Abstract
An inclusion complex of β-cyclodextrin and 4-aminothiophenol was assembled by hydrophobic interaction of the host (β-cyclodextrin) and guest (4-aminothiophenol). The complex was isolated as crystalline solid and studied by single crystal X-ray diffraction method along with NMR and IR spectroscopy. Two cyclodextrin rings each containing one disulfide form of 4-aminothiophenol were found to pair up by hydrogen bonding of the outer rim -OH groups. The phenyl disulfide moiety of 4-aminophenyl disulfide molecule was found in the core of β-cyclodextrin, while the amino functional groups were positioned to the exterior of the cyclodextrin ring. Phenyl rings of the guest molecule from each partner of the paired cyclodextrin complex were found parallel to each other, indicating possible π-π stacking interaction between them.
1. Introduction
Cyclodextrins are the most popular host molecule for supramolecular complexation. This has much to do with their efficacy in complexation with hydrophobic host molecules. Cyclodextrins have a hydrophilic exterior and hydrophobic interior. They are also available in various sizes. Among the available size variety, β-cyclodextrins are of suitable size to encapsulate aromatic compounds. β-CD forms inclusion complexes usually as dimers, due to its affinity to self-associate []. The dimers form because of hydrogen bonding between the hydroxyl groups on the rims of these molecules (Figure 1) [,].
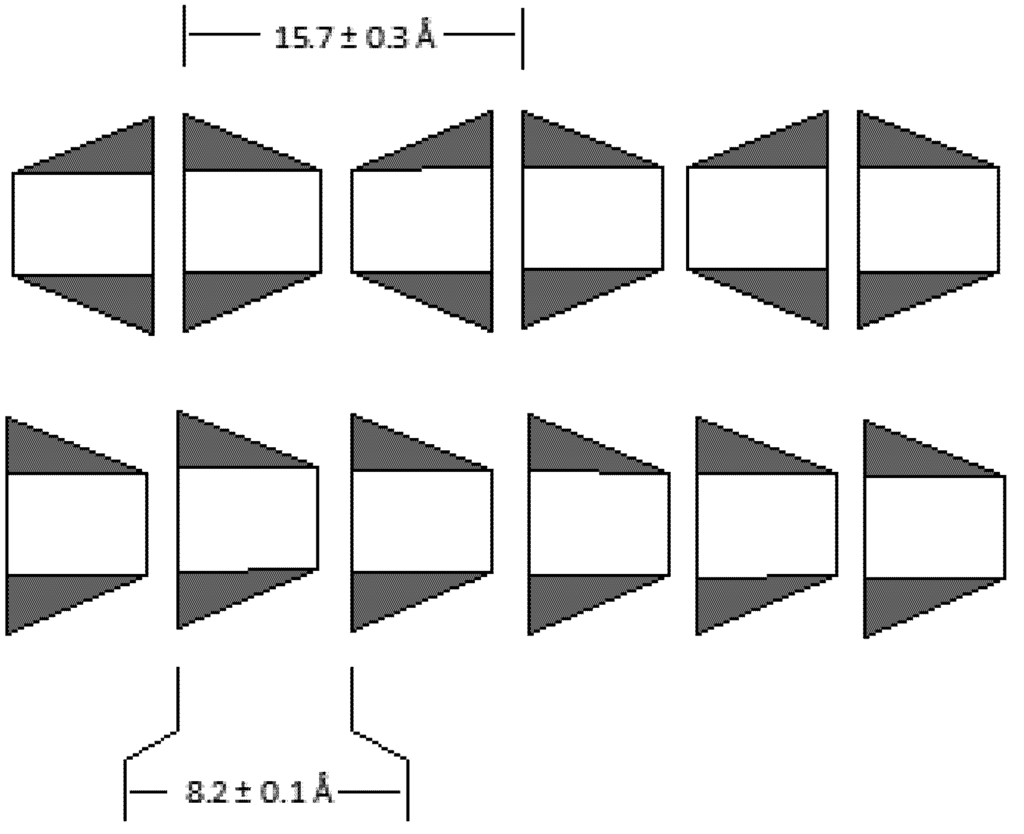
Figure 1.
Hydrogen bonded assembly of cyclodextrins in solid state. Head-to-head assembly of β-CD with a repeat distance of 15.7 ± 0.3 Å (top) and head-to-tail assembly of α-CD with repeat distance of 8.2 ± 0.1 Å (bottom).
