Assessment of the Technological Properties of Idebenone and Tocopheryl Acetate Co-Loaded Lipid Nanoparticles
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Lipid Nanoparticles
2.3. Transmission Electron Microscopy (TEM)
2.4. Photon Correlation Spectroscopy (PCS)
2.5. Stability Tests
2.6. Differential Scanning Calorimetry Analyses
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shirodkar, R.K.; Kumar, L.; Mutalik, S.; Lewis, S. Solid lipid nanoparticles and nanostructured lipid carriers: Emerging lipid based drug delivery systems. Pharm. Chem. J. 2019, 53, 440–453. [Google Scholar] [CrossRef]
- Paliwal, R.; Paliwal, S.R.; Kenwat, R.; Das Kurmi, B.; Sahu, M.K. Solid lipid nanoparticles: A review on recent perspectives and patents. Expert Opin. Ther. Patents 2020, 30, 179–194. [Google Scholar] [CrossRef]
- Montoto, S.S.; Muraca, G.; Ruiz, M.E. Solid lipid nanoparticles for drug delivery: Pharmacological and biopharmaceutical aspects. Front. Mol. Biosci. 2020, 7, 587997. [Google Scholar] [CrossRef]
- Haider, M.; Abdin, S.M.; Kamal, L.; Orive, G. Nanostructured lipid carriers for delivery of chemotherapeutics: A review. Pharmaceutics 2020, 12, 288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, V.; Bansal, K.K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J.M. Solid lipid nanoparticles: Emerging colloidal nano drug delivery systems. Pharmaceutics 2018, 10, 191. [Google Scholar] [CrossRef] [Green Version]
