Magnetic Amphiphilic Composites Applied for the Treatment of Biodiesel Wastewaters
Abstract
:1. Introduction
2. Experimental Section
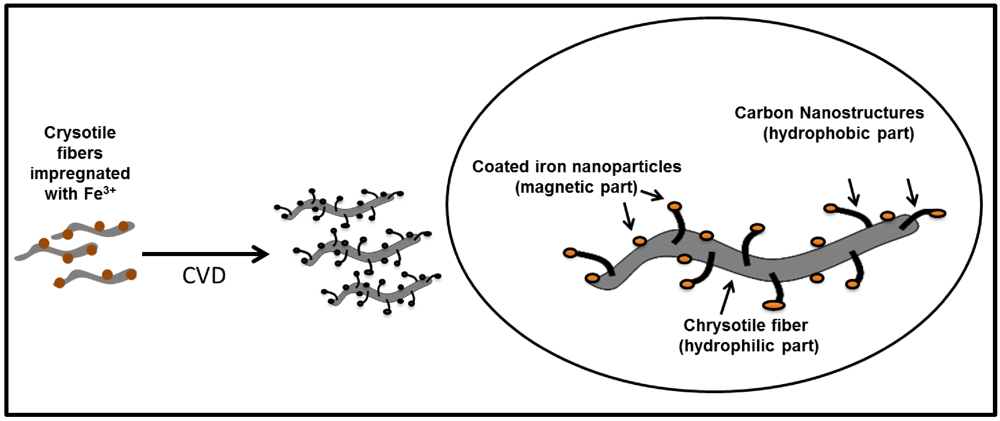
2.1. Synthesis and Characterization of Magnetic Amphiphilic Composites
2.2. Emulsion Studies
3. Results and Discussion
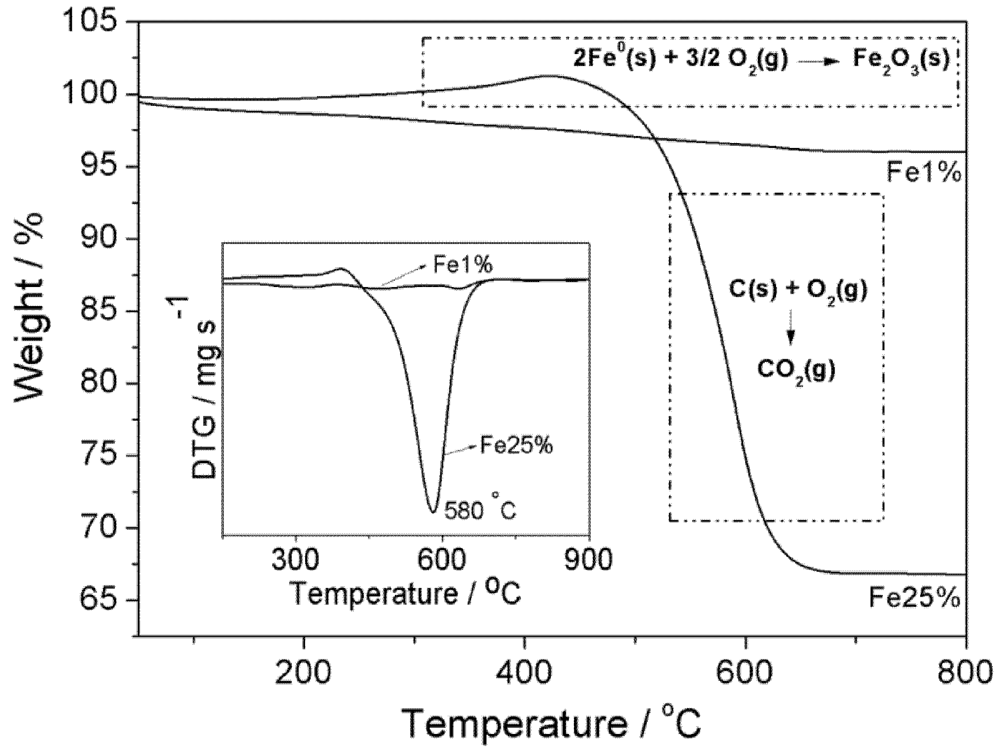
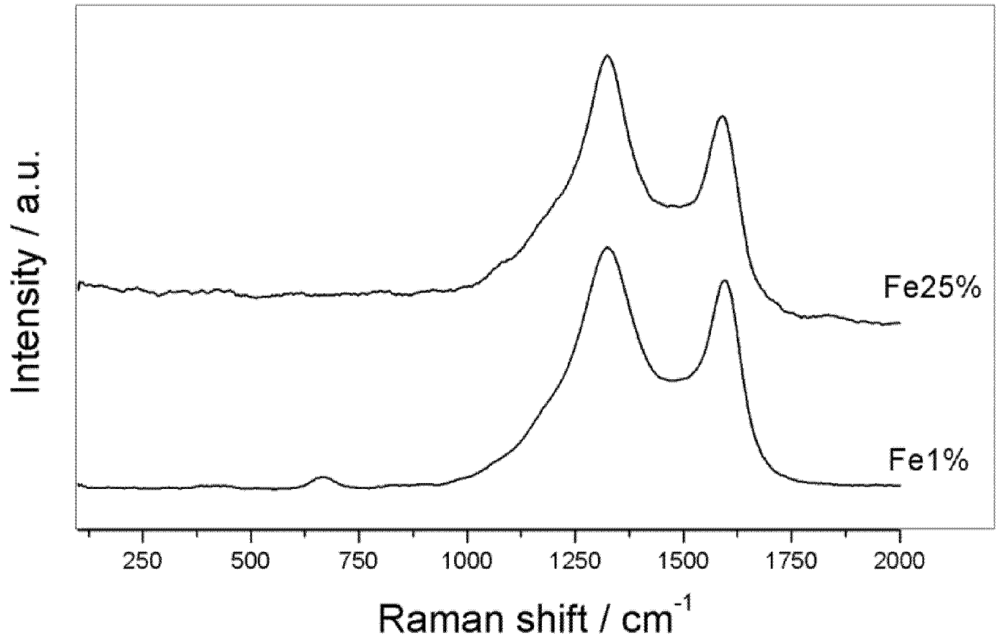
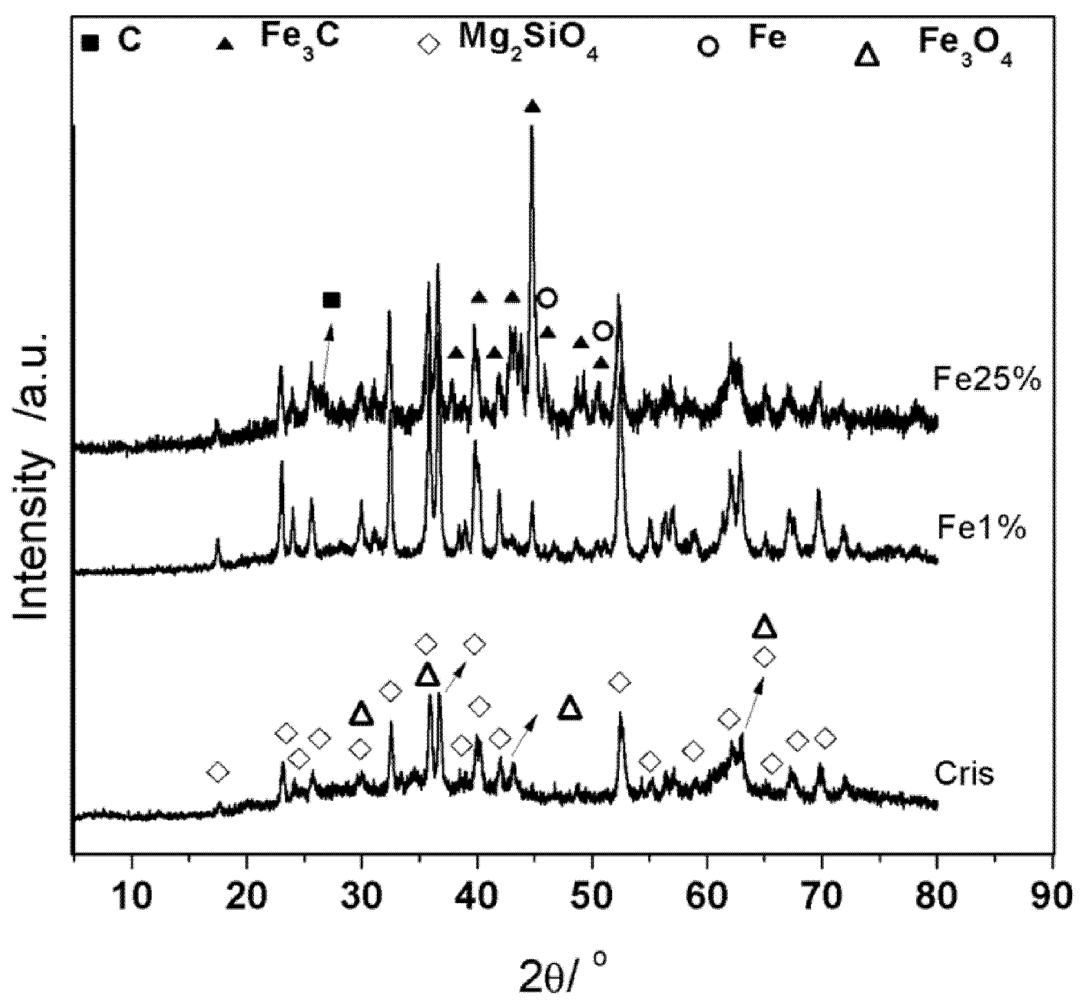
3.1. Synthesis and Characterization

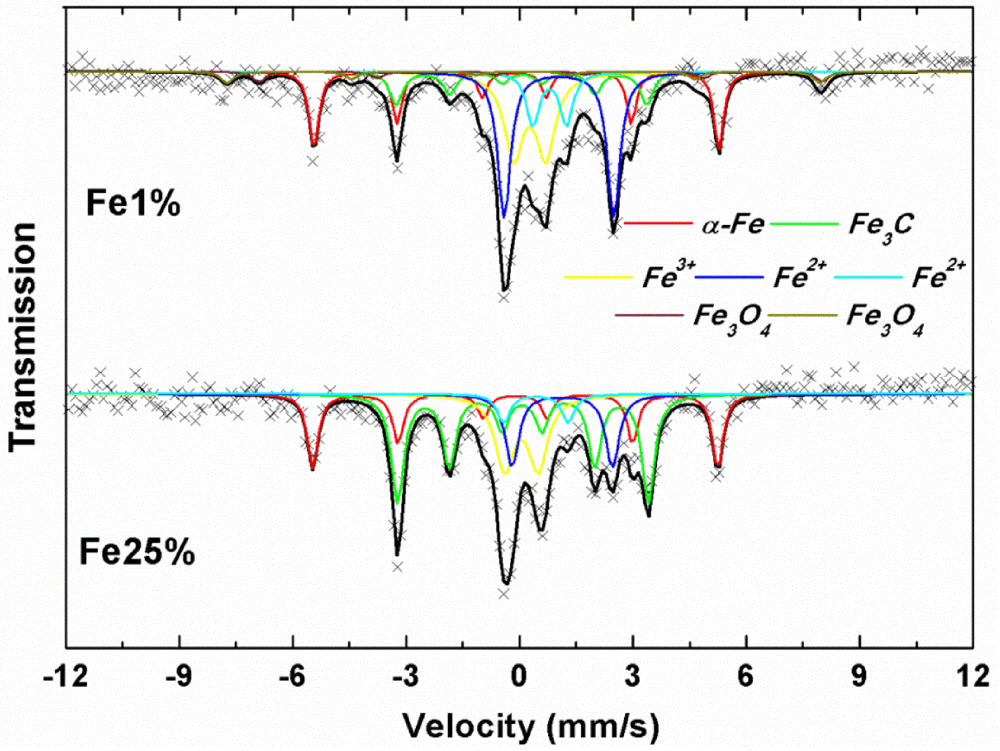
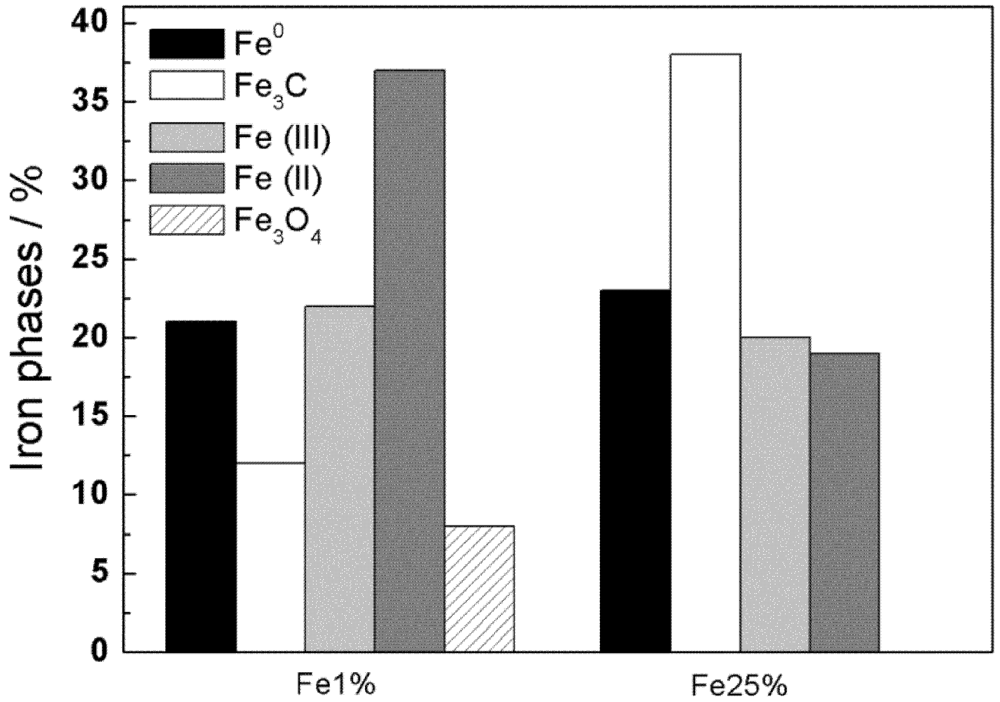
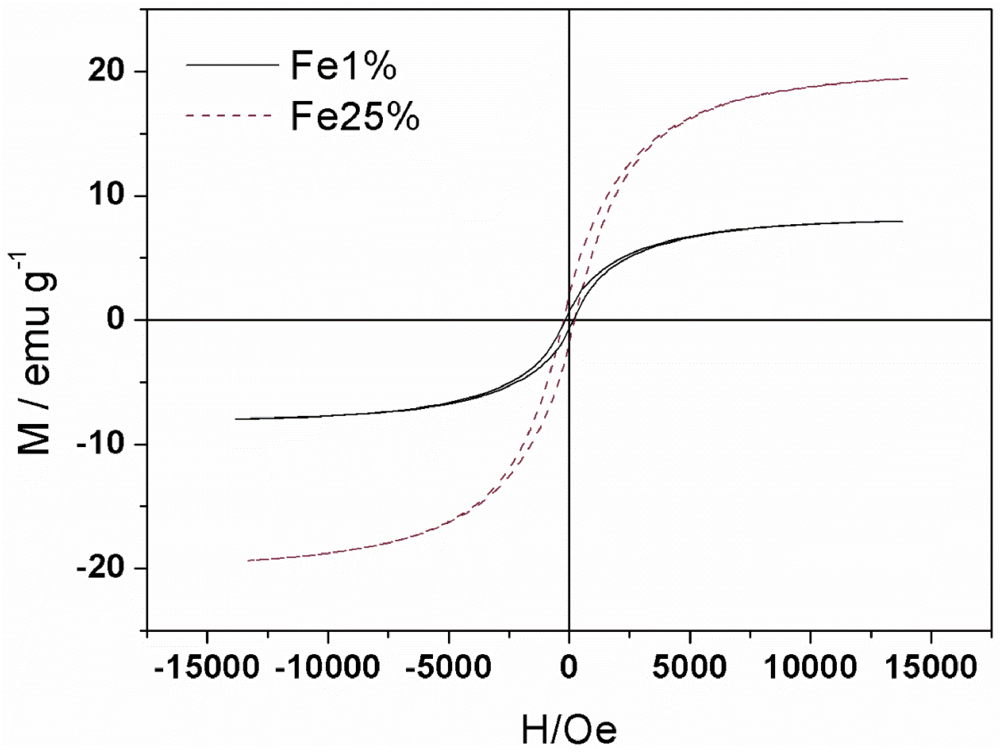
| Sample | MS(emu/g) | MR(emu/g) | S=MR/MS | HC(Oe) |
|---|---|---|---|---|
| Fe1% | 7.97 | 0.63 | 0.08 | 158 |
| Fe25% | 19.46 | 1.91 | 0.10 | 226 |
3.2. Emulsion Studies
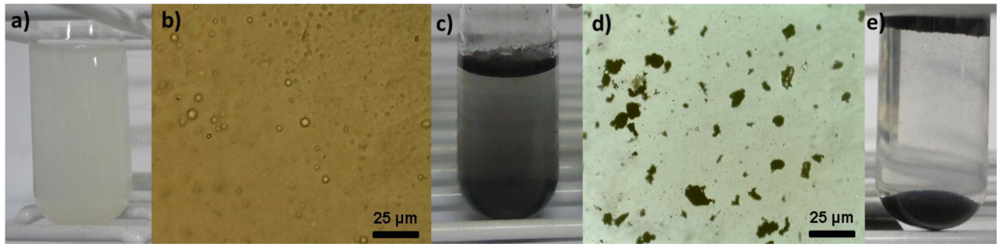
| Materials | Surface Area/m2·g−1 | Turbidity Reduction/% | Process |
|---|---|---|---|
| Fe1% | 7 | 86 | Mixture of composites and simple magnetic separation |
| Fe25% | 51 | 97 | Mixture of composites and simple magnetic separation |
| Chrysotile Fibers | 22 | 38 | Mixture of chrysotile and filtration |
