3.2. Xyl-O1 Purification
Xyl-O1 was purified by ultrafiltration through a PM-10 ultrafiltration membrane, gel filtration chromatography on Sephacryl S-100 and Sephadex G-50 and ion exchange chromatography on Q-Sepharose. In the first step, a xylanase activity of only 0.294 IU·mL
−1 was detected in the ultrafiltrate. Because this ultrafiltrate had low protein concentration, the solution was lyophilized prior to chromatography. The chromatographic profiles from the gel filtration columns (results not shown) initially showed that the enzyme sample subjected to chromatography on a Sephacryl S-100 column eluted in two peaks that had xylanase activity. The second peak was selected because it contained a xylanase activity that was approximately 3.5 times higher than the first peak and corresponded to the fractions with the greatest absorbance at 280 nm. The pooled fractions were applied onto a Sephadex G-50 column. The xylanase activity was eluted off this column in a single peak with a low absorbance at 280 nm. An additional peak was eluted with a greater absorbance at 280 nm and an unidentified colored substance. As shown in
Figure 1, fractionation on a Q-Sepharose column revealed that Xyl-O1 eluted before the salt gradient at pH 7.0. According to Wong
et al. [
32], there is a conserved relationship between molecular weight and p
I when xylanases from different species are compared. Low-molecular-weight xylanases tend to be basic, whereas high molecular weight is associated with a more acidic p
I. An exception to this relationship is the
Schizophyllum commune xylanase (21 kDa; p
I 4.5); however, the relationship is conserved even in yeast-like fungi that lack multiple xylanases, e.g.,
Cryptococcus sp.
Figure 1.
Ion exchange chromatography of Xyl-O1 on Q-Sepharose. Absorbance at 280 nm is represented by closed diamonds [
![Applsci 02 00754 i001]()
], xylanase activity is represented by thin Xs [
![Applsci 02 00754 i002]()
], and the linear gradient (0–1.0 mol·L
−1 NaCl in equilibration buffer) is represented by a solid line [
![Applsci 02 00754 i003]()
].
Figure 1.
Ion exchange chromatography of Xyl-O1 on Q-Sepharose. Absorbance at 280 nm is represented by closed diamonds [
![Applsci 02 00754 i001]()
], xylanase activity is represented by thin Xs [
![Applsci 02 00754 i002]()
], and the linear gradient (0–1.0 mol·L
−1 NaCl in equilibration buffer) is represented by a solid line [
![Applsci 02 00754 i003]()
].
Table 2.
Summary of purification of Xyl-O1.
Table 2.
Summary of purification of Xyl-O1.
| Purification steps | Total volume (mL) | Total protein (mg) | Total activity (IU) | Specific activity (IU·mg−1) * | Purification (fold) | Yield (%) |
|---|
| Filtered culture supernatant | 110.00 | 5.830 | 53.350 | 9.151 | 1.000 | 100.00 |
| Ultrafiltrate | 100.00 | 1.500 | 29.400 | 19.600 | 2.142 | 55.11 |
| Lyophilization | 4.50 | 0.828 | 1.647 | 1.989 | 0.217 | 3.08 |
| Sephacryl S-100 (fractions 58–64) | 21.00 | 0.441 | 7.833 | 17.762 | 1.941 | 14.68 |
| Sephadex G-50 (fractions 71–84) | 40.00 | 0.080 | 9.880 | 123.500 | 13.496 | 18.52 |
| Q-Sepharose FF (fractions 7–18) | 36.00 | 0.036 | 8.928 | 248.000 | 27.101 | 16.74 |
As noted in
Table 2, the xylanolytic activity remaining in the ultrafiltrate portion after lyophilization was considerably recovered during fractionation using gel filtration and ion exchange chromatography. In addition,
Table 2 also shows Xyl-O1 purification and yield values of 27.1% and 16.7%, respectively. These values are consistent with other studies involving xylanase purification in which the recovery was low [
33,
34,
35,
36]. The ultrafiltration procedure retained most of the β-xylanase activity in the concentrate. The absence of other isoforms or isoenzymes of xylanase in the ultrafiltrate portion, which act synergistically in the hydrolysis of the substrate, may have underestimated the purification and yield values during the final fractionation step [
14].
