Two-Photon Absorbing Molecules as Potential Materials for 3D Optical Memory
Abstract
:1. Introduction
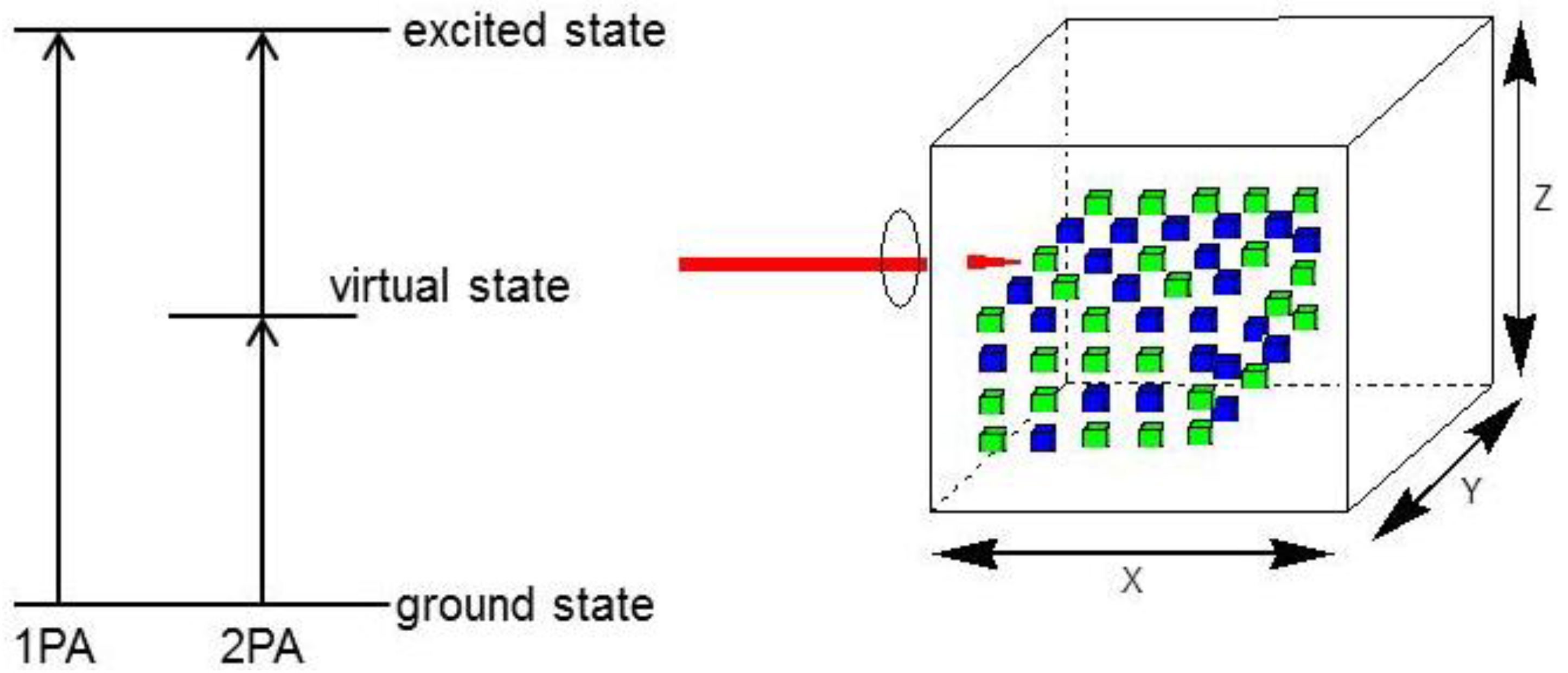
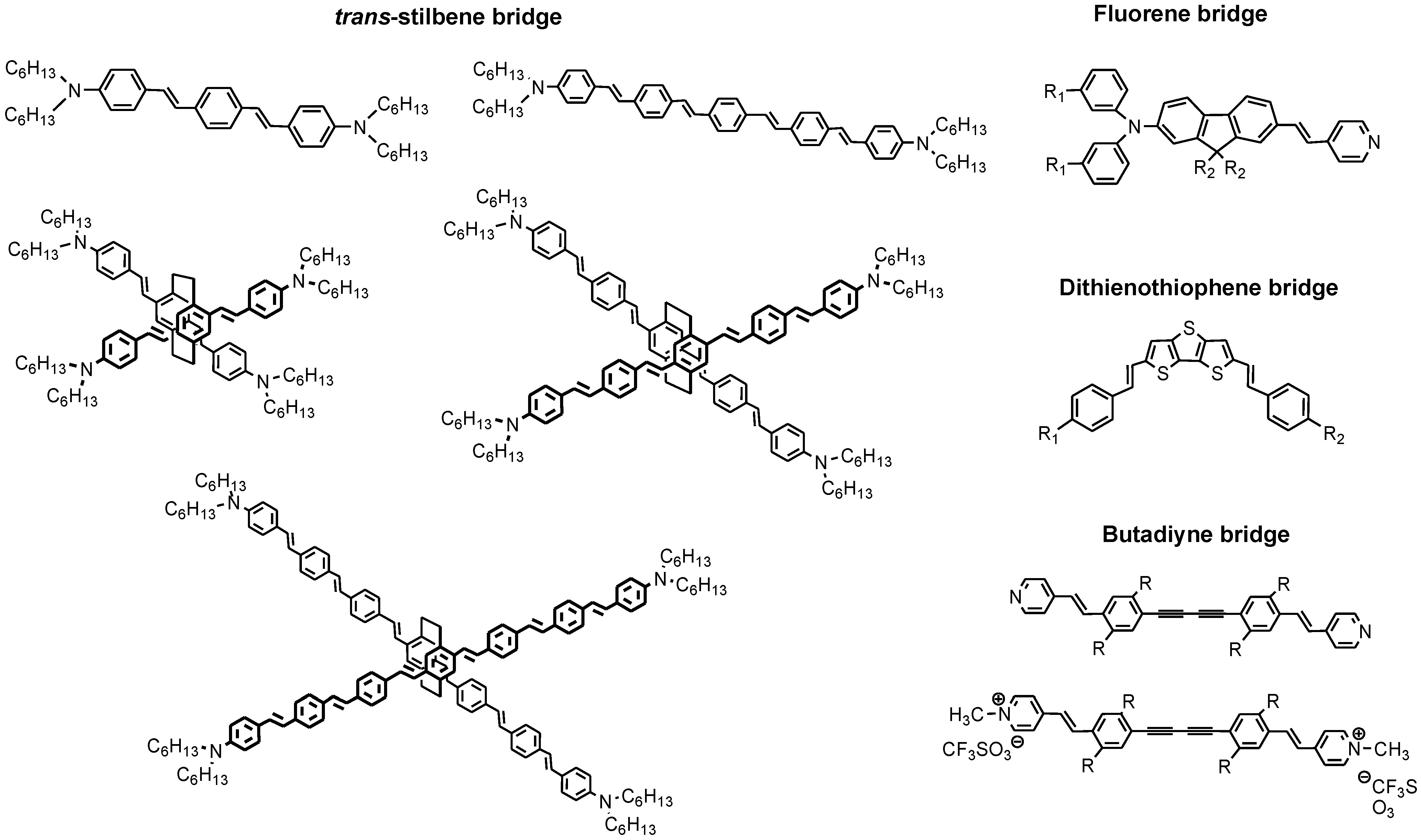
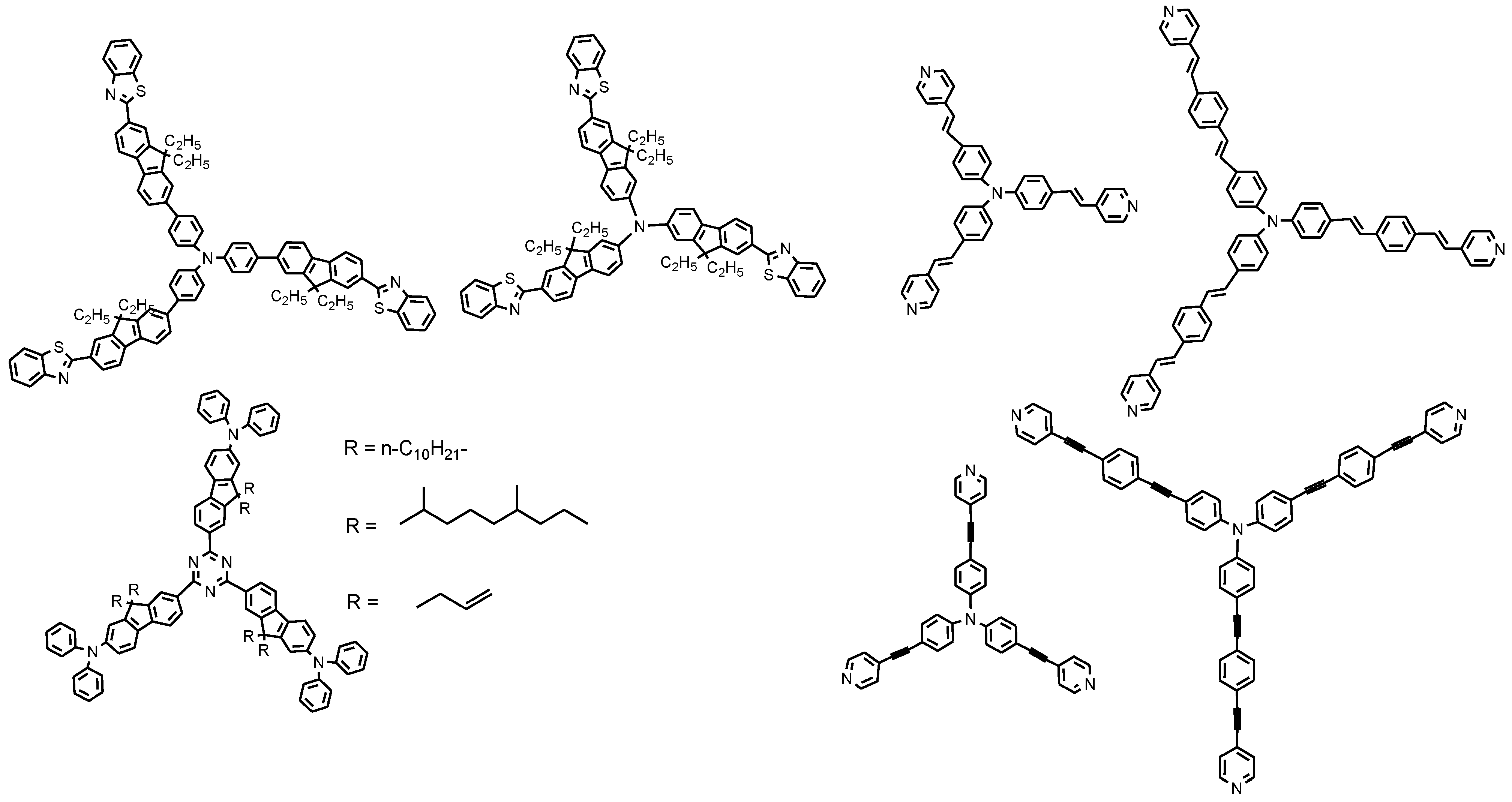

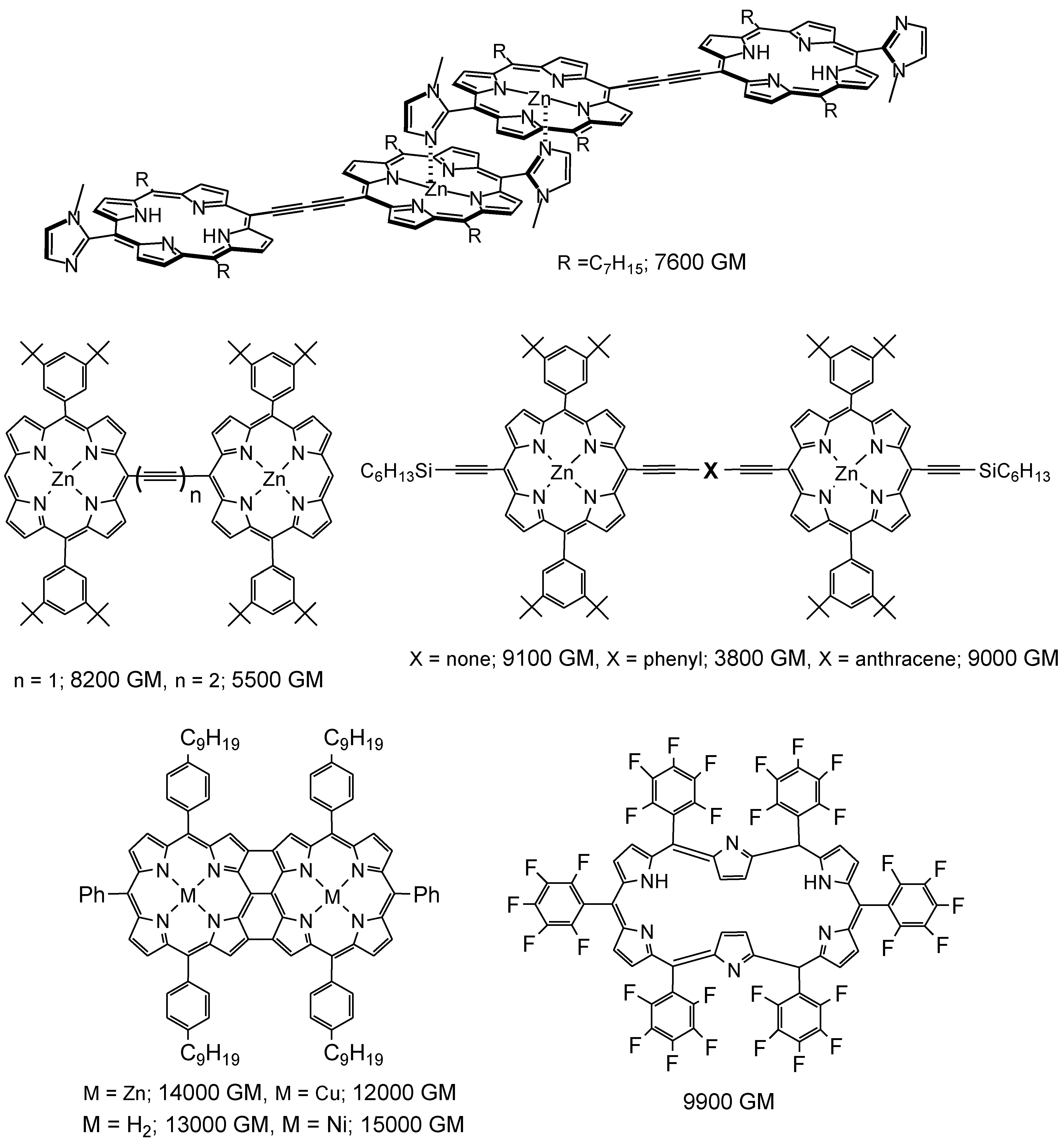
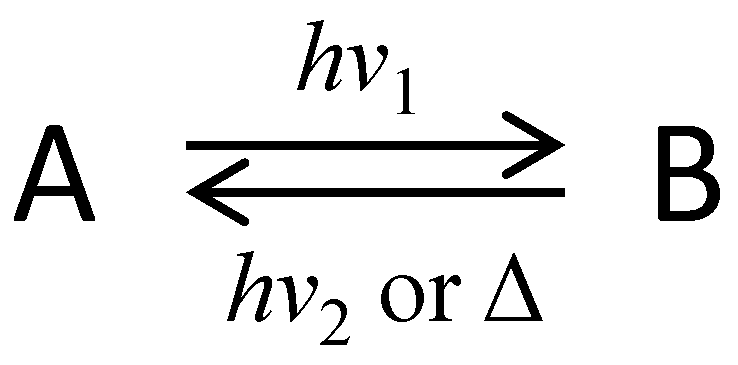

2. 3D Optical Memory Systems by Photochromic Molecules Using Two-Photon Absorption
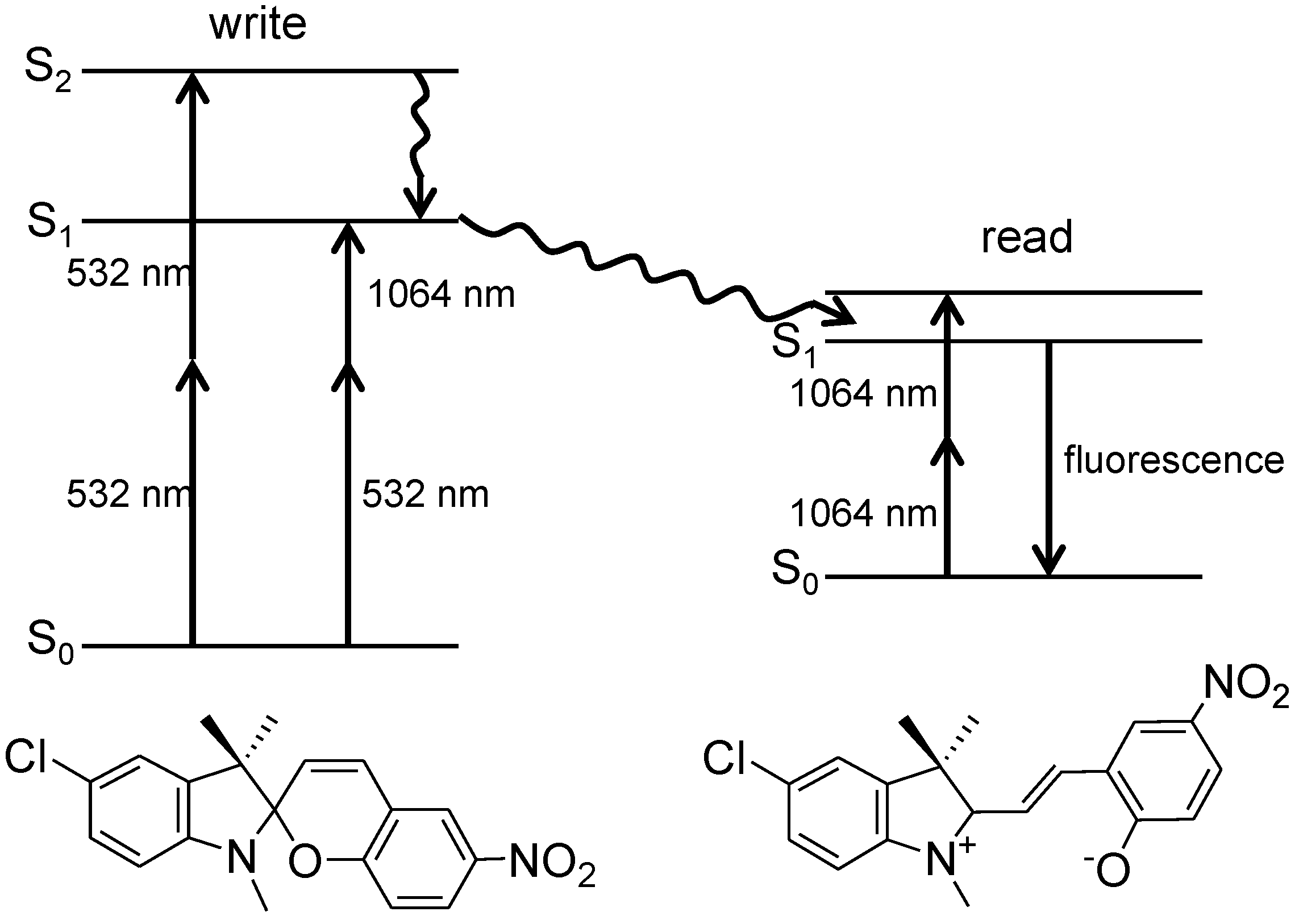
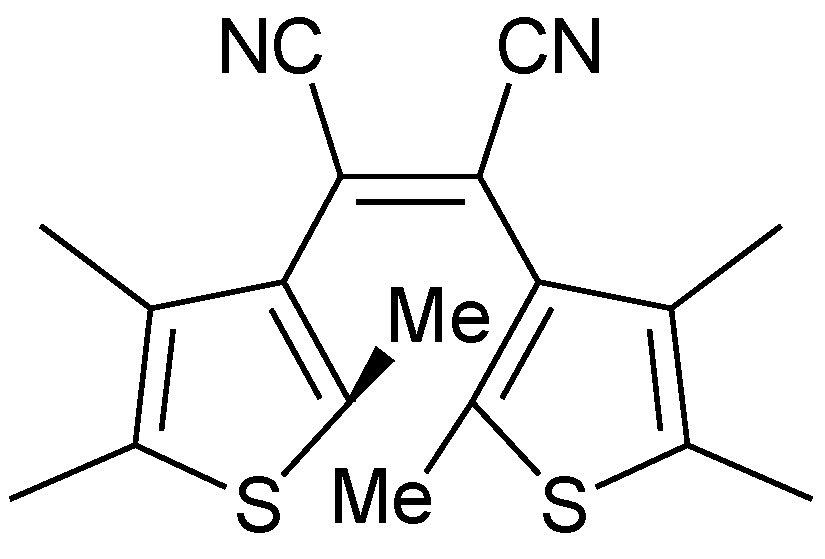
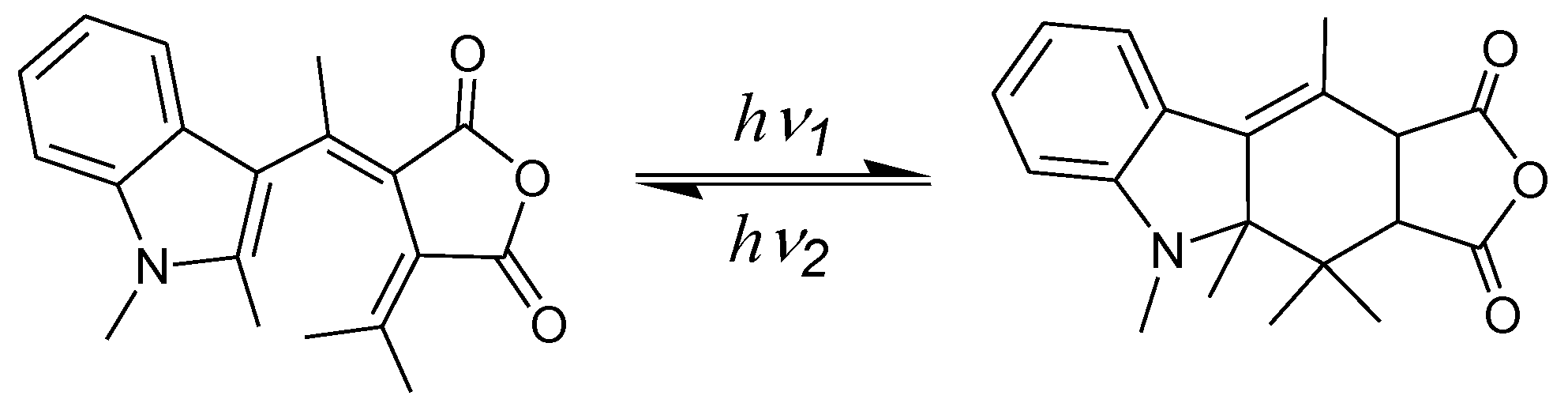

| Entry | Quantum yield | Conversion | |
|---|---|---|---|
| Cyclization | Cycloreversion | ||
| 3 | 0.17 | 0.63 | 47% |
| 4 | 0.32 | 0.026 | 93% |
| 5 | 0.12 | 0.017 | 76% |
| Entry | Wavelength/nm | 2PA cross-section/GM |
|---|---|---|
| 3a | 770 | 44 |
| 5a | 770 | 43 |
| 5a | 820 | 23 |
| 6 | 770 | 10 |
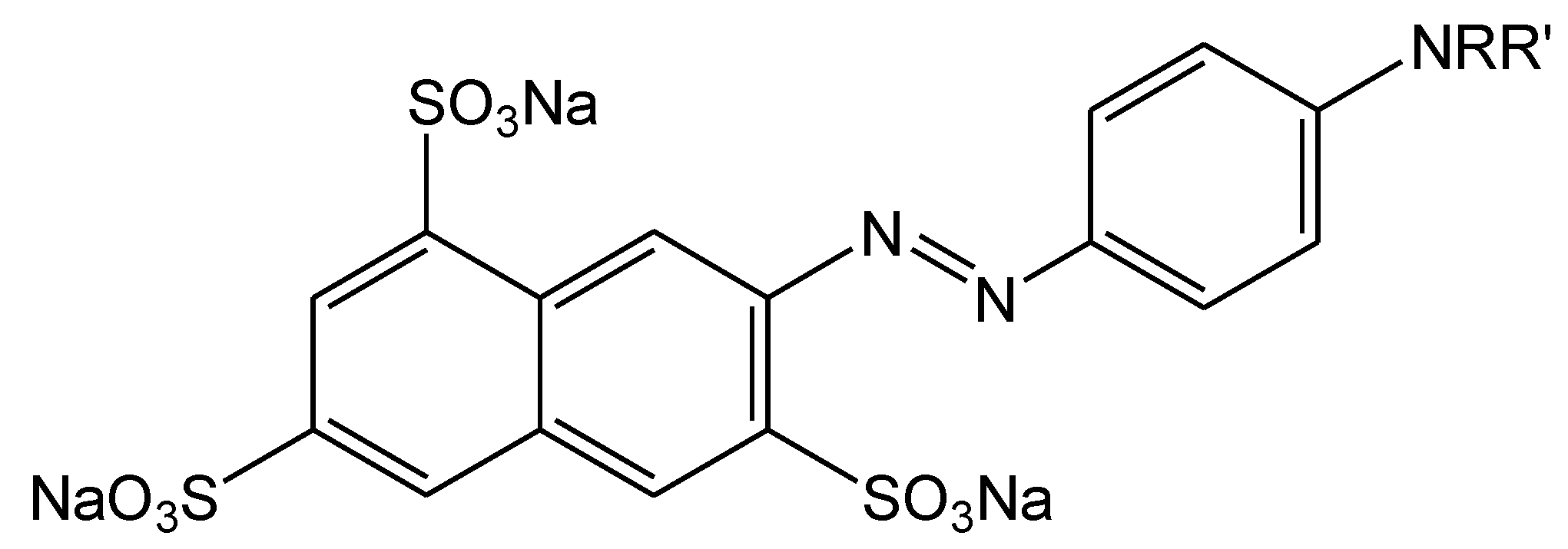
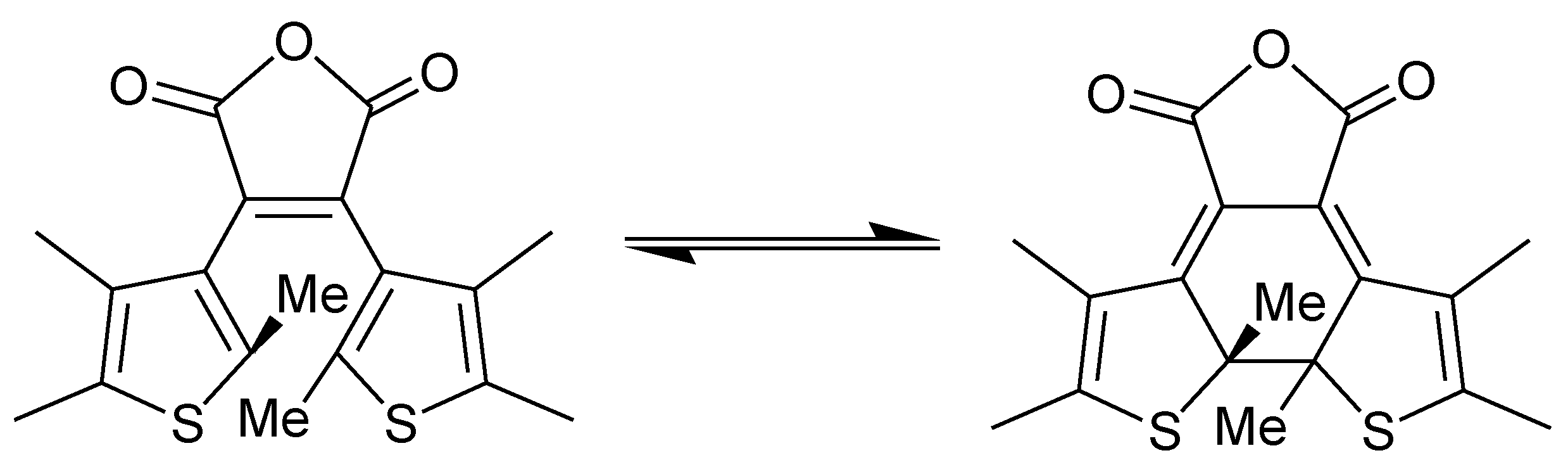
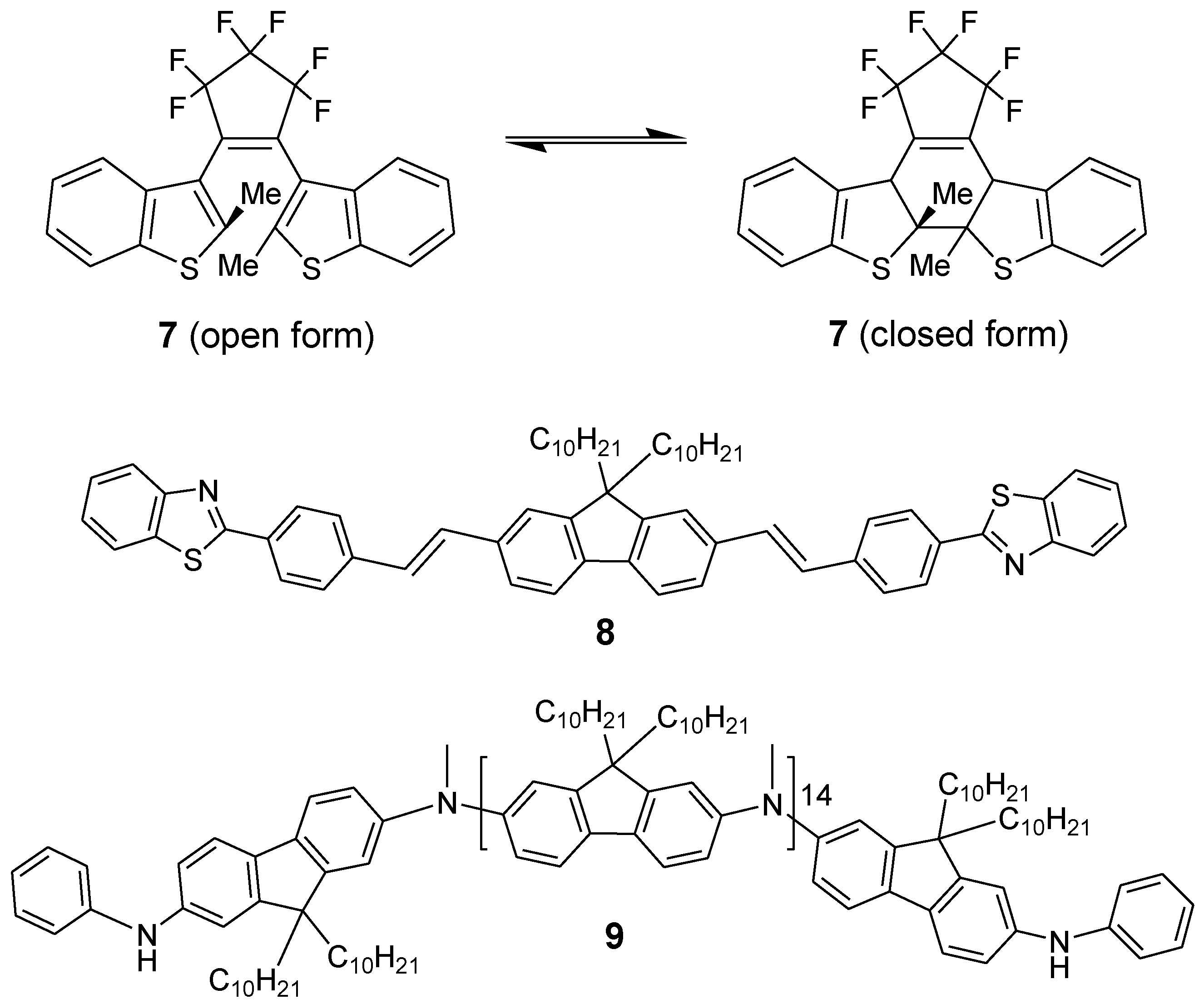

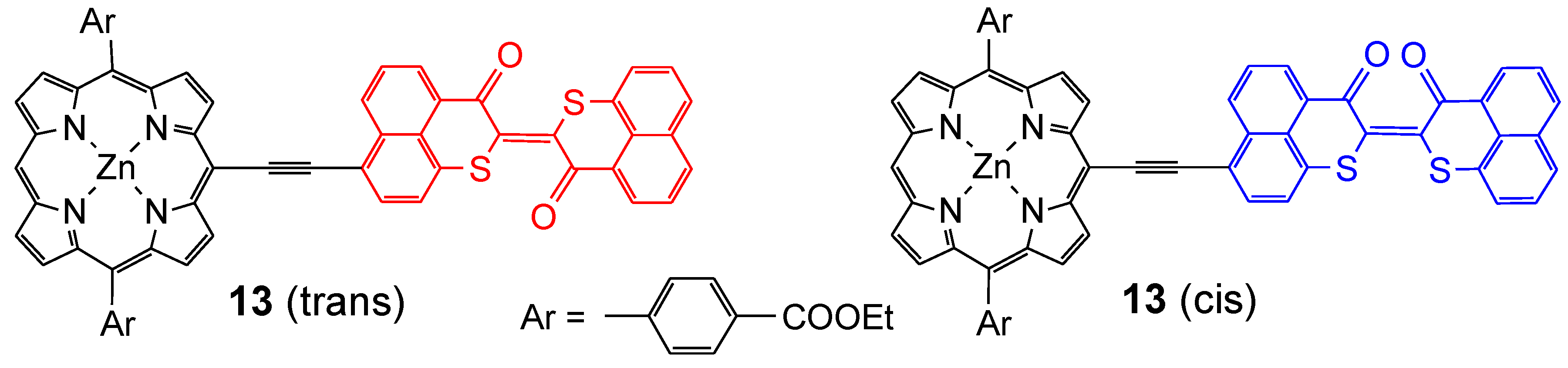
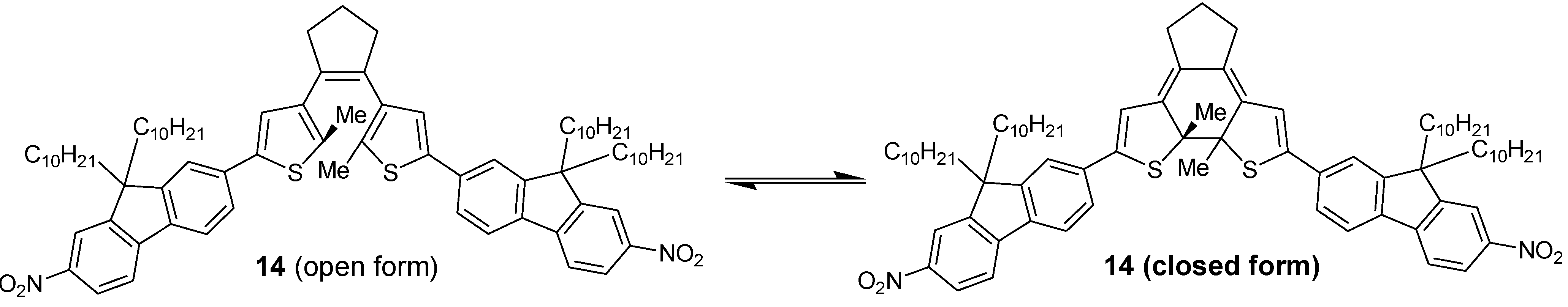
3. Conclusions
Conflicts of Interest
References
- Parthenopoulos, D.A.; Rentzepis, P.M. Three dimensional optical storage memory. Science 1989, 245, 843–845. [Google Scholar]
- Kawata, S.; Kawata, Y. Three-dimensional optical data storage using photochromic materials. Chem. Rev. 2000, 100, 1777–1788. [Google Scholar] [CrossRef]
- Bhawalkar, J.D.; Kumar, N.D.; Zhao, C.F.; Prasad, P.N. Two-photon photodynamic therapy. J. Clin. Laser. Med. Surg. 1997, 15, 201–204. [Google Scholar]
- Wachter, E.A.; Partridge, W.P.; Fisher, W.G.; Dees, H.C.; Petersen, M.G. Simultaneous two-photon excitation of photodynamic therapy agents. Proc. SPIE-Into. Soc. Opt. Eng. 1998, 3269, 68–74. [Google Scholar]