| Chrysotile Fibers/800 °C | 7 | 2 | Mixture of thermally treated chrysotile and filtration |
| AC | 866 | 77 | Mixture of activated carbon and filtration |
4. Conclusions
Acknowledgments
References
- Kraft, D.J.; Luigjes, B.; de Folter, J.W.J.; Philipse, A.P.; Kegel, W.K. Evolution of Equilibrium Pickering Emulsions-A Matter of Time Scales. J. Phys. Chem. B 2010, 114, 12257–12263. [Google Scholar]
- Wen, Y.; Cheng, H.; Lu, L.J.; Liu, J.; Feng, Y.; Guan, W.; Zhou, Q.; Huang, X.F. Analysis of biological demulsification process of water-in-oil emulsion by Alcaligenes sp S-XJ-1. Bioresource Technol. 2010, 101, 8315–8322. [Google Scholar]
- Alvarez, G.; Poteau, S.; Argillier, J.F.; Langevin, D.; Salager, J.L. Heavy oil-water interfacial properties and emulsion stability: Influence of dilution. Energ. Fuels 2009, 23, 294–299. [Google Scholar]
- Basheer, C.; Suresh, V.; Renu, R.; Lee, H.K. Development and application of polymer-coated hollow fiber membrane microextraction to the determination of organochlorine pesticides in water. J. Chromatogr. A 2004, 1033, 213–220. [Google Scholar]