Host–guest complexation with β-CD is commonly used in pharmaceuticals to solubilize water insoluble drugs []. Other uses for β-CD include the stabilization of gold nanoparticles and projects involving environmental remediation [,]. As an example of complexation of hydrophobic guest molecule pyrene and β-CD could be mentioned []. The solid state structure of this complex revealed that the pyrene molecule was positioned inside the cavity prepared by two cyclodextrin rings in a head-to-head fashion. The guest pyrene molecule was inside at the juncture of the two β-CD rings in a hydrophobic environment.
Thiols are a popular tool for the fabrication of nano-materials [,] as linkers and stabilizers. Aromatic bifunctional aminothiophenols have been employed for this purpose by making a host–guest complex with β-CD []. It is known that guest molecules may behave differently inside the host molecule [,,,,,,]. For example, highly conjugated cyanine dyes get a boost in fluorescence response when complexed with cyclodextrins [,]. As most molecular properties depend on structural arrangements, it is important to learn about the structural arrangement of host–guest complexes. In this article, we report some structural aspects of such complexation between β-CD and 4-aminothiophenol (4-ATP).
2. Experimental Section
β-CD, 4-ATP and ethanol were purchased from Sigma Aldrich and used without further purification. IR spectra were recorded on a Perkin Elmer ATR-FTIR spectrometer, 1H NMR was recorded on a 300 MHz Varian spectrometer. Crystallographic data was collected on a Rigaku instrument at 100 (±2) K temperature.
Assembling β-Cyclodextrin4-ATPInclusionComplex
A clear saturated solution of β-CD was prepared by dissolving 0.567 g β-CD hydrate in 40 mL water. In a separate container, a clear solution of ATP was made by dissolving 0.0640 g (0.511 mmol) of ATP in 10 mL of ethanol. The ethanolic ATP solution was slowly added to the aqueous β-CD solution. This mixture was left in the container overnight until crystals of the inclusion complex were noticed. Solvent was removed. The solid remaining was then washed three times with acetone and dried under atmosphere. Suitable crystals for diffraction studies were isolated from this sample.
IR (ν, cm−1): 3253 (m, br), 2929 (w, br), 1623 (w, sh), 1596 (w, sh), 1494 (w, sh), 1413 (w, sh), 1370 (w, sh), 1330 (w, sh), 1297 (w, sh), 1205(w, sh), 1155 (m, sh), 1079 (s, sh), 1024 (s, sh), 1000 (s, sh).
1H NMR (D2O) δ (ppm): 0.94 (t), 2.0 (s), 6.6 (d) and 7.0 (d), region 4-5 was noisy and no signal was detectable. This is because of disordered water molecules in the matrix of the inclusion complex which is released in D2O upon dissolution.
3. Results and Discussion
Host–guest interaction was studied between β-CD and 4-aminothiophenol (4-ATP). The host–guest complex was assembled by hydrophobic interaction for each other. Structural analysis of the complex comprises of IR, 1H NMR and single crystal X-ray diffraction studies. The guest 4-ATP is insoluble in aqueous solution, while the host β-CD is soluble in water (1.08 mg/mL). The complexation was induced by mixing an ethanolic solution of 4-ATP in an aqueous solution of β-CD. IR spectrum of the complex preserves most features of β-CD, while the bands at 1623 (w, sh), 1596 (w, sh), 1494 (w, sh) were unique. These were assigned due to the N-H bending modes and C-C stretching frequency of the aromatic ring as part of 4-ATP. The NMR spectrum of the complex bears a stronger indication of complex formation. 1H NMR of the complex had two doublets at δ 6.6 and 7.0. These are signals from the aromatic guest molecule. The 1H NMR of β-CD and the complex were recorded in D2O. In D2O the guest molecule is not soluble and hence there was signal from the aromatic proton of the guest molecule in the inclusion complex. The NMR spectra recorded were very noisy in the δ3.5-5 region because of the water molecules in the hydrophilic region of the complex shadowing the signals from the β-CD ring.
It is a common practice to use thiols for the fabrication of nanomaterials [,,] as linkers. In a separate study 4-ATP/β-CD complex was bonded to gold nanoparticles []. Thiols are known to oxidize to become disulfides. Evidently, previous reports ignored this common structural modification of the thiol molecules. Suitable crystals of the complex were isolated for single crystal X-ray diffraction studies. Scheme 1 illustrates the structural modification of 4-ATP to its disulfide form. The reason for oxidation was determined, though it was not measured if the oxidation of the guest molecule, 4-ATP, was accelerated in the β-CD environment. However, the suspected causes could be solely, or by a combination of, dissolved oxygen and atmospheric oxygen in the drying process.