- Sutherland, R.L. Handbook of Nonlinear Optics, 2nd ed.; Marcel Dekker: New York, NY, USA, 2003. [Google Scholar]
- Göppert-Mayer, M. Uber elementarakte mit zwei quantensprüngen. Ann. Phys. 1931, 9, 273–294. [Google Scholar] [CrossRef]
- Kaiser, W.; Garrett, C.G.B. Two-photon excitation in caf2: Eu2+. Phys. Rev. Lett. 1961, 7, 229–231. [Google Scholar] [CrossRef]
- Albota, M.; Beljonne, D.; Brédas, J.-L.; Ehrlich, J.E.; Fu, J.-Y.; Heikal, A.A.; Hess, S.E.; Kogej, T.; Levin, M.D.; Marder, S.R.; et al. Design of organic molecules with large two-photon absorption cross sections. Science 1998, 281, 1653–1656. [Google Scholar] [CrossRef]
- Reinhardt, B.A.; Brott, L.L.; Clarson, S.J.; Dillard, A.G.; Bhatt, J.C.; Kannan, R.; Yuan, L.X.; He, G.S.; Prasad, P.N. Highly active two-photon dyes: Design, synthesis, and characterization toward application. Chem. Mater. 1998, 10, 1863–1874. [Google Scholar] [CrossRef]
- Bartholomew, G.P.; Rumi, M.; Pond, S.J.K.; Perry, J.W.; Tretiak, S.; Bazan, G.C. Two-photon absorption in three-dimensional chromophores based on [2.2]-paracyclophane. J. Am. Chem. Soc. 2004, 126, 11529–11542. [Google Scholar] [CrossRef]
- Woo, H.Y.; Korystov, D.; Mikhailovsky, A.; Nguyen, T.-Q.; Bazan, G.C. Two-photon absorption in aqueous micellar solutions. J. Am. Chem. Soc. 2005, 127, 13794–13795. [Google Scholar] [CrossRef]
- Wang, C.; Macak, P.; Luo, Y.; Agren, H. Effects of centers and symmetry on two-photon absorption cross sections of organic chromophores. J. Chem. Phys. 2001, 114, 9813–9820. [Google Scholar] [CrossRef]
- Kamada, K.; Ohta, K.; Yoichiro, I.; Kondo, K. Two-photon absorption properties of symmetric substituted diacetylene: Drastic enhancement of the cross section near the one-photon absorption peak. Chem. Phys. Lett. 2003, 372, 386–393. [Google Scholar] [CrossRef]