- Lee, J.; Jha, A.K.; Bose, A.; Tripathi, A. Imaging new transient nanostructures using a microfluidic chip integrated with a controlled environment vitrification system for cryogenic transmission electron microscopy. Langmuir 2008, 24, 12738–12741. [Google Scholar]
- Ma, X.W.; Zhao, Y.L.; Liang, X.J. Theranostic nanoparticles engineered for clinic and pharmaceutics. Accounts Chem. Res. 2011, 44, 1114–1122. [Google Scholar] [CrossRef]
- Hebrant, M.; Tecilla, P.; Scrimin, P.; Tondre, C. Copper(II) complexation by hydrophobic single- and double-alkyl chain ligands solubilized in ammonium surfactant vesicles. Langmuir 1997, 13, 5539–5543. [Google Scholar] [CrossRef]
- Oliveira, A.A.S.; Teixeira, I.F.; Ribeiro, L.P.; Tristao, J.C.; Dias, A.; Lago, R.M. Magnetic amphiphilic composites based on carbon nanotubes and nanofibers grown on an inorganic matrix: Effect on water-oil interfaces. J. Brazil. Chem. Soc. 2010, 21, 2184–2188. [Google Scholar]
- Oliveira, A.A.S.; Tristao, J.C.; Ardisson, J.D.; Dias, A.; Lago, R.M. Production of nanostructured magnetic composites based on Fe(0) nuclei coated with carbon nanofibers and nanotubes from red mud waste and ethanol. Appl. Catal. B-Environ. 2011, 105, 163–170. [Google Scholar] [CrossRef]
- Teixeira, A.P.C.; Purceno, A.D.; Barrosa, A.S.; Lemos, B.R.S.; Ardisson, J.D.; Macedo, W.A.A.; Nassor, E.C.O.; Amorim, C.C.; Moura, F.C.C.; Hernández-Terrones, M.G.; Lago, F.M.P.M. Amphiphilic magnetic composites based on layered vermiculite and fibrous chrysotile with carbon nanostructures: Application in catalysis. Catal. Today 2012, in press.. [Google Scholar]
- Teixeira, A.P.D.C.; Lemos, B.R.S.; Magalhães, L.A.; Ardisson, J.D.; Lago, R.M.; Furtado, C.A.; Santos, A.P. Temperature programmed CVD: A novel technique to investigate carbon nanotube synthesis on FeMo/MgO Catalysts. J. Nanosci. Nanotechnol. 2012, 12, 2661–2667. [Google Scholar]
- Purceno, A.D.; Teixeira, A.P.C.; Souza, N.J.d.; Fernandez-Outon, L.E.; Ardisson, J.D.; Lago, R.M. Hybrid magnetic amphiphilic composites based on carbon nanotube/nanofibers and vermiculite as efficient adsorbent for ethynilestradiol. J. Colloid Interface Sci. 2012, in press.. [Google Scholar]