However, our fractionation method, beginning with protein concentration by lyophilization, resulted in higher purification and yield compared to other fractionation methods that are initiated by precipitation with (NH
4)
2SO
4 (results not shown). However, the total xylanase activity of the ultrafiltrate (29.400 IU) was significantly reduced to 1.647 IU after the lyophilization step. This finding suggests the presence of xylanase inhibitors that were produced as a result of PDCR degradation and that were also concentrated during the lyophilization step. According to Panagiotou and Olsson [
37], the lignin derivatives (furans, phenols and low-molecular-weight acids) that are found in the hydrolysate of pretreated wheat straw inactivated both a mixture of commercial enzymes and a crude enzyme sample from
Penicillium brasilianum IBT 20888 during the enzymatic digestion of filter paper and xylan. The purification procedures provided an apparently homogeneous preparation of xylanase activity from
A. oryzae. An increase in the specific activity from 9.15 IU·mg
−1 in the FCS to 248 IU·mg
−1 at the end of chromatography was observed. This is an approximately 27-fold increase and confirms the efficiency of the Xyl-O1 purification. The apparent purity of the enzyme was demonstrated by SDS-PAGE because under denaturing conditions, the gel showed a single band (
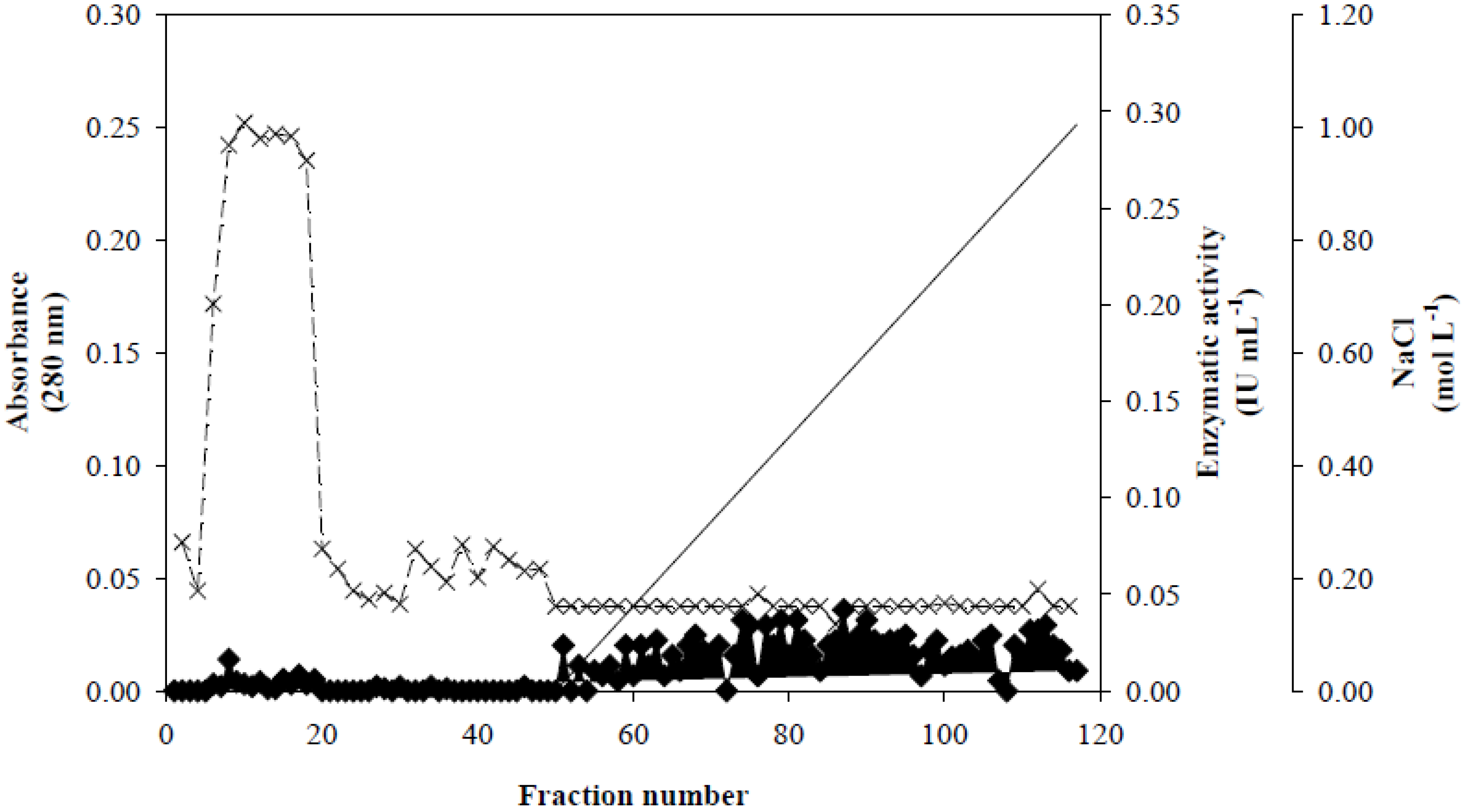
Figure 2).
