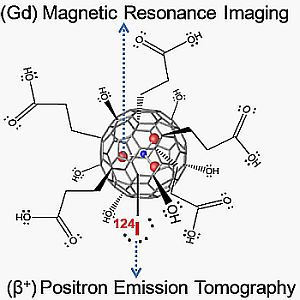
A Dual PET/MR Imaging Nanoprobe: 124I Labeled Gd3N@C80
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis and Surface Functionalization of Gd3N@C80
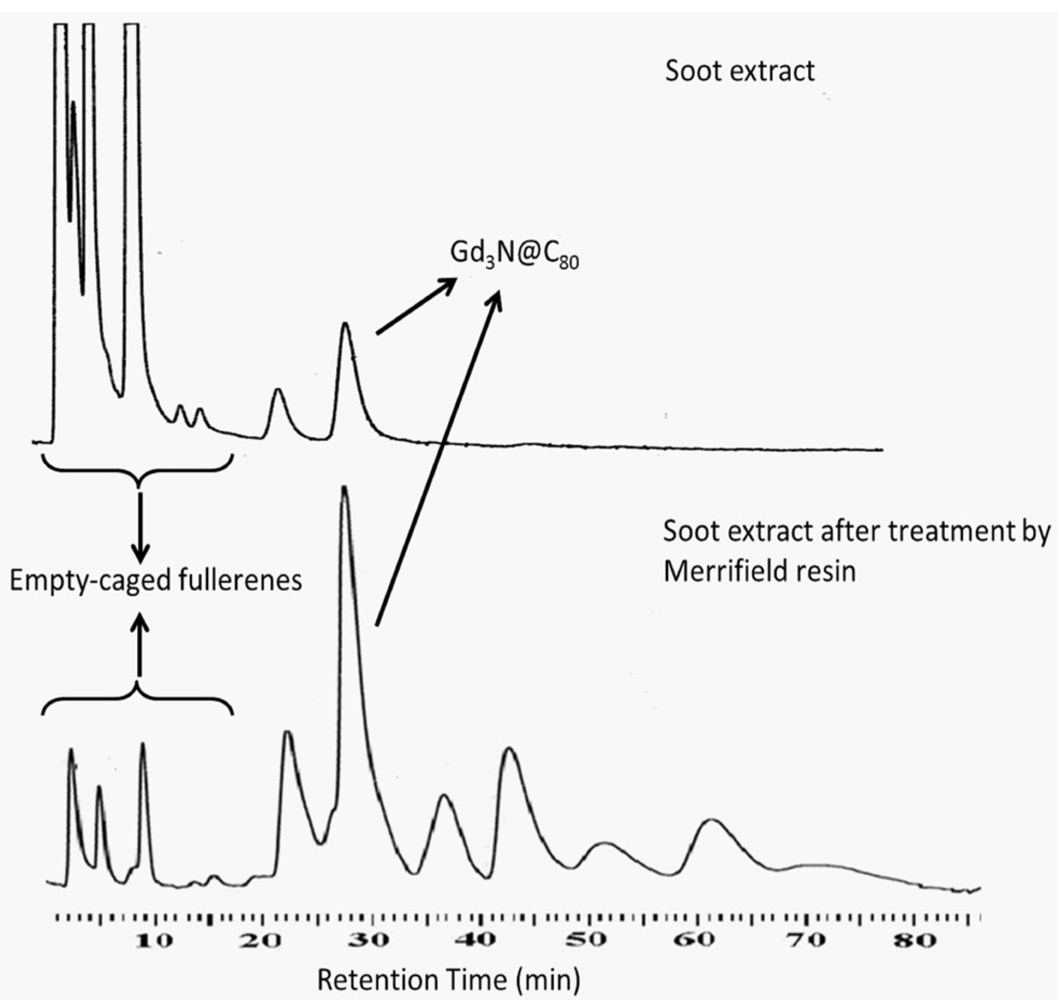
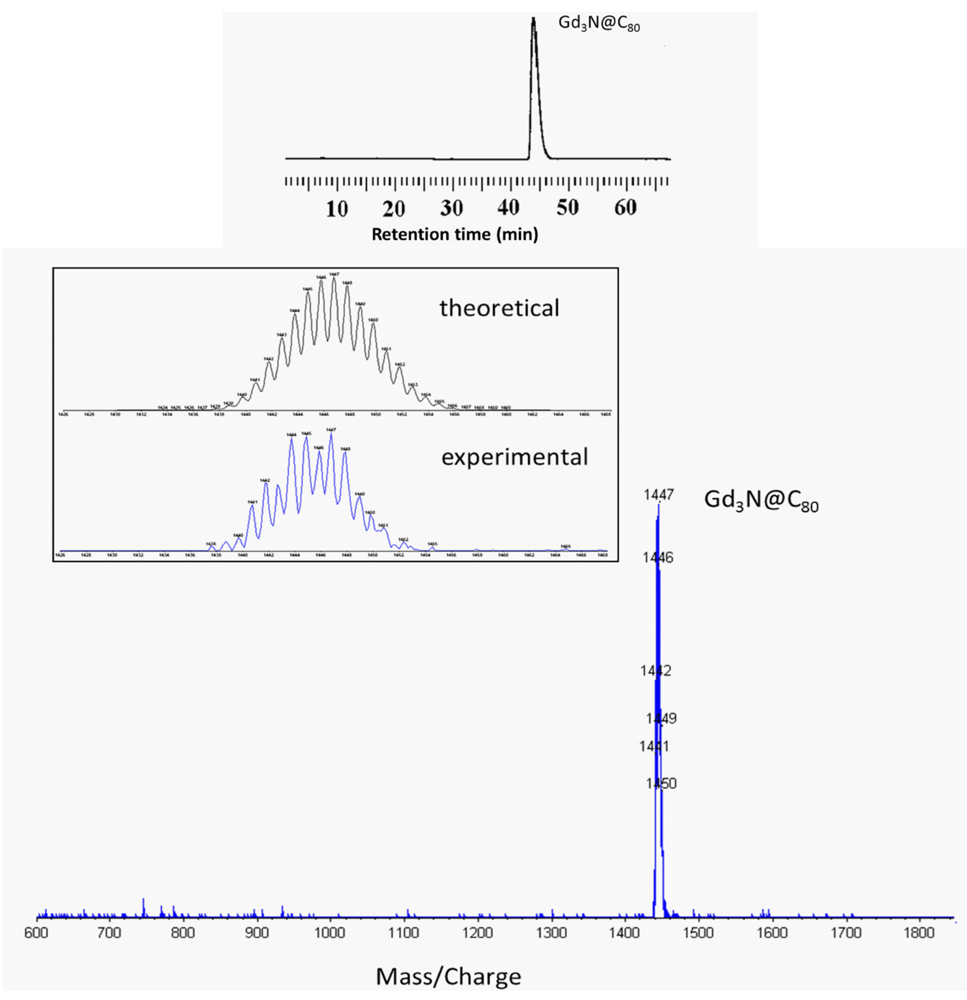
2.2. 124I Labeling of Carboxyl and Hydroxyl Functionalized Gd3N@C80
2.3. Cell Culture and Animal Model
2.4. In Vivo Imaging Methods: MRI and microPET
3. Results and Discussion




4. Conclusions
Acknowledgments
Conflict of Interest
References
- Ahn, P.H.; Garg, M.K. Positron emission tomography/computed tomography for target delineation in head and neck cancers. Semin. Nuclear Med. 2008, 38, 141–148. [Google Scholar] [CrossRef]
- Bockisch, A.; Freudenberg, L.S.; Schmidt, D.; Kuwert, T. Hybrid imaging by SPECT/CT and PET/CT: Proven outcomes in cancer imaging. Semin. Nuclear Med. 2009, 39, 276–289. [Google Scholar] [CrossRef]
- Delbeke, D.; Schöder, H.; Martin, W.H.; Wahl, R.L. Hybrid imaging (SPECT/CT and PET/CT): Improving therapeutic decisions. Semin. Nuclear Med. 2009, 39, 308–340. [Google Scholar] [CrossRef]
- Even-Sapir, E.; Keidar, Z.; Bar-Shalom, R. Hybrid imaging (SPECT/CT and PET/CT)—Improving the diagnostic accuracy of functional/metabolic and anatomic imaging. Semin. Nuclear Med. 2009, 39, 264–275. [Google Scholar] [CrossRef]
- Kaufmann, P.A.; di Carli, M.F. Hybrid SPECT/CT and PET/CT imaging: The next step in noninvasive cardiac imaging. Semin. Nuclear Med. 2009, 39, 341–347. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Gao, H.; Weir, V.; Yu, H.; Cong, W.; Xu, X.; Shen, H.; Bennett, J.; Wang, Y.; Vannier, M. Omni-tomography/multi—tomography-Integrating multiple modalities for simultaneous imaging. 2011; arXiv:11062124v1 [physicsmed-ph]. [Google Scholar]
- Shao, Y.; Cherry, S.R.; Farahani, K.; Meadors, K.; Siegel, S.; Silverman, R.W.; Marsden, P.K. Simultaneous PET and MR imaging. Phys. Med. Biol. 1997, 42, 1965–1970. [Google Scholar] [CrossRef]
- Beyer, T.; Townsend, D.W.; Brun, T.; Kinahan, P.E.; Charron, M.; Roddy, R.; Jerin, J.; Young, J.; Byars, L.; Nutt, R. A combined PET/CT scanner for clinical oncology. J. Nuclear Med. 2000, 41, 1369–1379. [Google Scholar]