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Müller, R.; Radtke, M.; Wissing, S. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54, S131–S155. [Google Scholar] [CrossRef]
- Montenegro, L.; Lai, F.; Offerta, A.; Sarpietro, M.G.; Micicchè, L.; Maccioni, A.M.; Valenti, D.; Fadda, A.M. From nanoemulsions to nanostructured lipid carriers: A relevant development in dermal delivery of drugs and cosmetics. J. Drug Deliv. Sci. Technol. 2016, 32, 100–112. [Google Scholar] [CrossRef]
- Kulkarni, J.A.; Cullis, P.R.; Van Der Meel, R. Lipid nanoparticles enabling gene therapies: From concepts to clinical utility. Nucleic Acid Ther. 2018, 28, 146–157. [Google Scholar] [CrossRef] [Green Version]
- Borges, A.; De Freitas, V.; Mateus, N.; Fernandes, I.; Oliveira, J. Solid lipid nanoparticles as carriers of natural phenolic compounds. Antioxidants 2020, 9, 998. [Google Scholar] [CrossRef]
- Montenegro, L. Lipid-based nanoparticles as carriers for dermal delivery of antioxidants. Curr. Drug Metab. 2017, 18, 469–480. [Google Scholar] [CrossRef]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid lipid nanoparticles and nanostructured lipid carriers: Structure, preparation and application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef] [Green Version]