- Purceno, A.D.; Barrioni, B.R.; Dias, A.; da Costa, G.M.; Lago, R.M.; Moura, F.C.C. Carbon nanostructures-modified expanded vermiculites produced by chemical vapor deposition from ethanol. Appl. Clay Sci. 2011, 54, 15–19. [Google Scholar] [CrossRef]
- Thompson, S.K.; Mason, E. Asbestos: Mineral and fibers. J. Chem. Health Saf. 2002, 21–23. [Google Scholar]
- Morgan, A. Acid leaching studies of chrysotile asbestos from mines in the Coalinga region of California and from Quebec and British Columbia. Ann. Occup. Hyg. 1997, 41, 249–265. [Google Scholar]
- Boni, L.d.; Goldani, E.; Milcharek, C.; Santos, F. Tratamento físico-químico da água de lavagem proveniente da purificação do biodiesel. Tche-química 2007, 7, 41–50. [Google Scholar]
- Trigueiro, J.P.C.; Silva, G.G.; Lavall, R.L.; Furtado, C.A.; Oliveira, S.; Ferlauto, A.S.; Lacerda, R.G.; Ladeira, L.O.; Liu, J.W.; Frost, R.L.; George, G.A. Purity evaluation of carbon nanotube materials by thermogravi metric, TEM, and SEM methods. J. Nanosci. Nanotechnol. 