- Tanihara, J.; Ogawa, K.; Kobuke, Y. Two-photon absorption properties of conjugated supramolecular porphyrins with electron donor and acceptor. J. Photochem. Photobiol. A 2006, 178, 140–149. [Google Scholar] [CrossRef]
- Kannan, R.; He, G.S.; Lin, T.C.; Prasad, P.N.; Vaia, R.A.; Tan, L.S. Toward highly active two-photon absorbing liquids. Synthesis and characterization of 1,3,5-triazine-based octupolar molecules. Chem. Mater. 2004, 16, 185–194. [Google Scholar] [CrossRef]
- Bhaskar, A.; Ramakrishna, G.; Lu, Z.K.; Twieg, R.; Hales, J.M.; Hagan, D.J.; van Stryland, E.; Goodson, T. Investigation of two-photon absorption properties in branched alkene and alkyne chromophores. J. Am. Chem. Soc. 2006, 128, 11840–11849. [Google Scholar] [CrossRef]
- Drobizhev, M.; Karotki, A.; Kruk, M.; Rebane, A. Resonance enhancement of two-photon absorption in porphyrins. Chem. Phys. Lett. 2002, 355, 175–182. [Google Scholar] [CrossRef]
- Drobizhev, M.; Karotki, A.; Kruk, M.; Mamardashvili, N.Z.; Rebane, A. Drastic enhancement of two-photon absorption in porphyrins associated with symmetrical electron-accepting substitution. Chem. Phys. Lett. 2002, 361, 504–512. [Google Scholar] [CrossRef]
- Goyan, R.L.; Cramb, D.T. Near-infrared two-photon excitation of proto-porphyrin IX: Photodynamics and photoproduct generation. Photochem. Photobiol. 2000, 72, 821–827. [Google Scholar] [CrossRef]
- Ogawa, K.; Ohashi, A.; Kobuke, Y.; Kamada, K.; Ohta, K. Strong two-photon absorption of self-assembled butadiyne-linked bisporphyrin. J. Am. Chem. Soc. 2003, 125, 13356–13357. [Google Scholar] [CrossRef]