- Liang, H.; Yang, Y.; Yang, K.; Wu, Y.; Boone, J.M.; Cherry, S.R. A microPET/CT system for in vivo small animal imaging. Phys. Med. Biol. 2007, 52, 3881–3894. [Google Scholar] [CrossRef]
- Pichler, B.J.; Kolb, A.; Nägele, T.; Schlemmer, H.P. PET/MRI: Paving the way for the next generation of clinical multimodality imaging applications. J. Nuclear Med. 2010, 51, 333–336. [Google Scholar] [CrossRef]
- Cho, Z.H.; Son, Y.D.; Kim, H.K.; Kim, N.B.; Choi, E.J.; Lee, S.Y.; Chi, J.G.; Park, C.W.; Kim, Y.B.; Ogawa, S. Observation of glucose metabolism in the thalamic nuclei by fusion PET/MRI. J. Nuclear Med. 2011, 52, 401–404. [Google Scholar] [CrossRef]
- Choi, J.S.; Park, J.C.; Nah, H.; Woo, S; Oh, J.; Kim, K.M.; Cheon, G.J.; Chang, Y.; Yoo, J.; Cheon, J. A hybrid nanoparticle probe for dual-modality positron emission tomography and magnetic resonance imaging. Angew. Chem. Int. Ed. 2008, 47, 6259–6262. [Google Scholar]
- Glaus, C.; Rossin, R.; Welch, M.J.; Bao, G. In vivo evaluation of Cu-64-labeled magnetic nanoparticles as a dual-modality PET/MR imaging agent. Bioconjugate Chem. 2010, 21, 715–722. [Google Scholar] [CrossRef]
- Lee, H.Y.; Li, Z.; Chen, K.; Hsu, A.R.; Xu, C.J.; Xie, J.; Sun, S.H.; Chen, X.Y. PET/MRI dual-modality tumor imaging using arginine-glycine-aspartic (RGD)—Conjugated radiolabeled iron oxide nanoparticles. J. Nuclear Med. 2008, 49, 1371–1379. [Google Scholar] [CrossRef]
- Uppal, R.; Catana, C.; Ay, I.; Benner, T.; Sorensen, A.G.; Caravan, P. Bimodal thrombus imaging: Simultaneous PET/MR imaging with a fibrin-targeted dual PET/MR probe—Feasibility study in rat model. Radiology 2011, 258, 812–820. [Google Scholar]
- Catana, C.; Procissi, D.; Wu, Y.B.; Judenhofer, M.S.; Qi, J.Y.; Pichler, B.J.; Jacobs, R.E.; Cherry, S.R. Simultaneous in vivo positron emission tomography and magnetic resonance imaging. Proc. Natl. Acad. Sci. USA 2008, 105, 3705–3710. [Google Scholar]