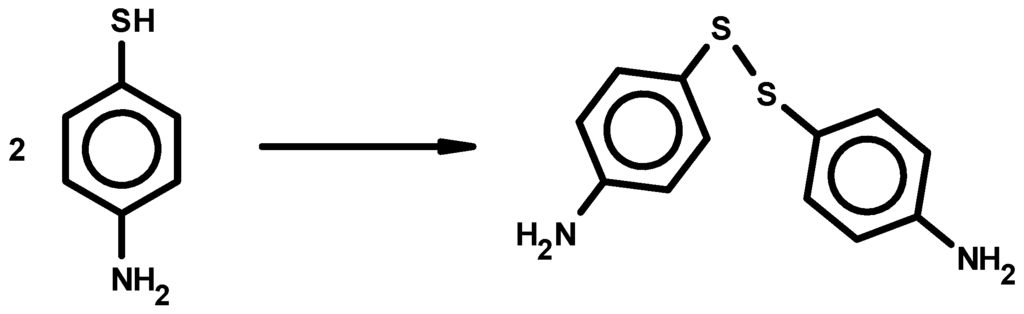
Scheme 1.
Modification of 4-ATP to 4,4'-diaminodiphenyl disulfide.
The solid-state structure of the complex demonstrated the host–guest interactions between the modified 4-ATP and β-CD (Figure 2). Each β-CD ring has one 4,4'-diaminodiphenyl disulfide guest molecule. Two of these complexes are in close proximity indicating noncovalent interactions. The β-CD rings maintain their preferred head-to-head interaction. Here, the hydrogen bonding is dominant between the –OH groups in the bigger rim of the cyclodextrin ring. It is also likely that there might be active π-π stacking interactions between the aromatic rings of two guest molecules from the neighboring complexes, as well. On average, a rough measurement of distance between such rings is 3.7(±4) Å. Usually, parallel aromatic rings separated by a distance less than 4 Å are considered to be involved in π-π stacking interactions. These rings are parallel but not completely superimposable to each other with a slight skid and twist away for carbon-to-carbon alignment of the two rings. Generally, the T-type π-π stacking is more stable than overlap-type π-π. In this situation, the π-π stacking interaction is a later type. This was probably due to the strong H-bonding of the cyclodextrin rim controlling the π-π stacking interaction. However, the guest molecule when crystallized by itself also demonstrates overlap-type π-π stacking interaction. The amine group of the guest molecules is positioned outside the β-CD ring. This perhaps contributes to the slight enhancement of the solubility of the complex (1.18 mg/mL) compared to the host β-CD (1.08 mg/mL). The crystal lattice had disordered water molecules (not shown in Figure 2).
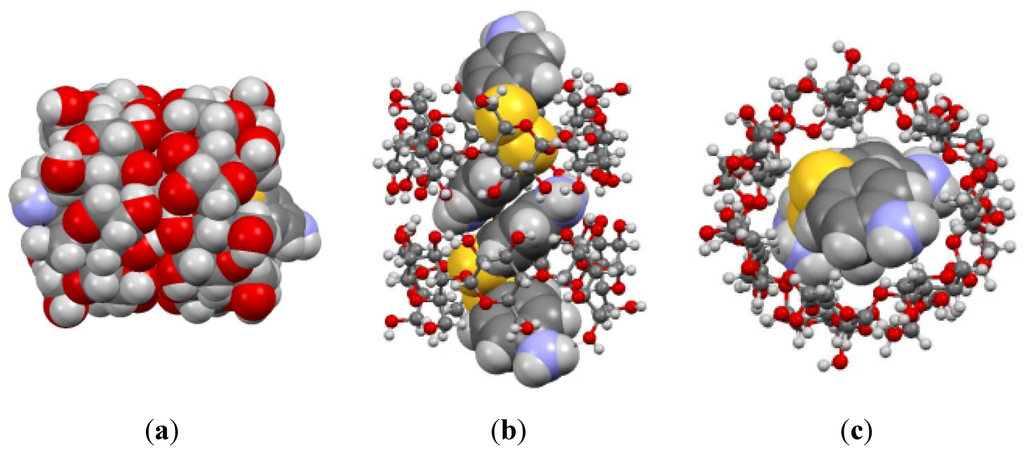
Figure 2.
(a) Side view space filling model of the host–guest complex, (b) Side view space filling-ball stick (hybrid) model of the complex and (c) top view hybrid model of the complex.
The crystal structure of 4-ATP alone reveals no similar arrangements of the aromatic rings []. Figure 3 shows the packing diagram of pure 4-ATP crystal and the 4-ATP as guest molecule in the lattice of 4-ATP/β-CD complex. The cyclodextrin molecules have been eliminated for clarity.