2007, 7, 3477–3486. [Google Scholar]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman spectroscopy of carbon nanotubes. Phys. Rep.-Rev. Sect. Phys. Lett. 2005, 409, 47–99. [Google Scholar]
- Jorio, A.; Pimenta, M.A.; Souza, A.G.; Saito, R.; Dresselhaus, G.; Dresselhaus, M.S. Characterizing carbon nanotube samples with resonance Raman scattering. New J. Phys. 2003, 5, 139:1–139:17. [Google Scholar]
- Cattaneo, A.; Gualtieri, A.F.; Artioli, G. Kinetic study of the dehydroxylation of chrysotile asbestos with temperature by in situ XRPD. Phys. Chem. Miner. 2003, 30, 177–183. [Google Scholar] [CrossRef]
- Sarvaramini, A.; Larachi, F. Mössbauer Spectroscopy and Catalytic Reaction Studies of Chrysotile-Catalyzed Steam Reforming of Benzene. J. Phys. Chem. C 2011, 115, 6841–6848. [Google Scholar] [CrossRef]
- Ristic, M.; Czako-Nagy, I.; Music, S.; Vertes, A. Spectroscopic characterization of chrysotile asbestos from different regions. J. Mol. Struct. 2011, 993, 120–126. [Google Scholar] [CrossRef]