- Farahani, K.; Slates, R.; Shao, Y.P.; Silverman, R.; Cherry, S. Contemporaneous positron emission tomography and MR imaging at 1.5 T. J. Magn. Reson. Imaging 1999, 9, 497–500. [Google Scholar] [CrossRef]
- Judenhofer, M.S.; Wehrl, H.F.; Newport, D.F.; Catana, C.; Siegel, S.B.; Becker, M.; Thielscher, A.; Kneilling, M.; Lichy, M.P.; Eichner, M.; Klingel, K.; Reischl, G.; Widmaier, S.; Rocken, M.; Nutt, R.E.; Machulla, H.J.; Uludag, K.; Cherry, S.R.; Claussen, C.D.; Pichler, B.J. Simultaneous PET-MRI: A new approach for functional and morphological imaging. Nat. Med. 2008, 14, 459–465. [Google Scholar]
- Pichler, B.J.; Judenhofer, M.S.; Catana, C.; Walton, J.H.; Kneilling, M.; Nutt, R.E.; Siegel, S.B.; Claussen, C.D.; Cherry, S.R. Performance test of an LSO-APD detector in a 7-T MRI scanner for simultaneous PET/MRI. J. Nuclear Med. 2006, 47, 639–647. [Google Scholar]
- Schlemmer, H.P.W.; Pichler, B.J.; Schmand, M.; Burbar, Z.; Michel, C.; Ladebeck, R.; Jattke, K.; Townsend, D.; Nahmias, C.; Jacob, P.K.; Heiss, W.D.; Claussen, C.D. Simultaneous MR/PET imaging of the human brain: Feasibility study. Radiology 2008, 248, 1028–1035. [Google Scholar] [CrossRef]
- Jennings, L.E.; Long, N.J. ‘Two is better than one’-probes for dual-modality molecular imaging. Chem. Commun. 2009, 3511–3524. [Google Scholar] [CrossRef]
- Johnston, H.J.; Hutchison, G.R.; Christensen, F.M.; Aschberger, K.; Stone, V. The biological mechanisms and physicochemical characteristics responsible for driving fullerene toxicity. Toxicol. Sci. 2010, 114, 162–182. [Google Scholar] [CrossRef]
- Levi, N.; Hantgan, R.R.; Lively, M.O.; Carroll, D.L.; Prasad, G.L. C60-fullerenes: Detection of intracellular photoluminescence and lack of cytotoxic effects. J. Nanobiotechnol. 2006, 4. [Google Scholar] [CrossRef]
- Xia, X.R.; Monteiro-Riviere, N.A.; Riviere, J.E. Intrinsic biological property of colloidal fullerene nanoparticles (nC60): Lack of lethality after high dose exposure to human epidermal and bacterial cells. Toxicol. Lett. 2010, 197, 128–134. [Google Scholar] [CrossRef]
