfactometry (GC-O) methods. J. Agric. Food Chem. 2013, 61, 4728–4736. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zu, Y.; Yang, L. A novel approach for isolation of essential oil from fresh leaves of Magnolia sieboldii using microwave-assisted simultaneous distillationand extraction. Sep. Purif. Technol. 2015, 154, 271–280. [Google Scholar] [CrossRef]
- Zhao, C.; Li, Z.; Li, C.; Yang, L.; Yao, L.; Fu, Y.; He, X.; Shi, K.; Lu, Z. Optimized extraction of polysaccharides from Taxus chinensis var. mairei fruits and its antitumor activity. Int. J. Biol. Macromol. 2015, 75, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y. High-Performance Liquid Chromatography (HPLC): Principles, Procedures and Practices; Nova Science Publisher: New York, NY, USA, 2014. [Google Scholar]
- Zhao, C.; Li, C.; Wang, L.; Zu, Y.; Yang, L. Determination of camptothecin and 10-hydroxycamptothecin in Camptotheca acuminata by LC-ESI-MS/MS. Anal. Lett. 2010, 43, 2681–2693. [Google Scholar] [CrossRef]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured: Carol Stream, IL, USA, 2007. [Google Scholar]
- Bezerra, M.; Santelli, R.; Oliveira, E.; Villar, L.; Escaleira, L. Response surface methodology (RSM) as a tool for optimization in analytical. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Bica, K.; Gaertner, P.; Rogers, R. Ionic liquids and fragrances—Direct isolation of orange essential oil. Green Chem. 2011, 13, 1997–1999. [Google Scholar] [CrossRef]
- Fukaya, Y.; Hayashi, K.; Wada, M.; Ohno, H. Cellulose dissolution with polar ionic liquids under mild conditions: Required factors for anions. Green Chem. 2008, 10, 44–46. [Google Scholar] [CrossRef]
- Huddleston, J.; Visser, A.; Reichert, W.; Willauer, H.; Broker, G.; Rogers, R. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem. 2001, 3, 156–164. [Google Scholar] [CrossRef]
- Pinkert, A.; Marsh, K.; Pang, S.; Staiger, M. Ionic liquids and their interaction with cellulose. Chem. Rev. 2009, 109, 6712–6728. [Google Scholar] [CrossRef] [PubMed]
- Remsing, R.; Swatloski, R.; Rogers, R.; Moyna, G. Mechanism of cellulose dissolution in the ionic liquid 1-n-butyl-3-methylimidazolium chloride: A 13C and 35/37Cl NMR relaxation study on model systems. Chem. Commun. 2006, 12, 1271–1273. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Wang, H.; Zhu, S.; Chen, S.; Zhang, M.; Wang, Z. Ionic liquids based simultaneous ultrasonic and microwave assisted extraction of phenolic compounds from burdock leaves. Anal. Chim. Acta 2012, 716, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Lu, Y.; Hu, R.; Chen, J.; Zhang, Z.; Pan, Y. Application of ionic liquids based microwave-assisted extraction of three alkaloids N-nornuciferine, O-nornuciferine, and nuciferine from lotus leaf. Talanta 2010, 80, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Henderson, L.; Byrne, N. Rapid and efficient protic ionic liquid-mediated pinacol rearrangements under microwave irradiation. Green Chem. 2011, 13, 813–816. [Google Scholar] [CrossRef]