- Subramaniam, B.; Siddik, Z.H.; Nagoor, N.H. Optimization of nanostructured lipid carriers: Understanding the types, designs, and parameters in the process of formulations. J. Nanoparticle Res. 2020, 22, 1–29. [Google Scholar] [CrossRef]
- Montenegro, L.; Messina, C.M.; Manuguerra, S.; Santagati, L.M.; Pasquinucci, L.; Turnaturi, R.; Parenti, C.; Arena, R.; Santulli, A. In vitro antioxidant activity and in vivo topical efficacy of lipid nanoparticles co-loading idebenone and tocopheryl acetate. Appl. Sci. 2019, 9, 845. [Google Scholar] [CrossRef] [Green Version]
- Jaber, S.; Polster, B.M. Idebenone and neuroprotection: Antioxidant, pro-oxidant, or electron carrier? J. Bioenerg. Biomembr. 2015, 47, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Becker, C.; Bray-French, K.; Drewe, J. Pharmacokinetic evaluation of idebenone. Expert Opin. Drug Metab. Toxicol. 2010, 6, 1437–1444. [Google Scholar] [CrossRef]
- Thiele, J.J.; Ekanayake-Mudiyanselage, S. Vitamin E in human skin: Organ-specific physiology and considerations for its use in dermatology. Mol. Asp. Med. 2007, 28, 646–667. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Evidence for beneficial effects of vitamin E. Korean J. Intern. Med. 2015, 30, 571–579. [Google Scholar] [CrossRef]