- Yan, L.A.; Zhao, F.; Li, S.J.; Hu, Z.B.; Zhao, Y.L. Low-toxic and safe nanomaterials by surface-chemical design, carbon nanotubes, fullerenes, metallofullerenes, and graphenes. Nanoscale 2011, 3, 362–382. [Google Scholar] [CrossRef]
- Baker, G.L.; Gupta, A.; Clark, M.L.; Valenzuela, B.R.; Staska, L.M.; Harbo, S.J.; Pierce, J.T.; Dill, J.A. Inhalation toxicity and lung toxicokinetics of C60 fullerene nanoparticles and microparticles. Toxicol. Sci. 2008, 101, 122–131. [Google Scholar]
- Jia, G.; Wang, H.; Yan, L.; Wang, X.; Pei, R.; Yan, T.; Zhao, Y.; Guo, X. Cytotoxicity of carbon nanomaterials: Single-wall nanotube, multi-wall nanotube, and fullerene. Environ. Sci. Technol. 2005, 39, 1378–1383. [Google Scholar] [CrossRef]
- Liang, X.J.; Meng, H.; Wang, Y.; He, H.; Meng, J.; Lu, J.; Wang, P.C.; Zhao, Y.; Gao, X.; Sun, B.; et al. Metallofullerene nanoparticles circumvent tumor resistance to cisplatin by reactivating endocytosis. Proc. Natl. Acad. Sci. USA 2010, 107, 7449–7454. [Google Scholar]
- Roursgaard, M.; Poulsen, S.S.; Kepley, C.L.; Hammer, M.; Nielsen, G.D.; Larsen, S.T. Polyhydroxylated C60 fullerene (fullerenol) attenuates neutrophilic lung inflammation in mice. Basic Clin. Pharmacol. Toxicol. 2008, 103, 386–388. [Google Scholar] [CrossRef]
- Sayes, C.M.; Marchione, A.A.; Reed, K.L.; Warheit, D.B. Comparative pulmonary toxicity assessments of C60 water suspensions in rats: Few differences in fullerene toxicity in vivo in contrast to in vitro profiles. Nano Lett. 2007, 7, 2399–2406. [Google Scholar] [CrossRef]
- Tsao, N.; Kanakamma, P.P.; Luh, T.Y.; Chou, C.K.; Lei, H.Y. Inhibition of Escherichia coli-induced meningitis by carboxyfullerence. Antimicrob. Agents Chemother. 1999, 43, 2273–2277. [Google Scholar]
- Wang, J.; Chen, C.; Li, B.; Yu, H.; Zhao, Y.; Sun, J.; Li, Y.; Xing, G.; Yuan, H.; Tang, J.; et al. Antioxidative function and biodistribution of [Gd@C82(OH)22]n nanoparticles in tumor-bearing mice. Biochem. Pharmacol. 2006, 71, 872–881. [Google Scholar]
- Ehrich, M.; Van Tassell, R.; Li, Y.B.; Zhou, Z.G.; Kepley, C.L. Fullerene antioxidants decrease organophosphate-induced acetylcholinesterase inhibition in vitro. Toxicol. In Vitro 2011, 25, 301–307. [Google Scholar] [CrossRef]