- Ogawa, K.; Ohashi, A.; Kobuke, Y.; Kamada, K.; Ohta, K. Two-photon absorption properties of self-assemblies of butadiyne-linked bis(imidazolylporphyrin). J. Phys. Chem. B 2005, 109, 22003–22012. [Google Scholar] [CrossRef]
- Drobizhev, M.; Karotki, A.; Kruk, M.; Krivokapic, A.; Anderson, H.L.; Rebane, A. Upconversion fluorescence in porphyrins: One-photon hot-band absorption versus two-photon absorption. Chem. Phys. Lett. 2003, 380, 690–699. [Google Scholar]
- Screen, T.E.O.; Thorne, J.R.G.; Denning, R.G.; Bucknall, D.G.; Anderson, H.L. Two methods for amplifying the optical nonlinearity of a conjugated porphyrin polymer: trans-metallation and self-assembly. J. Mater. Chem. 2003, 13, 2796–3909. [Google Scholar] [CrossRef]
- Karotki, A.; Drobizhev, M.; Dzenis, Y.; Taylor, P.N.; Anderson, H.L.; Rebane, A. Drastic enhancement of intrinsic two-photon absorption in a conjugated porphyrin dimer. Phys. Chem. Chem. Phys. 2004, 6, 7–10. [Google Scholar] [CrossRef]
- Drobizhev, M.; Stepanenko, Y.; Dzenis, Y.; Karotki, A.; Rebane, A.; Taylor, P.N.; Anderson, H.L. Understanding strong two-photon absorption in pi-conjugated porphyrin dimers via double-resonance enhancement in a three-level model. J. Am. Chem. Soc. 2004, 126, 15352–15353. [Google Scholar] [CrossRef]
- Drobizhev, M.; Stepanenko, Y.; Rebane, A.; Wilson, C.J.; Screen, T.E.O.; Anderson, H.L. Strong cooperative enhancement of two-photon absorption in double-strand conjugated porphyrin ladder arrays. J. Am. Chem. Soc. 2006, 128, 12432–12433. [Google Scholar] [CrossRef]
- Kim, D.Y.; Ahn, T.K.; Kwon, J.H.; Kim, D.; Ikeue, T.; Aratani, N.; Osuka, A.; Shigeiwa, M.; Maeda, S. Large two-photon absorption (TPA) cross-section of directly linked fused diporphyrins. J. Phys. Chem. A 2005, 109, 2996–2999. [Google Scholar] [CrossRef]