Figure 2.
SDS-PAGE (12%) of purified Xyl-O1. The gel was stained with (A) silver nitrate or (B) 0.1% Congo red. MW—molecular weight marker (phosphorylase b (97 kDa), albumin (66 kDa), ovalbumin (45 kDa), carbonic anhydrase (30 kDa), trypsin inhibitor (20.1 kDa), and α-lactalbumin (14.4 kDa)); Lane 1—FCS from Aspergillus oryzae (10 µg); Lane 2—Purified Xyl-O1 (5.0 µg); Lane 3—FCS from A. oryzae; Lane 4—Purified Xyl-O1.
Figure 2.
SDS-PAGE (12%) of purified Xyl-O1. The gel was stained with (A) silver nitrate or (B) 0.1% Congo red. MW—molecular weight marker (phosphorylase b (97 kDa), albumin (66 kDa), ovalbumin (45 kDa), carbonic anhydrase (30 kDa), trypsin inhibitor (20.1 kDa), and α-lactalbumin (14.4 kDa)); Lane 1—FCS from Aspergillus oryzae (10 µg); Lane 2—Purified Xyl-O1 (5.0 µg); Lane 3—FCS from A. oryzae; Lane 4—Purified Xyl-O1.
The molecular weight of the purified Xyl-O1 was 21.5 kDa, as estimated by the relative migration of the standard markers. A low-molecular-weight xylanase can be useful in pulp bleaching, since smaller enzymes can penetrate further the fiber wall structure and alter more efficiently pulp properties. The molecular weight of Xyl-O1 was also estimated by gel filtration chromatography on Sephadex G-50; however, the value was less than 10 kDa (result not shown), suggesting that the enzyme interacted with the matrix. The same phenomenon has been described for β-xylosidase from
Trichoderma harzianum strains [
17] and for a low-molecular-weight xylanase from
A. fumigatus Fresenius [
34]. According to Poutanen [
35], the reason for the lower apparent molecular weight that is obtained by gel filtration could be the retarded migration of the enzyme in the gel matrix.
3.3. Enzyme Characterization
The xylanase activities of the FCS and purified Xyl-O1 were greatest in the temperature range of 45–60 °C and the pH range of 5.5 to 6.0. The two samples were stable in the temperature range of 45–60 °C, with xylanase activity greater than 70%. The greatest FCS activity was observed at 55 °C, and the greatest Xyl-O1 activity was at 50 °C. The two enzyme samples presented the best activity at pH 6.0 in sodium acetate buffer. The activity of the two samples was greater than 90% in the pH range of 5.5 to 6.0. Other studies involving the purification and characterization of endo-β-1,4-xylanases from filamentous fungi have indicated that 50 °C is the most effective temperature for enzyme activity [
33,
38,
39]. Our results are consistent with Kimura
et al. [
40], who found that the greatest activity for a xylanase, XynG2, that was isolated from a genomic library of
A. oryzae KBN616 was at pH 6.0. However, its greatest enzymatic activity was at 58 °C, and it displayed a different Km value (5.1 mg·mL
−1) on birchwood as the substrate. The K
m values (mg·mL
−1) of Xyl-O1 on a soluble fraction of oat spelt and birchwood xylans were 10.05 and 3.34, respectively. The apparent K
m values indicated that the purified Xyl-O1 had a preference for birchwood xylan as a substrate. Birchwood xylan contains 90% xylose, whereas oat spelt xylan contains 75% xylose, 10% arabinose and 15% glucose residues. The preference of Xyl-O1 for substrates containing a higher proportion of
D-xylose residues is a relevant property that can be used in the future targeting of this enzyme for alternative methods of cellulose pulp biobleaching.