- Shu, C.Y.; Corwin, F.D.; Zhang, J.F.; Chen, Z.J.; Reid, J.E.; Sun, M.H.; Xu, W.; Sim, J.H.; Wang, C.R.; Fatouros, P.P.; Esker, A.R.; Gibson, H.W.; Dorn, H.C. Facile preparation of a new gadofullerene-based magnetic resonance imaging contrast agent with high H-1 relaxivity. Bioconjugate Chem. 2009, 20, 1186–1193. [Google Scholar] [CrossRef]
- Zhang, J.F.; Fatouros, P.P.; Shu, C.Y.; Reid, J.; Owens, L.S.; Cai, T.; Gibson, H.W.; Long, G.L.; Corwin, F.D.; Chen, Z.J.; Dorn, H.C. High relaxivity trimetallic nitride (Gd3N) metallofullerene MRI contrast agents with optimized functionality. Bioconjugate Chem. 2010, 21, 610–615. [Google Scholar]
- Iezzi, E.B.; Duchamp, J.C.; Fletcher, K.R.; Glass, T.E.; Dorn, H.C. Lutetium-based trimetallic nitride endohedral metallofullerenes: New contrast agents. Nano Lett. 2002, 2, 1187–1190. [Google Scholar] [CrossRef]
- Shultz, M.D.; Duchamp, J.C.; Wilson, J.D.; Shu, C.Y.; Ge, J.C.; Zhang, J.Y.; Gibson, H.W.; Fillmore, H.L.; Hirsch, J.I.; Dorn, H.C.; Fatouros, P.P. Encapsulation of a radiolabeled cluster inside a fullerene cage, (LuxLu(3-x)N)-Lu-177@C-80: An interleukin-13-conjugated radiolabeled metallofullerene platform. J. Am. Chem. Soc. 2010, 132, 4980–4981. [Google Scholar]
- Wharton, T.; Wilson, L.J. Highly-iodinated fullerene as a contrast agent for X-ray imaging. Bioorg. Med. Chem. 2002, 10, 3545–3554. [Google Scholar] [CrossRef]
- Wharton, T.; Wilson, L.J. Toward fullerene-based X-ray contrast agents: Design and synthesis of non-ionic, highly-iodinated derivatives of C60. Tetrahedron Lett. 2002, 43, 561–564. [Google Scholar] [CrossRef]
- Yuguo, L.; Xiaodong, Z.; Qingnuan, L.; Wenxin, L. Radioiodination of C60 derivative C60(OH)xOy. J. Radioanal. Nuclear Chem. 2001, 250, 363–364. [Google Scholar] [CrossRef]
- Shultz, M.D.; Wilson, J.D.; Fuller, C.E.; Zhang, J.; Dorn, H.C.; Fatouros, P.P. Metallofullerene-based nanoplatform for brain tumor brachytherapy and longitudinal imaging in a murine orthotopic xenograft model. Radiology 2011, 261, 136–143. [Google Scholar] [CrossRef]