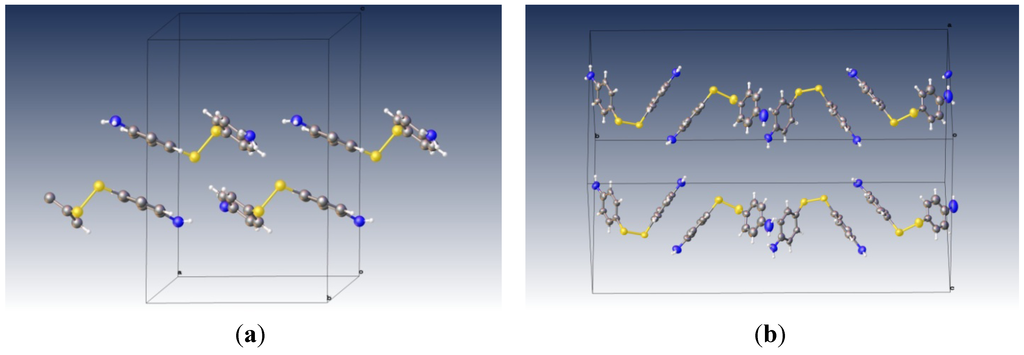
Figure 3.
(a) The packing diagram of pure 4,4'-diaminodiphenyl disulfide molecule (view: slightly rotated from along b-axis); (b) The packing diagram of 4,4'-diaminodiphenyl disulfide inside the cavity of β-CD (with the cyclodextrin molecules removed). In this view, the crystallographic b-axis is horizontal.
The solid-state structural data has been submitted to the Cambridge Crystallographic Data Center. The structure deposition number is CCDC 879687. Crystallographic data table for the complex is provided in Table 1.

Table 1.
Crystal data and structure refinement for 4-ATP/β-CD.
| Parameter | Crystal Data |
|---|---|
| CCDC deposition number | 879687 |
| Empirical formula | C54 H84 N2 O46.75 S2 |
| Formula weight | 1,573.35 |
| Temperature | 100 (±2) K |
| Wavelength | 1.54187 Å |
| Crystal system | Monoclinic |
| Space group | P21 |
| Unit cell dimensions | a = 14.8541 (±3) Å α = 90° |
| b = 32.3999 (±6) Å β = 103.750(7)° | |
| c = 15.7645 (±11) Å γ = 90° | |
| Volume | 7369.6(±6) Å3 |
| Z | 4 |
| Density (calculated) | 1.418 Mg/m3 |
| Absorption coefficient | 1.596 mm−1 |
| F(000) | 3312 |
| Crystal size | 0.09 × 0.07 × 0.05 mm3 |
| Theta range for data collection | 2.73 to 68.23° |
| Index ranges | −17 ≤ h ≤ 17, −38 ≤ k ≤ 38, −18 ≤ l ≤ 14 |
| Reflections collected | 72735 |
| Independent reflections | 25731 (R(int) = 0.0906) |
| Completeness to theta = 68.23° | 99.7% |
| Max. and min. transmission | 0.9245 and 0.8697 |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 25731/1243/1908 |
| Goodness-of-fit on F2 | 1.035 |
| Final R indices (I > 2θ(I)) | R1 = 0.1128, wR2 = 0.2285 |
| R indices (all data) | R1 = 0.2441, wR2 = 0.3136 |
| Absolute structure parameter | 0.01 (±4) |
| Extinction coefficient | 0.00061 (±7) |
| Largest diff. peak and hole | 0.964 and −0.458 e·Å−3 |
4. Conclusions
The host–guest complex of 4-ATP and β-CD was studied for structural insight. 4-ATP was found to be converted to 4,4'-diaminodiphenyl disulfide. The β-CD units were found to be involved in hydrogen bonding via the –OH groups of the larger rims of cyclodextrins in a head-to-head fashion. The modified guest molecule, 4,4'-diaminodiphenyl disulfide from two neighboring complexes were found to be parallel to each other with an average distance of 3.7(±4) Å, thereby suggesting active π-π interaction. The amine terminal of the guest molecule is positioned outside the host cavity, indicating possible contribution towards the solubility of the complex in water.
Acknowledgments
TAS acknowledges the partial support of this work by a grant from the US DOE, fund number 222174-13407.
Conflict of Interest
The authors declare no conflict of interest.