The Xyl-O1 enzyme activity was reduced to 86 and 8% in the presence of Hg
2+ at concentrations of 1.0 and 10 mmol·L
−1, respectively. The same inhibitory effect was observed for Ag
+ at a concentration of 10 mmol·L
−1. The inhibitory effects of Hg
2+ and Ag
+ on xylanase activity are known [
7,
25,
41,
42]. According to Sandrim
et al. [
41], the addition of Hg
2+ drastically inhibits xylanolytic activity, suggesting the existence of thiol groups in the catalytic site of the enzyme. In general, purified Xyl-O1 remained stable in the presence of most of the ions that we tested. However, Mn
2+, which is commonly referred to as an activator of xylanase activity, showed an inhibitory effect at a concentration of 10 mmol·L
−1 and promoted a 10% reduction in activity compared to the control. The same inhibitory effect was observed when the enzyme was pre-incubated for 40 min at 28 °C with the same concentration of Mn
2+. Conversely, Carmona
et al. [
43] and Teixeira
et al. [
39] reported an activating effect of 10 mmol·L
−1 Mn
2+ on xylanase from
A. versicolor and
A. awamori 2B.361 U2/1. Here, purified Xyl-O1 in the presence of amino acid-modifying reagents and amino acids remained relatively stable or was activated by certain reagents (
Table 3). The enzyme retained 74% of its xylanolytic activity in the presence of 20 mmol·L
−1 SDS.
The reaction mixture containing 5.0 mmol·L
−1 L-tryptophan did not have increased enzymatic activity. However, for compounds containing thiol groups, such as
L-cysteine, DTT and β-mercaptoethanol, the xylanase activity increased by 40, 14 and 37%, respectively. The enzyme was stable in the presence of NBS and EDC reagents at concentrations of 1.0 and 5.0 mmol·L
−1, respectively. However, the enzyme activity was completely inhibited by NBS at a concentration of 10 mmol·L
−1 (result not shown). Teixeira
et al. [
39] also reported the inhibition of xylanase from
A. awamori by NBS at the same concentration. NBS is an effective inhibitor of xylanases [
44,
45] and promotes the oxidation of tryptophan residues that are involved in enzymatic catalysis. Xyl-O1 was slightly inhibited by the alkylating reagent iodoacetamide, indicating the need for thiol groups in catalysis. DEPC and DTP reduced the Xyl-O1 activity by 15 and 12%, respectively (
Table 3).
Table 3.
Effect of modifying agents and amino acids on the xylanolytic activity of purified Xyl-O1.
Table 3.
Effect of modifying agents and amino acids on the xylanolytic activity of purified Xyl-O1.
| Modifying agents or amino acids | Activity (IU·mL−1) | Relative activity (%) | Concentration (mmol·L−1) |
|---|
| H2O control | 0.262 ± 0.021 | 100.00 | --- |
| 4% Ethanol control | 0.285 ± 0.026 | 100.00 | --- |
| NBS | 0.285 ± 0.079 | 108.42 | 1 |
| DTNB * | 0.399 ± 0.025 | 140.02 | 2 |
| DTT | 0.300 ± 0.037 | 114.35 | 20 |
| L-Cysteine | 0.367 ± 0.076 | 140.04 | 20 |
| | | | |
| H2O control | 0.290 ± 0.029 | 100.00 | --- |
| 4% Ethanol control | 0.322 ± 0.006 | 100.00 | --- |
| DTP * | 0.285 ± 0.025 | 88.43 | 2 |
| L-tryptophan | 0.285 ± 0.012 | 98.13 | 5 |
| Iodoacetamide | 0.255 ± 0.046 | 87.71 | 5 |
| DEPC | 0.248 ± 0.037 | 85.32 | 5 |
| EDC | 0.291 ± 0.046 | 100.08 | 5 |
| β-Mercaptoethanol | 0.397 ± 0.026 | 136.68 | 5 |
| | | | |
| H2O control | 0.306 ± 0.031 | 100.00 | --- |
| SDS | 0.225 ± 0.025 | 73.56 | 20 |
The assays investigating the effect of incubation time on Xyl-O1 activity at pH 7.0 revealed that at 50 °C, the enzyme remained active for 5.0 h, retaining 60% of its activity. Conversely, at 55 °C, the activity decreased to less than 30% within the first hour of incubation. The thermostability of Xyl-O1 at 50 °C was greatly increased in the presence of 20 mmol·L
−1 L-cysteine, and the activity remained close to 100% for an incubation period of 6.0 h (
Figure 3(A)). At 55 °C, the xylanase activity was enhanced in the presence of
L-cysteine and remained stable for 1.0 h, with the catalytic activity remaining greater than 75% (
Figure 3(B)).