- Montenegro, L.; Campisi, A.; Sarpietro, M.G.; Carbone, C.; Acquaviva, R.; Raciti, G.; Puglisi, G. In vitroevaluation of idebenone-loaded solid lipid nanoparticles for drug delivery to the brain. Drug Dev. Ind. Pharm. 2011, 37, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Trapani, A.; Latrofa, A.; Puglisi, G. In vitro evaluation on a model of blood brain barrier of idebenone-loaded solid lipid nanoparticles. J. Nanosci. Nanotechnol. 2012, 12, 330–337. [Google Scholar] [CrossRef]
- Ruktanonchai, U.; Limpakdee, S.; Meejoo, S.; Sakulkhu, U.; Bunyapraphatsara, N.; Junyaprasert, V.; Puttipipatkhachorn, S. The effect of cetyl palmitate crystallinity on physical properties of gamma-oryzanol encapsulated in solid lipid nanoparticles. Nanotechnology 2008, 19, 095701. [Google Scholar] [CrossRef]
- Izquierdo, P.; Feng, J.; Esquena, J.; Tadros, T.F.; Dederen, J.C.; Garcia, M.J.; Azemar, N.; Solans, C. The influence of surfactant mixing ratio on nano-emulsion formation by the pit method. J. Colloid Interface Sci. 2005, 285, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Jenning, V.; Schäfer-Korting, M.; Gohla, S. Vitamin A-loaded solid lipid nanoparticles for topical use: Drug release properties. J. Control. Release 2000, 66, 115–126. [Google Scholar] [CrossRef]
- Sarpietro, M.G.; Accolla, M.L.; Puglisi, G.; Castelli, F.; Montenegro, L. Idebenone loaded solid lipid nanoparticles: Calorimetric studies on surfactant and drug loading effects. Int. J. Pharm. 2014, 471, 69–74. [Google Scholar] [CrossRef]