- Ahn, T.K.; Kwon, J.H.; Kim, D.Y.; Cho, D.W.; Jeong, D.H.; Kim, S.K.; Suzuki, M.; Shimizu, S.; Osuka, A.; Kim, D. Comparative photophysics of [26] and [28]hexaphyrins(1.1.1.1.1.1): Large two-photon absorption cross section of aromatic [26]hexaphyrins(1.1.1.1.1.1). J. Am. Chem. Soc. 2005, 127, 12856–12861. [Google Scholar] [CrossRef]
- Ahn, T.K.; Kim, K.S.; Kim, D.Y.; Noh, S.B.; Aratani, N.; Ikeda, C.; Osuka, A.; Kim, D. Relationship between two-photon absorption and the π-conjugation pathway in porphyrin arrays through dihedral angle control. J. Am. Chem. Soc. 2006, 128, 1700–1704. [Google Scholar]
- Durr, H.; Bouas-Laurent, H. Photochromism: Molecules and Systems; Elsevier: Amsterdam, The Netherlands, 1990. [Google Scholar]
- Berkovic, G.; Krongauz, V.; Weiss, V. Spiropyrans and spirooxazines for memories and switches. Chem. Rev. 2000, 100, 1741–1754. [Google Scholar] [CrossRef]
- Irie, M. Diarylethenes for memories and switches. Chem. Rev. 2000, 100, 1685–1716. [Google Scholar] [CrossRef]
- Yokoyama, Y. Fulgides for memories and switches. Chem. Rev. 2000, 100, 1717–1740. [Google Scholar] [CrossRef]
- Dvornikov, A.S.; Walker, E.P.; Rentzepis, P.M. Two-photon three-dimensional optical storage memory. J. Phys. Chem. A 2009, 113, 13633–13644. [Google Scholar] [CrossRef]
- Mikhailov, I.; Belfield, K.D.; Masunov, A.E. Dft-based methods in the design of two-photon operated molecular switches. J. Phys. Chem. A 2009, 113, 7080–7089. [Google Scholar] [CrossRef]
- Patel, P.D.; Mikhailov, I.A.; Belfield, K.D.; Masunov, A.M.E. Theoretical study of photochromic compounds, part 2: Thermal mechanism for byproduct formation and fatigue resistance of diarylethenes used as data storage materials. Int. J. Quantum Chem. 2009, 109, 3711–3722. [Google Scholar] [CrossRef]