- Choi, J.S.; Park, J.C.; Nah, H.; Woo, S.; Oh, J.; Kim, K.M.; Cheon, G.J.; Chang, Y.; Yoo, J.; Cheon, J. A hybrid nanoparticle probe for dual-modality positron emission tomography and magnetic resonance imaging. Angew. Chem. Int. Ed. 2008, 47, 6259–6262. [Google Scholar]
- Stevenson, S.; Rice, G.; Glass, T.; Harich, K.; Cromer, F.; Jordan, M.R.; Craft, J.; Hadju, E.; Bible, R.; Olmstead, M.M.; Maitra, K.; Fisher, A.J.; Balch, A.L.; Dorn, H.C. Small-bandgap endohedral metallofullerenes in high yield and purity. Nature 1999, 401, 55–57. [Google Scholar]
- Ge, Z.; Duchamp, J.; Cai, T.; Gibson, H.; Dorn, H. Purification of endohedral trimetallic nitride fullerenes in a single, facile step. J. Am. Chem. Soc. 2005, 127, 16292–16298. [Google Scholar]
- Vaidyanathan, G.; Zalutsky, M.R. Protein radiohalogenation: Observations on the design of N-succinimidyl ester acylation agents. Bioconjugate Chem. 1990, 1, 269–273. [Google Scholar] [CrossRef]
- Fatouros, P.P.; Corwin, F.D.; Chen, Z.J.; Broaddus, W.C.; Tatum, J.L.; Kettenmann, B.; Ge, Z.; Gibson, H.W.; Russ, J.L.; Leonard, A.P.; Duchamp, J.C.; Dorn, H.C. In vitro and in vivo imaging studies of a new endohedral metallofullerene nanoparticle. Radiology 2006, 240, 756–764. [Google Scholar] [CrossRef]
- Qian, M., Ong. Qian, M., Ong, S.V., Khanna, N., and Knickelbein, M.B. Phys. Rev. B 2007, 75, 104424:1–104424:6. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Luo, J.; Wilson, J.D.; Zhang, J.; Hirsch, J.I.; Dorn, H.C.; Fatouros, P.P.; Shultz, M.D. A Dual PET/MR Imaging Nanoprobe: 124I Labeled Gd3N@C80. Appl. Sci. 2012, 2, 465-478. https://doi.org/10.3390/app2020465
Luo J, Wilson JD, Zhang J, Hirsch JI, Dorn HC, Fatouros PP, Shultz MD. A Dual PET/MR Imaging Nanoprobe: 124I Labeled Gd3N@C80. Applied Sciences. 2012; 2(2):465-478. https://doi.org/10.3390/app2020465
Chicago/Turabian StyleLuo, Jianqiao, John D. Wilson, Jianyuan Zhang, Jerry I. Hirsch, Harry C. Dorn, Panos P. Fatouros, and Michael D. Shultz. 2012. "A Dual PET/MR Imaging Nanoprobe: 124I Labeled Gd3N@C80" Applied Sciences 2, no. 2: 465-478. https://doi.org/10.3390/app2020465