Figure 3.
Effect of 20 mmol·L
−1 L-cysteine on the thermostability of purified Xyl-O1 at pH 7.0 and (
A) 50 °C or (
B) 55 °C [
![Applsci 02 00754 i004]()
]. The control without
L-cysteine [
![Applsci 02 00754 i005]()
].
Figure 3.
Effect of 20 mmol·L
−1 L-cysteine on the thermostability of purified Xyl-O1 at pH 7.0 and (
A) 50 °C or (
B) 55 °C [
![Applsci 02 00754 i004]()
]. The control without
L-cysteine [
![Applsci 02 00754 i005]()
].
The purified Xyl-O1 efficiently hydrolyzed the substrates that contained
D-xylose residues, especially the soluble fraction of oat spelt and the birchwood and beechwood xylans (
Table 4). These results suggest that Xyl-O1 is a potential candidate for the pulp bleaching processes, whereas the application of xylanases in the pulp and paper industry requires a cellulase-free activity [
8,
11,
41,
46]. However, the enzyme activity was significantly reduced in the insoluble fractions of the same substrates, especially for oat spelt xylan, which had a relative activity of less than 1.0%. The enzyme also showed a hydrolysis efficiency of 49.87% when tested with 4-
O-methyl-glucurono-
D-xylan as the substrate. The relative activities for the birchwood and beechwood xylans were 85 and 84.4%, respectively (
Table 4). The residual activity was less than 10% for filter paper, CM-cellulose, laminarin, mannan and pectin, indicating that these substrates might have been contaminated with pentosan, which is susceptible to hydrolysis [
14]. Furthermore, Xyl-O1 was completely inactive against the avicel and
p-nitrophenylglycoside substrates (
Table 4).
Table 4.
Substrate specificity of purified Aspergillus oryzae Xyl-O1.
Table 4.
Substrate specificity of purified Aspergillus oryzae Xyl-O1.
| Substrate | Main chain linkage | Activity (IU·mL−1) | Purified Xyl-O1 (% RA)
a |
|---|
| Oat spelt xylan (S)
b | β-1,4 | 0.659 ± 0.005 | 100.00 |
| Oat spelt xylan (I)
c | β-1,4 | 0.003 ± 0.004 | 0.39 |
| Birchwood xylan (S) | β-1,4 | 0.560 ± 0.011 | 85.03 |
| Birchwood xylan (I) | β-1,4 | 0.228 ± 0.025 | 34.60 |
| Beechwood xylan (S) | β-1,4 | 0.556 ± 0.030 | 84.38 |
| Beechwood xylan (I) | β-1,4 | 0.024 ± 0.030 | 3.58 |
| 4-
O-methyl-glucurono-D-xylan (I) | β-1,4 | 0.328 ± 0.028 | 49.87 |
| Filter paper | β-1,4 | 0.010 ± 0.007 | 1.46 |
| CM-cellulose | β-1,4 | 0.045 ± 0.004 | 6.82 |
| Avicel | β-1,4 | 0.000 ± 0.002 | 0.00 |
| Laminarin | β-1,3 | 0.039 ± 0.018 | 5.93 |
| Pectin | β-1,4 | 0.024 ± 0.019 | 3.62 |
| Mannan | β-1,4 | 0.002 ± 0.003 | 0.37 |
| PNPX | PNP-β-1,4 | 0.000 ± 0.000 | 0.00 |
| PNPA | PNP-α-1,4 | 0.000 ± 0.000 | 0.00 |
| PNPG | PNP-β-1,4 | 0.000 ± 0.000 | 0.00 |
| PNPM | PNP-β-1,4 | 0.000 ± 0.000 | 0.00 |
The specificity of Xyl-O1 was determined by analyzing the hydrolysis products of the soluble and insoluble birchwood xylan using the DNS method and HPLC on a Dionex system. The results presented in
Table 5 show that the enzyme was more active on the soluble fraction, with a maximum hydrolysis of approximately 33% obtained after 12 h of incubation. A similar effect was observed with the insoluble fraction, but the hydrolysis reached only 17.5%. These results are consistent with the observations of Ryan
et al. [
25], who also found higher rates of hydrolysis for soluble xylan. The preference of Xyl-O1 for the soluble fraction of the substrate may indicate a catalytic efficiency for the branched sites of
O-acetyl-(4-
O-methyl-
D-glucurono) in the structure of the birchwood xylan. According to Coughlan and colleagues, certain constituents in the structure of the xylan act as binding sites for the catalytic activity of some xylanases [
11]. The residues that comprise these sites in the branching structure were partially removed during the preparative alkaline and acid extractions that were performed on the soluble and insoluble fractions of the substrate. However, as shown in
Table 5, we observed that 18– and 24 h hydrolysis periods did not increase the amount of reducing sugar that was released. It is possible that Xyl-O1-promoted hydrolysis is more efficient after 12 h and represents a plateau of activity that remains constant for longer incubations.