- Zuo, Y.; Zhang, K.; Wu, J.; Rego, C.; Fritz, J. An accurate and non-destructive GC method for determination of cocaine on US paper currency. J. Sep. Sci. 2008, 31, 2444–2450. [Google Scholar] [CrossRef] [PubMed]

| Runs | Factors | Yields (mg/g) | |||||
|---|---|---|---|---|---|---|---|
| X1 (mL/g) a | X2 (min) b | X3 (W) c | CPT | HCPT | VCS-LT | EOs | |
| 1 | 1 (16) | −1 (20) | −1 (350) | 1.267 | 0.115 | 0.181 | 0.423 |
| 2 | −1 (8) | −1 (20) | 1 (650) | 1.303 | 0.091 | 0.192 | 0.405 |
| 3 | −1 (8) | −1 (20) | −1 (350) | 1.187 | 0.084 | 0.145 | 0.388 |
| 4 | 1 (16) | 1 (40) | 1 (650) | 2.154 | 0.154 | 0.274 | 0.647 |
| 5 | 1 (16) | 1 (40) | −1 (350) | 1.328 | 0.108 | 0.186 | 0.415 |
| 6 | −1 (8) | 1 (40) | −1 (350) | 1.099 | 0.062 | 0.167 | 0.361 |
| 7 | 0 (12) | 0 (30) | 0 (500) | 2.445 | 0.160 | 0.292 | 0.841 |
| 8 | 1 (16) | −1 (20) | 1 (650) | 1.428 | 0.102 | 0.229 | 0.481 |
| 9 | 0 (12) | 0 (30) | 0 (500) | 2.463 | 0.164 | 0.297 | 0.790 |
| 10 | −1 (8) | 1 (40) | 1 (650) | 1.532 | 0.084 | 0.236 | 0.459 |
| 11 | 0 (12) | 0 (30) | 0 (500) | 2.353 | 0.157 | 0.286 | 0.805 |
| 12 | 0 (12) | 0 (30) | 0 (500) | 2.286 | 0.153 | 0.281 | 0.738 |
| 13 | −1.68 (5.27) | 0 (30) | 0 (500) | 1.184 | 0.031 | 0.157 | 0.379 |
| 14 | 0 (12) | 0 (30) | −1.68 (247.73) | 1.221 | 0.117 | 0.142 | 0.392 |
| 15 | 0 (12) | −1.68 (13.18) | 0 (500) | 1.094 | 0.079 | 0.163 | 0.339 |
| 16 | 0 (12) | 0 (30) | 0 (500) | 2.385 | 0.149 | 0.295 | 0.778 |
| 17 | 0 (12) | 1.68 (46.82) | 0 (500) | 1.657 | 0.118 | 0.243 | 0.501 |
| 18 | 0 (12) | 0 (30) | 1.68 (752.27) | 2.115 | 0.148 | 0.253 | 0.657 |
| 19 | 0 (12) | 0 (30) | 0 (500) | 2.296 | 0.154 | 0.276 | 0.789 |
| 20 | 1.68 (18.73) | 0 (30) | 0 (500) | 1.739 | 0.092 | 0.256 | 0.529 |
| Source a | DF | CPT | HCPT | VCS-LT | EO | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sum of Square | Mean Aquare | F Value | p-Value b | Sum of Squares | Mean Aquare | F Value | p-Value b | Sum of Square | Mean Aquare | F Value | p-Value b | Sum of Square | Mean Aquare | F Value | p-Value b | ||
| Model | 9 | 5.09 | 0.57 | 66.67 | 0.000 ++ | 2.63 × 10−2 | 2.93 × 10−3 | 41.03 | 0.000 ++ | 5.67 × 10−2 | 6.30 × 10−3 | 45.85 | 0.000 ++ | 0.59 | 6.57 × 10−2 | 51.89 | 0.000 ++ |
| X1 | 1 | 1.58 | 1.58 | 186.56 | 0.000 ++ | 1.84 × 10−2 | 1.84 × 10−2 | 257.45 | 0.000 ++ | 1.72 × 10−2 | 1.72 × 10−2 | 125.24 | 0.000 ++ | 0.19 | 0.19 | 147.09 | 0.000 ++ |
| X2 | 1 | 8.99 × 10−2 | 8.99 × 10−2 | 10.59 | 0.010 | 1.74 × 10−5 | 1.74 × 10−5 | 0.24 | 0.633 | 3.58 × 10−3 | 3.58 × 10−3 | 26.08 | 0.001 ++ | 6.16 × 10−3 | 6.16 × 10−3 | 4.87 | 0.055 |
| X3 | 1 | 0.36 | 0.36 | 42.97 | 0.000 ++ | 6.29 × 10−4 | 6.29 × 10−4 | 8.82 | 0.016 | 9.12 × 10−3 | 9.12 × 10−3 | 66.42 | 0.000 ++ | 2.51 × 10−2 | 2.51 × 10−2 | 19.87 | 0.002 ++ |