- Eisenbach, M.; Nicholson, D.M.; Rusanu, A.; Brown, G. First principles calculation of finite temperature magnetism in Fe and Fe3C. J. Appl. Phys. 2011, 109, 07E138:1–07E138:2. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lemos, B.R.S.; Teixeira, A.P.C.; Ardisson, J.D.; Macedo, W.A.A.; Fernandez-Outon, L.E.; Amorim, C.C.; Moura, F.C.C.; Lago, R.M. Magnetic Amphiphilic Composites Applied for the Treatment of Biodiesel Wastewaters. Appl. Sci. 2012, 2, 513-524. https://doi.org/10.3390/app2020513
Lemos BRS, Teixeira APC, Ardisson JD, Macedo WAA, Fernandez-Outon LE, Amorim CC, Moura FCC, Lago RM. Magnetic Amphiphilic Composites Applied for the Treatment of Biodiesel Wastewaters. Applied Sciences. 2012; 2(2):513-524. https://doi.org/10.3390/app2020513
Chicago/Turabian StyleLemos, Bruno R. S., Ana Paula C. Teixeira, José D. Ardisson, Waldemar A. A. Macedo, Luis E. Fernandez-Outon, Camila C. Amorim, Flávia C. C. Moura, and Rochel M. Lago. 2012. "Magnetic Amphiphilic Composites Applied for the Treatment of Biodiesel Wastewaters" Applied Sciences 2, no. 2: 513-524. https://doi.org/10.3390/app2020513