Table 5.
Hydrolysis of soluble or insoluble birchwood xylan catalyzed by purified Xyl-O1.
Table 5.
Hydrolysis of soluble or insoluble birchwood xylan catalyzed by purified Xyl-O1.
| Hydrolysis time (h) | Soluble | Insoluble |
|---|
| Specific reducing sugar (mg) * | Hydrolysis (%) | Specific reducing sugar (mg) * | Hydrolysis (%) |
|---|
| 0 | 0.000 ± 0.000 | 0.00 | 0.031 ± 0.006 | 1.55 |
| 6 | 0.434 ± 0.011 | 21.72 | 0.209 ± 0.036 | 10.47 |
| 12 | 0.667 ± 0.029 | 33.35 | 0.351 ± 0.057 | 17.54 |
| 18 | 0.554 ± 0.025 | 27.69 | 0.345 ± 0.039 | 17.23 |
| 24 | 0.554 ± 0.025 | 27.70 | 0.350 ± 0.037 | 17.50 |
The identification and quantitative analysis of the soluble xylooligosaccharides that were released seem to indicate that the enzyme catalyzes the random cleavage of internal glycosidic linkages in the soluble and insoluble fractions of the birchwood xylan (
Table 6). A similar activity has been described for other enzymes, including the endo-β-1,4-xylanases produced by
Penicillium capsulatum [
25],
Cephalosporium sp. RYM-202 strain [
38],
A. fumigatus Fresenius [
34] and
Acrophialophora nainiana [
33]. As shown in
Table 6, the hydrolysis products X2-X6 predominated during the first 12 h of incubation. During the subsequent 12 h, either the X4-X6 xylooligomers predominated, or it was not possible to identify the products, as shown for the insoluble fraction after 24 h (
Table 6). The decreased identification or quantification of the hydrolysis products by HPAEC-PAD indicated the accumulation of higher xylooligomers after the elution of the xylohexaose standard primarily during incubation periods of 18 and 24 h (results not shown). This suggests that the purified Xyl-O1 is involved in transglycosylation reactions at certain times. In this case, the chromatographic profile showed irregular peaks presenting more intense electrochemical signals and longer retention times than those of established xylooligomers patterns. Because only xylooligosaccharides containing six or fewer xylose units could be detected with the selected chromatographic parameters, we postulated that the products with a higher degree of polymerization (DP) could not be identified by the absence of xylooligomer patterns with longer retention times. With regard to the highest hydrolysis percentage obtained with the DNS method, we hypothesize that the identification of the hydrolysis products by their retention times results in greater specificity. Conversely, the detection of reducing sugars by the DNS method, even using xylose as reference, would not distinguish between the reducing ends of the xylose or more complex xylooligomers. Indeed, the effect of transglycosylation could explain the constant rate of hydrolysis obtained by the DNS method after 12 h of incubation (
Table 5). As reported by Biely and Vranská [
47], the transfer of glycosyl groups is a well-known activity of glycosidases and glycanases. Consistent with the findings of these authors, the enzyme acts on oligosaccharides or aryl glycosides (e.g., invertase, β-galactosidase,
A. niger β-glucosidase) to catalyze the formation of various positional isomers of the oligosaccharides. These positional isomers retain the configuration of the glycosidic linkage without specificity for the saccharide acceptor molecule and one particular linkage. For endo-acting glycanases, such as lysozyme, α-amylases and cellulases, the specificity is evident, and the same type of glycosidic linkage in the saccharide acceptors is formed from the oligosaccharide glycosyl donors that were cleaved previously. In addition, the xylanase produced by
Anoxybacillus flavithermus BC [
48] catalyzes this transglycosylation activity. In other studies, endo-1,4-β-xylanases and β-xylosidases have been used for the synthesis of specific oligosaccharides [
49,
50] to obtain a cellulose-xylan polymer hybrid [
51]. Our results suggest that the transglycosylation reaction catalyzed by Xyl-O1 can occur after a period of effective hydrolysis, in this case, after a 12 h incubation at 28 °C. However, specific testing of the transglycosylation reaction is required to confirm this hypothesis of Xyl-O1-promoted catalysis.