- Basso, L.G.; Rodrigues, R.Z.; Naal, R.M.; Costa-Filho, A.J. Effects of the antimalarial drug primaquine on the dynamic structure of lipid model membranes. Biochim. Biophys. Acta (BBA) Biomembr. 2011, 1808, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Prado, A.H.D.; Araújo, V.H.S.; Eloy, J.O.; Fonseca-Santos, B.; Pereira-Da-Silva, M.A.; Peccinini, R.G.; Chorilli, M. Synthesis and characterization of nanostructured lipid nanocarriers for enhanced sun protection factor of octyl p-methoxycinnamate. AAPS PharmSciTech 2020, 21, 125. [Google Scholar] [CrossRef]
- Kheradmandnia, S.; Vasheghani-Farahani, E.; Nosrati, M.; Atyabi, F. Preparation and characterization of ketoprofen-loaded solid lipid nanoparticles made from beeswax and carnauba wax. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 753–759. [Google Scholar] [CrossRef]
- Montenegro, L.; Turnaturi, R.; Parenti, C.; Pasquinucci, L. Idebenone: Novel strategies to improve its systemic and local efficacy. Nanomaterials 2018, 8, 87. [Google Scholar] [CrossRef] [Green Version]
- Montenegro, L.; Panico, A.M.; Santagati, L.M.; Siciliano, E.A.; Intagliata, S.; Modica, M.N. Solid lipid nanoparticles loading idebenone ester with pyroglutamic acid: In vitro antioxidant activity and in vivo topical