- Patel, P.D.; Masunov, A.E. Theoretical study of photochromic compounds: Part 3. Prediction of thermal stability. J. Phys. Chem. C 2011, 115, 10292–10297. [Google Scholar] [CrossRef]
- Masunov, A.E.; Mikhailov, I.A. Theory and computations of two-photon absorbing photochromic chromophores. Eur. J. Chem. 2010, 1, 142–161. [Google Scholar] [CrossRef]
- Toriumi, A.; Kawata, S.; Gu, M. Reflectionconfocal microscope readout system for three-dimensional photochromic optical data storage. Opt. Lett. 1998, 23, 1924–1926. [Google Scholar] [CrossRef]
- Belfield, K.D.; Liu, Y.; Negres, R.A.; Fan, M.; Pan, G.; Hagan, D.J.; Hernandez, F.E. Two-photon photochromism of an organic material for holographic recording. Chem. Mater. 2002, 14, 3663–3667. [Google Scholar] [CrossRef]
- Shiono, T.; Itoh, T.; Nishino, S. Two-photon absorption recording in photochromic diarylethenes using laser diode for three-dimensional optical memory. Jpn. J. Appl. Phys. 2005, 44, 3559–3563. [Google Scholar] [CrossRef]
- Saita, S.; Yamaguchi, T.; Kawai, T.; Irie, M. Two-photon photochromism of diarylethene dimer derivatives. Chemphyschem 2005, 6, 2300–2306. [Google Scholar] [CrossRef]
- Magennis, S.W.; Mackay, F.S.; Jones, A.C.; Tait, K.M.; Sadler, P.J. Two-photon-induced photoisomerization of an azo dye. Chem. Mater. 2005, 17, 2059–2062. [Google Scholar] [CrossRef]
- Corredor, C.C.; Belfield, K.D.; Bondar, M.V.; Przhonska, O.V.; Hernandez, F.E.; Kachkovsky, O.D. One- and two-photon photochromism of 3,4-bis-(2,4,5-trimethyl-thiophen-3-yl)furan-2,5-dione. J. Photochem. Photobiol. A 2006, 184, 177–183. [Google Scholar] [CrossRef]