| X1X2 | 1 | 5.22 × 10−2 | 5.22 × 10−2 | 6.15 | 0.035 | 6.85 × 10−4 | 6.85 × 10−4 | 9.60 | 0.013 | 3.20 × 10−5 | 3.20 × 10−5 | 0.23 | 0.641 | 2.10 × 10−3 | 2.10 × 10−3 | 1.66 | 0.230 |
| X1X3 | 1 | 2.40 × 10−2 | 2.40 × 10−2 | 2.83 | 0.127 | 2.00 × 10−6 | 2.00 × 10−6 | 0.03 | 0.871 | 5.00 × 10−5 | 5.00 × 10−5 | 0.36 | 0.561 | 3.84 × 10−3 | 3.84 × 10−3 | 3.04 | 0.115 |
| X2X3 | 1 | 0.12 | 0.12 | 14.20 | 0.004 ++ | 6.85 × 10−4 | 6.85 × 10−4 | 9.60 | 0.013 | 4.81 × 10−4 | 4.81 × 10−4 | 3.50 | 0.094 | 8.17 × 10−3 | 8.17 × 10−3 | 6.46 | 0.032 |
| X12 | 1 | 1.56 | 1.56 | 184.34 | 0.000 ++ | 1.48 × 10−2 | 1.48 × 10−2 | 208.01 | 0.000 ++ | 1.12 × 10−2 | 1.12 × 10−2 | 81.19 | 0.000 ++ | 0.20 | 0.20 | 160.19 | 0.000 ++ |
| X22 | 1 | 1.87 | 1.87 | 219.92 | 0.000 ++ | 5.20 × 10−3 | 5.20 × 10−3 | 72.99 | 0.000 ++ | 1.22 × 10−2 | 1.22 × 10−2 | 88.57 | 0.000 ++ | 0.25 | 0.25 | 194.12 | 0.000 ++ |
| X32 | 1 | 0.95 | 0.95 | 111.72 | 0.000 ++ | 7.04 × 10−4 | 7.04 × 10−4 | 9.87 | 0.012 | 1.38 × 10−2 | 1.38 × 10−2 | 100.82 | 0.000 ++ | 0.13 | 0.13 | 100.02 | 0.000 ++ |
| Residual | 9 | 7.64 × 10−2 | 8.49 × 10−3 | 6.42 × 10−4 | 7.13 × 10−5 | 1.24 × 10−3 | 1.37 × 10−4 | 1.14 × 10−2 | 1.27 × 10−3 | ||||||||
| Lack of Fit | 5 | 5.19 × 10−2 | 1.04 × 10−2 | 1.70 | 0.314 | 5.64 × 10−4 | 1.13 × 10−4 | 5.82 | 0.057 | 9.10 × 10−4 | 1.82 × 10−4 | 2.23 | 0.229 | 5.79 × 10−3 | 1.16 × 10−3 | 0.83 | 0.589 |
| Pure Error | 4 | 2.45 × 10−2 | 6.11 × 10−3 | 7.75 × 10−5 | 1.94 × 10−5 | 3.27 × 10−4 | 8.16 × 10−5 | 5.60 × 10−3 | 1.40 × 10−3 | ||||||||
| Cor Total | 19 | 5.17 | 2.73 × 10−2 | 5.82 × 10−2 | 0.60 | ||||||||||||
| R2 | 0.9852 | 0.9762 | 0.9787 | 0.9811 | |||||||||||||
| No. | Retention Time (min) | Compounds | RI a | CAS Number | Molecular Formula | Relative Area Percentage (%) | |
|---|---|---|---|---|---|---|---|
| HDE | IL-UMASDE | ||||||
| 1 | 7.334 | Dodecanal | 1408 | 112-54-9 | C12H24O | 7.10 | 6.52 |
| 2 | 8.575 | Dihydro-β-ionone | 1434 | 17283-81-7 | C13H22O | 3.09 | 2.74 |
| 3 | 8.761 | 4,6-Dimethyl-dodecane | 1437 | 61141-72-8 | C14H30 | 6.49 | 5.78 |
| 4 | 9.51 | Geranylacetone | 1454 | 3796-70-1 | C13H22O | 5.93 | 5.55 |
| 5 | 9.875 | 1-Dodecanol | 1462 | 112-53-8 | C12H26O | 2.95 | 2.68 |
| 6 | 10.184 | 4-Decalactone | 1468 | 706-14-9 | C10H18O2 | 3.52 | 3.26 |
| 7 | 10.395 | Allyl n-decanoate | 1473 | 57856-81-2 | C13H24O2 | nd b | 2.35 |
| 8 | 10.645 | Trans-β-ionone | 1478 | 79-77-6 | C13H20O | 5.31 | 5.12 |
| 9 | 11.156 | 2H-pyran-2-one | 1489 | 705-86-2 | C10H18O2 | 2.49 | 2.28 |
| 10 | 11.516 | 2-Tridecanone | 1496 | 593-08-8 | C13H26O | 6.29 | 5.97 |
| 11 | 12.586 | Tridecanal | 1519 | 10486-19-8 | C13H26O | 4.24 | 3.74 |
| 12 | 13.322 | 3,5,9-undecatrien-2-one | 1534 | 141-10-6 | C13H20O | 2.91 | 2.75 |
| 13 | 14.334 | Nerolidol | 1556 | 7212-44-4 | C15H26O | 5.95 | 5.43 |
| 14 | 14.767 | Dodecanoic acid | 1565 | 143-07-7 | C12H24O2 | 3.92 | 3.57 |