Table 6 shows the general profile of hydrolysis over the 6.0–24 h incubation period, confirming the predominance of X2 because of the action of Xyl-O1 on the soluble and insoluble fractions of the substrate. For the insoluble and soluble fractions, Xyl-O1 showed a hydrolysis profile with a more relaxed specificity, allowing for the quantification of the products X1 to X6 within six hours of incubation. During this incubation period, xylobiose was the main hydrolysis product released through the activity of the enzyme (
Table 6).
Table 6.
Identification and quantitative analysis by high-performance anion exchange chromatography coupled with pulsed amperometric detection (HPAEC-PAD) of the xylooligosaccharides released during the hydrolysis reactions catalyzed by purified Xyl-O1.
Table 6.
Identification and quantitative analysis by high-performance anion exchange chromatography coupled with pulsed amperometric detection (HPAEC-PAD) of the xylooligosaccharides released during the hydrolysis reactions catalyzed by purified Xyl-O1.
| Xylooligosaccharide products * | Hydrolysis (%) Soluble Fraction | Hydrolysis (%) Insoluble Fraction |
|---|
| Incubation time (h) | Incubation time (h) |
|---|
| 6 | 12 | 18 | 24 | 6 | 12 | 18 | 24 |
|---|
| X1 | 0.005 | 0.000 | 0.000 | 0.000 | 0.015 | 0.000 | 0.000 | 0.000 |
| X2 | 0.178 | 0.280 | 0.000 | 0.000 | 0.245 | 0.000 | 0.000 | 0.000 |
| X3 | 0.078 | 0.000 | 0.008 | 0.000 | 0.083 | 0.047 | 0.000 | 0.000 |
| X4 | 0.022 | 0.000 | 0.045 | 0.016 | 0.102 | 0.063 | 0.027 | 0.000 |
| X5 | 0.031 | 0.000 | 0.005 | 0.005 | 0.066 | 0.055 | 0.005 | 0.000 |
| X6 | 0.033 | 0.000 | 0.005 | 0.000 | 0.052 | 0.022 | 0.010 | 0.000 |
The reduced percentage of X1, especially during the incubation period of 6 h, suggests that Xyl-O1 has an endo-acting mechanism, whereas the predominant hydrolysis product released by Xyl-O1 was X2, corresponding to 0.458% in the range of 6 to 12 h for the soluble fraction and 0.245% for the insoluble fraction after 12 h of incubation (
Table 6). Additionally, the total hydrolysis percentages obtained by the release of X4, X5 and X6 from the insoluble fraction were 0.192, 0.126 and 0.084%, respectively, in the range of 6 to 18 h. This result shows that the purified Xyl-O1 presents catalytic flexibility by acting on both soluble and insoluble substrates.
 ], xylanase activity is represented by thin Xs [
], xylanase activity is represented by thin Xs [  ], and the linear gradient (0–1.0 mol·L−1 NaCl in equilibration buffer) is represented by a solid line [
], and the linear gradient (0–1.0 mol·L−1 NaCl in equilibration buffer) is represented by a solid line [  ].
].
 ], xylanase activity is represented by thin Xs [
], xylanase activity is represented by thin Xs [ 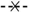 ], and the linear gradient (0–1.0 mol·L−1 NaCl in equilibration buffer) is represented by a solid line [
], and the linear gradient (0–1.0 mol·L−1 NaCl in equilibration buffer) is represented by a solid line [  ].
].
 ]. The control without L-cysteine [
]. The control without L-cysteine [  ].
].
 ]. The control without L-cysteine [
]. The control without L-cysteine [  ].
].