- Belfield, K.D.; Bondar, M.V.; Corredor, C.C.; Hernandez, F.E.; Przhonska, O.V.; Yao, S. Two-photon photochromism of a diarylethene enhanced by forster resonance energy transfer from two-photon absorbing fluorenes. Chemphyschem 2006, 7, 2514–2519. [Google Scholar] [CrossRef]
- Corredor, C.C.; Huang, Z.-L.; Belfield, K.D. Two-photon 3d optical data storage via fluorescence modulation of an efficient fluorene dye by a photochromic diarylethene. Adv. Mater. 2006, 18, 2910–2914. [Google Scholar] [CrossRef]
- Corredor, C.C.; Huang, Z.-L.; Belfield, K.D.; Morales, A.R.; Bondar, M.V. Photochromic polymer composites for two-photon 3d optical data. Chem. Mater. 2007, 19, 5165–5173. [Google Scholar] [CrossRef]
- Belfield, K.D.; Morales, A.R.; Kang, B.S.; Hales, J.M.; Hagan, D.J.; Van Stryland, E.W.; Chapela, V.M.; Percino, J. Synthesis, characterization, and optical properties of new two-photon-absorbing fluorene derivatives. Chem. Mater. 2004, 16, 4634–4641. [Google Scholar] [CrossRef]
- Belfield, K.D.; Morales, A.R.; Hales, J.M.; Hagan, D.J.; van Stryland, E.W.; Chapela, V.M.; Percino, J. Linear and two-photon photophysical properties of a series of symmetrical diphenylaminofluorenes. Chem. Mater. 2004, 16, 2267–2273. [Google Scholar] [CrossRef]
- Yanez, C.O.; Andrade, C.D.; Yao, S.; Luchita, G.; Bondar, M.V.; Belfield, K.D. Photosensitive polymeric materials for two-photon 3D worm optical data storage systems. ACS Appl. Mater. Interfaces 2009, 1, 2219–2229. [Google Scholar] [CrossRef]
- Belfield, K.D.; Schafer, K.J. A new photosensitive polymeric material for worm optical data storage using multichannel two-photon fluorescence readout. Chem. Mater. 2002, 14, 3656–3662. [Google Scholar] [CrossRef]