| 15 | 15.87 | Dodecanoic | 1588 | 106-33-2 | C14H28O2 | 4.29 | 3.84 |
| 16 | 18.691 | β-Acorenol | 1638 | 28400-11-5 | C15H26O | 3.02 | 2.86 |
| 17 | 20.125 | Trans-Farnesol | 1662 | 106-28-5 | C15H26O | nd | 2.71 |
| 18 | 20.304 | Ar-Turmerone | 1665 | 532-65-0 | C15H20O | 2.21 | 2.13 |
| 19 | 20.548 | (E) 2-tetradecenal | 1669 | 51534-36-2 | C14H26O | 3.70 | 3.51 |
| 20 | 21.035 | γ-dodecalactone | 1677 | 2305-5-7 | C12H22O2 | nd | 1.83 |
| 21 | 21.967 | 2-pentadecanone | 1693 | 2345-28-0 | C15H30O | 1.99 | 1.94 |
| 22 | 22.499 | Pentadecane | 1702 | 1921-70-6 | C19H40 | 5.41 | 4.81 |
| 23 | 23.347 | Farnesol | 1716 | 4602-84-0 | C15H26O | 4.20 | 3.91 |
| 24 | 24.057 | Methyl tetradecanoate | 1728 | 124-10-7 | C15H30O2 | 2.52 | 2.39 |
| 25 | 27.351 | Ethyl myristate | 1784 | 124-06-1 | C16H32O2 | 2.51 | 2.44 |
| Total identified compounds | - | - | - | 90.03 | 90.11 | ||
| Methods | CPT | HCPT | VCS-LT | EO | ||||
|---|---|---|---|---|---|---|---|---|
| K (min−1) | R2 | K (min−1) | R2 | K (min−1) | R2 | K (min−1) | R2 | |
| IL-UMASDE | 0.2187 | 0.9790 | 0.2111 | 0.9953 | 0.2033 | 0.9862 | 0.2397 | 0.9838 |
| IL-MASDE | 0.0403 | 0.9840 | 0.0620 | 0.9944 | 0.0402 | 0.9839 | 0.0369 | 0.9769 |
| IL-UAE | 0.0257 | 0.9967 | 0.0238 | 0.9964 | 0.0291 | 0.9983 | - | - |
| Ethanol-UAE | 0.0311 | 0.9969 | 0.0341 | 0.9975 | 0.0326 | 0.9972 | - | - |
| Ethanol-HRE | 0.0176 | 0.9816 | 0.0194 | 0.9908 | 0.0216 | 0.9963 | - | - |
| Water-UMASDE | - | - | - | - | - | - | 0.0160 | 0.9915 |
| Water-MASDE | - | - | - | - | - | - | 0.0311 | 0.9969 |
| HDE | - | - | - | - | - | - | 0.0245 | 0.9977 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Zhang, Y.; Li, C.; He, X.; Yang, L.; Fu, Y.; Zhang, J.; Zhao, W.; Zu, Y. Development of an Ionic Liquid-Based Ultrasonic/Microwave-Assisted Simultaneous Distillation and Extraction Method for Separation of Camptothecin, 10-Hydroxycamptothecin, Vincoside-Lactam, and Essential Oils from the Fruits of Camptotheca acuminata Decne. Appl. Sci. 2016, 6, 293. https://doi.org/10.3390/app6100293
Zhao C, Zhang Y, Li C, He X, Yang L, Fu Y, Zhang J, Zhao W, Zu Y. Development of an Ionic Liquid-Based Ultrasonic/Microwave-Assisted Simultaneous Distillation and Extraction Method for Separation of Camptothecin, 10-Hydroxycamptothecin, Vincoside-Lactam, and Essential Oils from the Fruits of Camptotheca acuminata Decne. Applied Sciences. 2016; 6(10):293. https://doi.org/10.3390/app6100293
Chicago/Turabian StyleZhao, Chunjian, Yukun Zhang, Chunying Li, Xin He, Lei Yang, Yujie Fu, Jingjing Zhang, Wenyan Zhao, and Yuangang Zu. 2016. "Development of an Ionic Liquid-Based Ultrasonic/Microwave-Assisted Simultaneous Distillation and Extraction Method for Separation of Camptothecin, 10-Hydroxycamptothecin, Vincoside-Lactam, and Essential Oils from the Fruits of Camptotheca acuminata Decne" Applied Sciences 6, no. 10: 293. https://doi.org/10.3390/app6100293