References
- Mavridis, I.M.; Hadjoudis, E.; Tsoucaris, G. The crystal structure of the inclusion complex of cyclomaltoheptaose (β-cyclodextrin) with 3,3-dimethylbutylamine. Carbohyd. Res. 1991, 220, 11–21. [Google Scholar] [CrossRef]
- Makedonopoulou, S.; Mavridis, I.M. Structure of the inclusion complex of β-cyclodextrin with 1,12-dodecanedioic acid using synchrotron radiation data; a detailed dimeric β-cyclodextrin structure. Acta Crystrallogr. B 2000, B56, 322–331. [Google Scholar] [CrossRef]
- Frampton, M.J.; Anderson, H.L. Insulated molecular wires. Angew. Chem. Int. Ed. 2007, 46, 1028–1064. [Google Scholar] [CrossRef]
- Schlenk, H.; Sand, D.M. The association of α- and β-cyclodextrins with organic acids. J. Am. Chem. Soc. 1961, 83, 2312–2320. [Google Scholar] [CrossRef]
- Gopalan, A.I.; Lee, K.-P.; Manesh, K.M.; Santhosh, P.; Kim, J.H. Gold nanoparticles dispersed into poly(aminothiophenol) as a novel electrocatalyst-Fabrication of modified electrode and evaluation of electrocatalytic activities for dioxygen reduction. J. Mol. Catal. A 2006, 256, 335–345. [Google Scholar] [CrossRef]
- Udachin, K.A.; Ripmeester, J.A. A novel mode of inclusion for pyrene in β-cyclodextrin compounds: The crystal structures of β-cyclodextrin with cyclohexanol and pyrene, and with n-octanol and pyrene. J. Am. Chem. Soc. 1998, 120, 1080–1081. [Google Scholar] [CrossRef]
- Huang, T.; Meng, F.; Qi, L. Facile synthesis and one-dimensional assembly of cyclodextrin-capped gold nanoparticles and their applications in catalysis and surface-enhanced Raman scattering. J. Phys. Chem. C 2009, 113, 13636–13642. [Google Scholar] [CrossRef]
- Nelles, G.; Weisser, M.; Back, R.; Wohlfart, P.; Wenz, G.; Mittler-neher, S. Controlled orientation of cyclodextrin derivatives immobilized on gold surfaces. J. Am. Chem. Soc. 1996, 118, 5039–5046. [Google Scholar] [CrossRef]
- Park, J.S.; Wilson, J.N.; Hardcastle, K.I.; Bunz, U.H.F.; Srinivasarao, M. Reduced fluorescence quenching of cyclodextrin-acetylene dye rotaxanes. J. Am. Chem. Soc. 2006, 128, 7714–7715. [Google Scholar]
- Buston, J.E.H.; Young, J.R.; Anderson, H.L. Rotaxane-encapsulated cyanine dyes: Enhanced fluorescence efficiency and photostability. Chem. Commun. 2000, 905–906. [Google Scholar]
- Matsuzawa, Y.; Tamura, S.-I.; Matsuzawa, N.; Ata, M. Light stability of a β-cyclodextrin inclusion complex of a cyanine dye. J. Chem. Soc. Faraday Trans. 1994, 90, 3517–3520. [Google Scholar] [CrossRef]
- Buston, J.E.H.; Marken, F.; Anderson, H.L. Enhanced chemical reversibility of redox processes in cyanine dye rotaxanes. Chem. Commun. 2001, 1046–1047. [Google Scholar]
- Stanier, C.A.; O’Connell, M.J.; Clegg, W.; Anderson, H.L. Synthesis of fluorescent stilbene and tolan rotaxanes by Suzuki coupling. Chem. Commun. 2001, 493–494. [Google Scholar]
- Terao, J.; Tang, A.; Michels, J.J.; Krivokapic, A.; Anderson, H.L. Synthesis of poly(para-phenylenevinylene) rotaxanes by aqueous Suzuki coupling. Chem. Commun. 2004, 56–57. [Google Scholar]
- Cacialli, F.; Wilson, J.S.; Michels, J.J.; Daniel, C.; Silva, C.; Friend, R.H.; Sevrin, N.; Samori, P.; Rabe, J.P.; O’Connell, M.J.; et al. Cyclodextrin-threaded conjugated polyrotaxanes as insulated molecular wires with reduced interstrand interactions. Nat. Mater. 2002, 1, 160–164. [Google Scholar] [CrossRef]
- Breslow, R.; Zhang, B. Cholesterol recognition and binding by cyclodextrin dimers. J. Am. Chem. Soc. 1996, 118, 8495–8496. [Google Scholar] [CrossRef]
- Vittal, J.J.; Anjali, K.S. Sinusoidal ribbon waves in the crystal structure of bis(4-aminophenyl) disulfide. Cryst. Eng. 1998, 1, 147–152. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).