- Dy, J.T.; Maeda, R.; Nagatsuka, Y.; Ogawa, K.; Kamada, K.; Ohta, K.; Kobuke, Y. A photochromic porphyrin-perinaphthothioindigo conjugate and its two-photon absorption properties. Chem. Commun. 2007, 2007, 5170–5172. [Google Scholar]
- Ogawa, K.; Dy, J.; Maeda, R.; Nagatsuka, Y.; Kamada, K.; Kobuke, Y. Synthesis and photophysical properties of a porphyrin-perinaphthothioindigo dye. J. Porphyrins. Phthalocyanines. 2013, 17, 821–830. [Google Scholar] [CrossRef]
- Ogawa, K.; Kobuke, Y. Design of two-photon absorbing materials for molecular optical memory and photodynamic therapy. Organ. Biomol. Chem. 2009, 7, 2241–2246. [Google Scholar] [CrossRef]
- Irie, M.; Ishida, H.; Tsujioka, T. Rewritable near-field optical recording on photochromic perinaphthothioindigo thin films: readout by fluorescence. Jpn. J. Appl. Phys. 1999, 38, 6114–6117. [Google Scholar] [CrossRef]
- Luchita, G.; Bondar, M.V.; Yao, S.; Mikhailov, I.A.; Yanez, C.O.; Przhonska, O.V.; Masunov, A.E.; Belfield, K.D. Efficient photochromic transformation of a new fluorenyl diarylethene: One- and two-photon absorption spectroscopy. ACS Appl. Mater. Interfaces 2011, 3, 3559–3567. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ogawa, K. Two-Photon Absorbing Molecules as Potential Materials for 3D Optical Memory. Appl. Sci. 2014, 4, 1-18. https://doi.org/10.3390/app4010001
Ogawa K. Two-Photon Absorbing Molecules as Potential Materials for 3D Optical Memory. Applied Sciences. 2014; 4(1):1-18. https://doi.org/10.3390/app4010001
Chicago/Turabian StyleOgawa, Kazuya. 2014. "Two-Photon Absorbing Molecules as Potential Materials for 3D Optical Memory" Applied Sciences 4, no. 1: 1-18. https://doi.org/10.3390/app4010001