efficacy. Nanomaterials 2018, 9, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinelli, C.; Battaglini, M.; Pucci, C.; Gioi, S.; Caracci, C.; Macaluso, G.; Doccini, S.; Santorelli, F.M.; Ciofani, G. Development of nanostructured lipid carriers for the delivery of idebenone in autosomal recessive spastic ataxia of charlevoix-saguenay. ACS Omega 2020, 5, 12451–12466. [Google Scholar] [CrossRef]
- Delanty, N.; Dichter, M.A. Antioxidant therapy in neurologic disease. Arch. Neurol. 2000, 57, 1265–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keen, M.A.; Hassan, I. Vitamin E in dermatology. Indian Dermatol. Online J. 2016, 7, 311–315. [Google Scholar] [CrossRef]
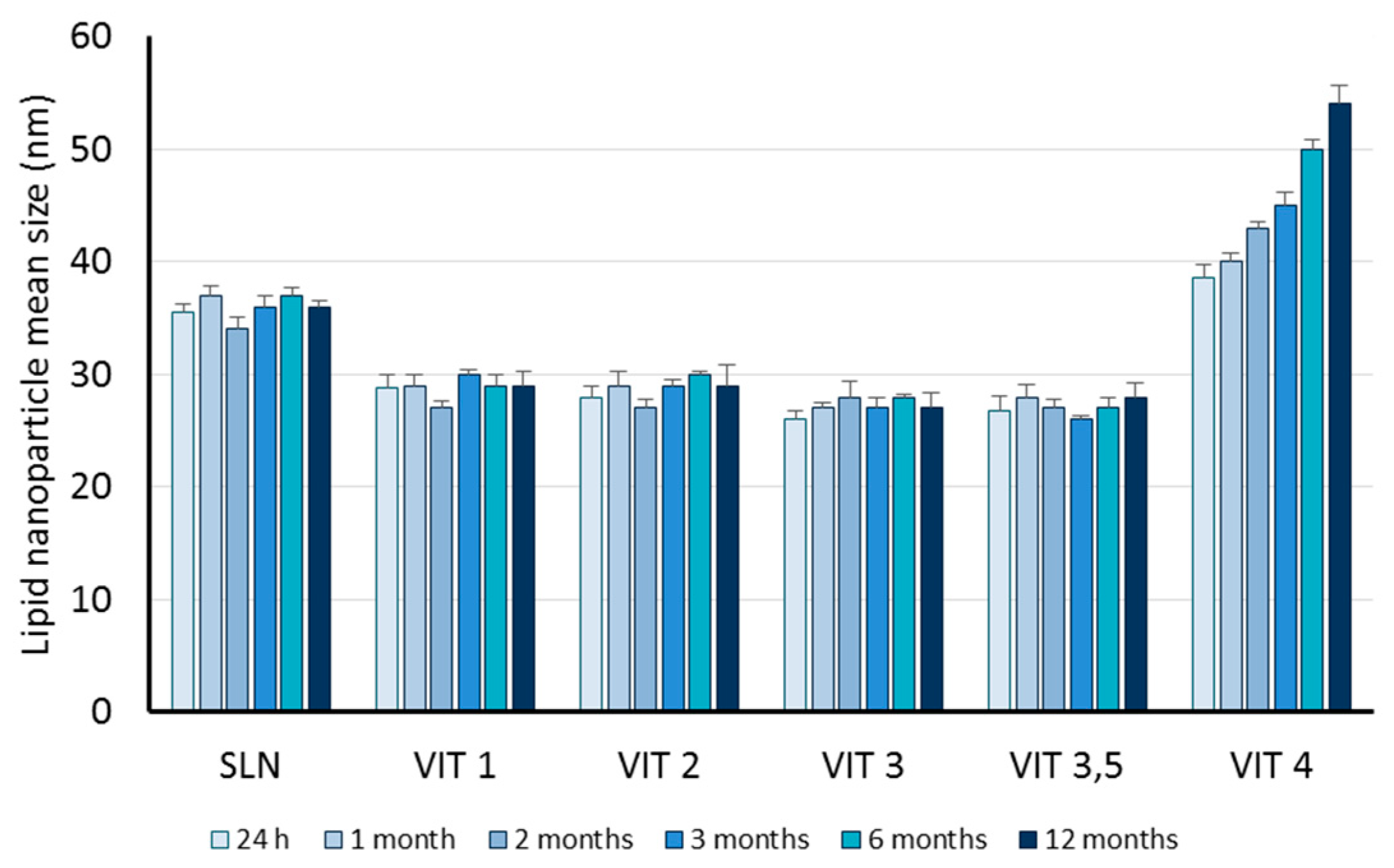


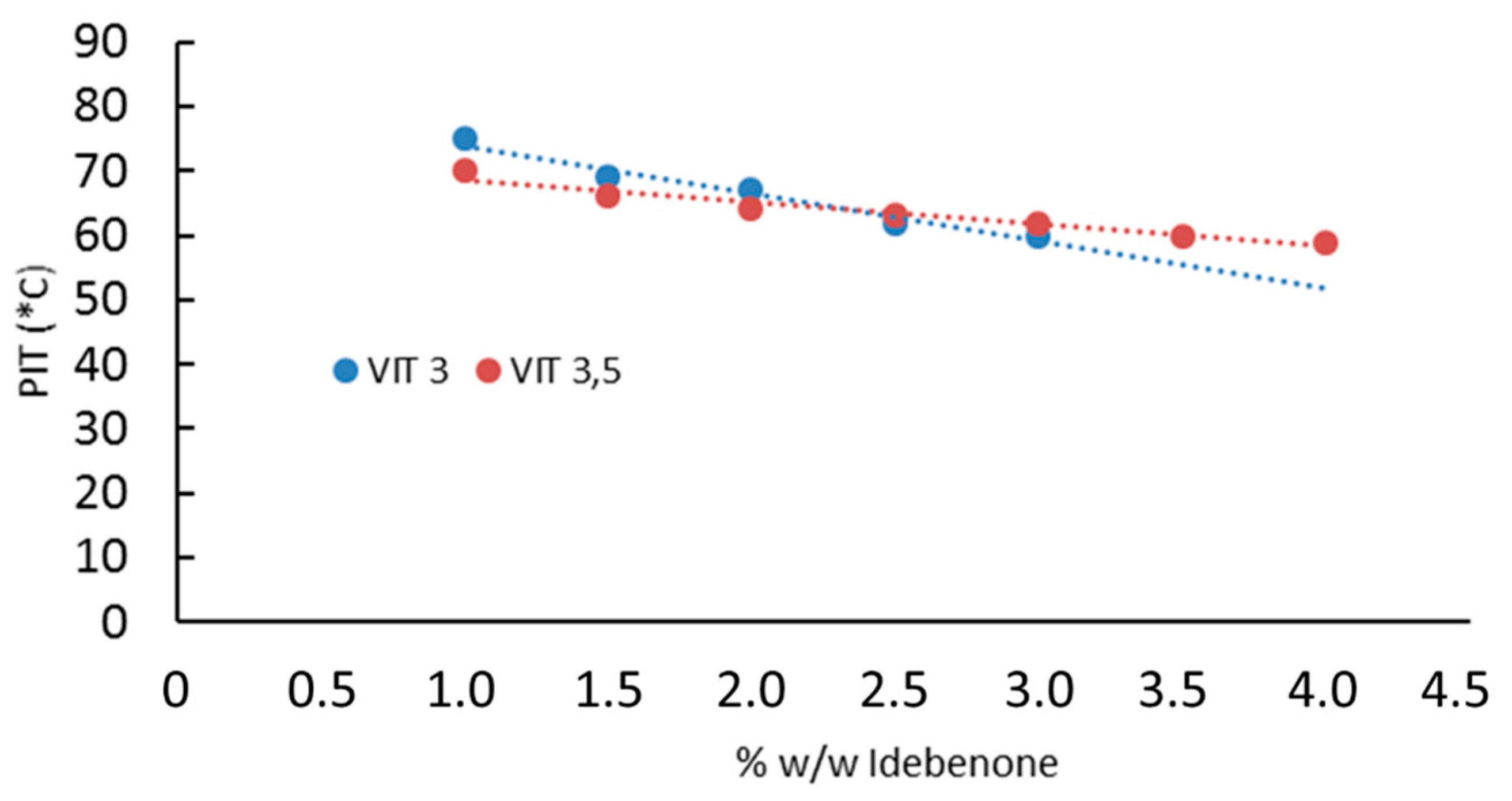
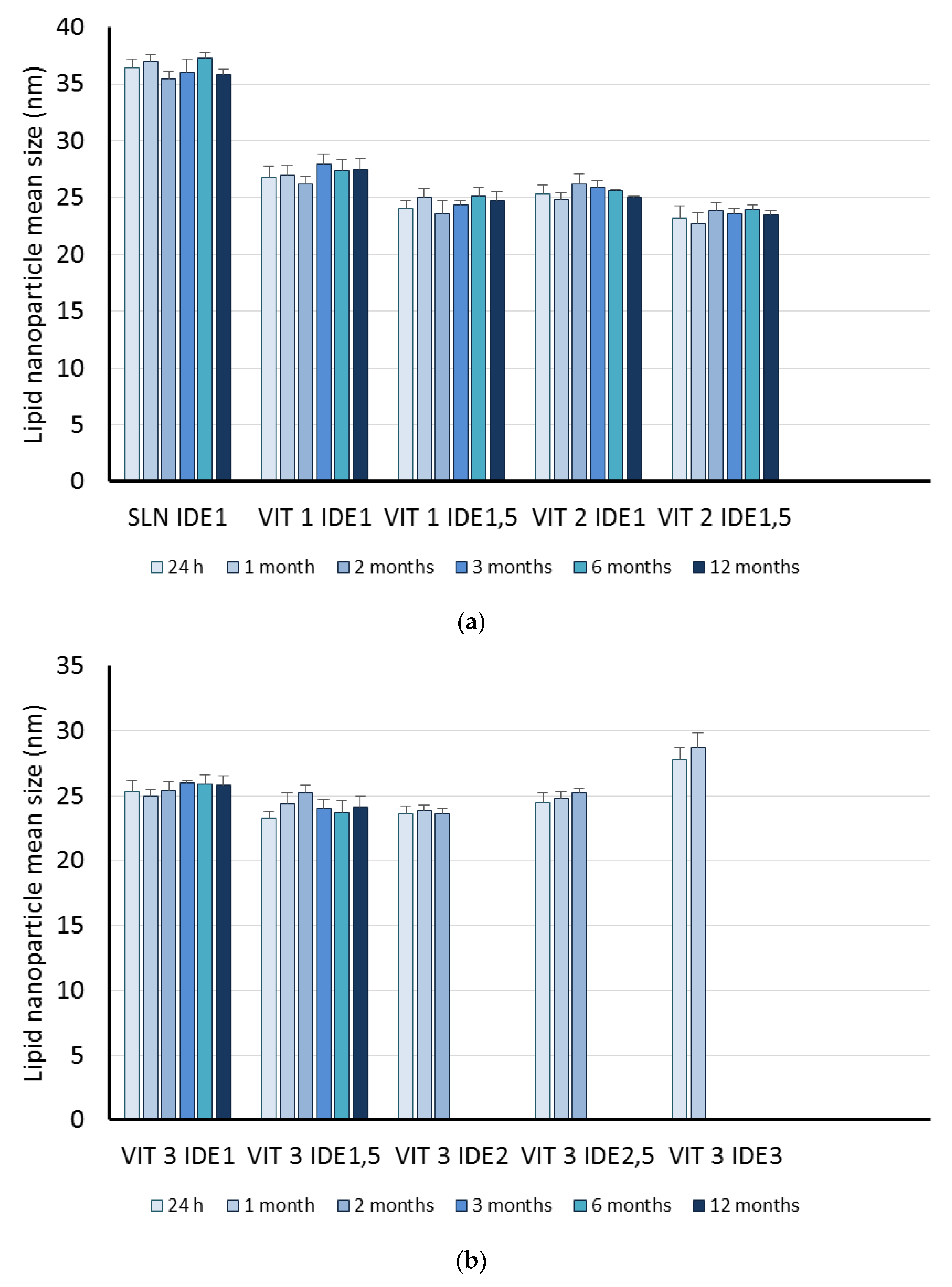
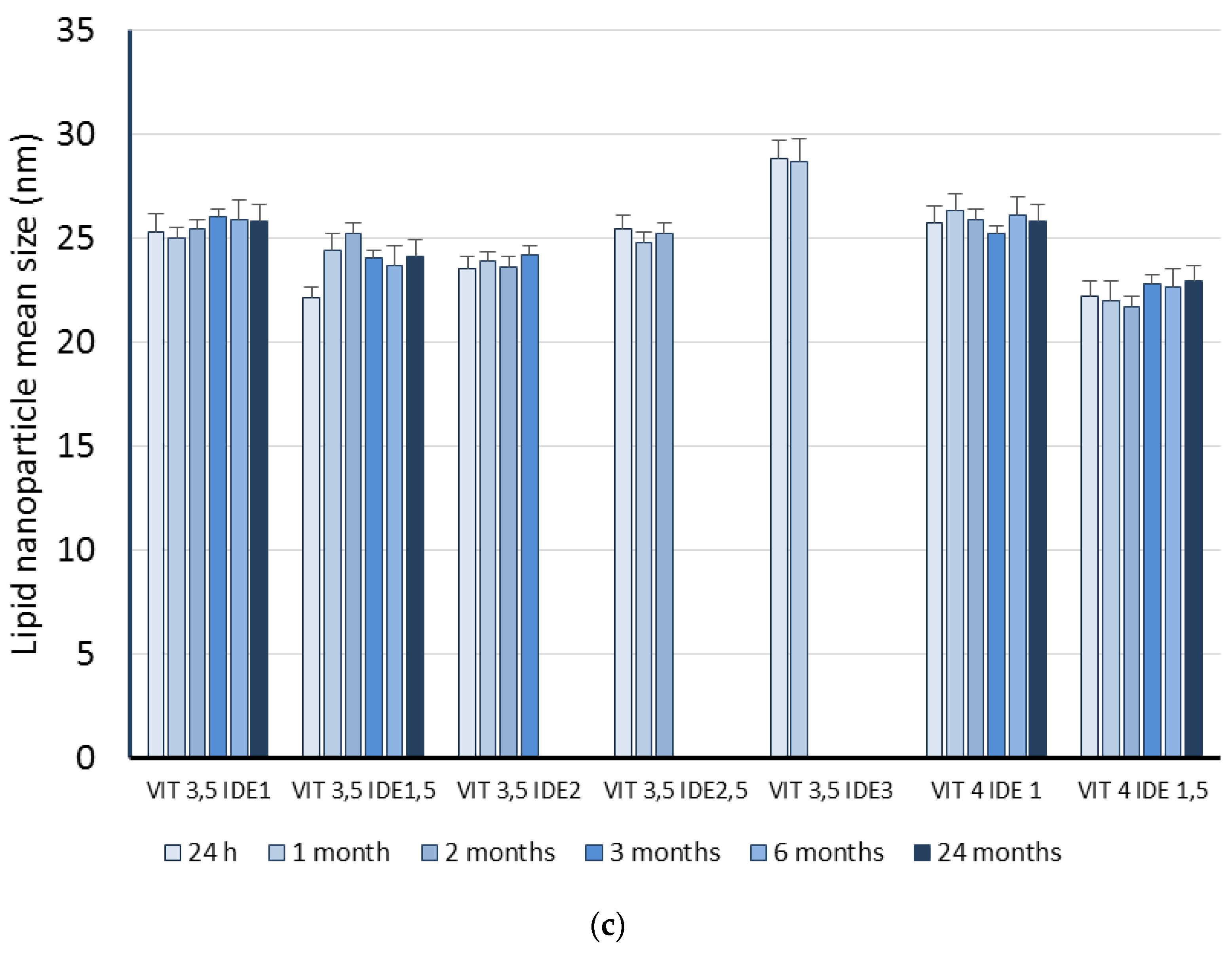


| Code | Oleth-20 | GO | CP | TA | IDE |
|---|---|---|---|---|---|
| SLN | 8.7 | 4.4 | 7.0 | - | - |
| VIT 1 | 8.7 | 4.4 | 6.0 | 1.0 | - |
| VIT 2 | 8.7 | 4.4 | 5.0 | 2.0 | - |
| VIT 3 | 8.7 | 4.4 | 4.0 | 3.0 | - |
| VIT 3,5 | 8.7 | 4.4 | 3.5 | 3.5 | - |
| VIT 4 | 8.7 | 4.4 | 3.0 | 4.0 | - |
| SLN IDE1 | 8.7 | 4.4 | 7.0 | - | 1.0 |
| VIT 1 IDE1 | 8.7 | 4.4 | 6.0 | 1.0 | 1.0 |
| VIT 1 IDE1,5 | 8.7 | 4.4 | 6.0 | 1.0 | 1.5 |
| VIT 1 IDE2 | 8.7 | 4.4 | 6.0 | 1.0 | 2.0 |
| VIT 2 IDE1 | 8.7 | 4.4 | 5.0 | 2.0 | 1.0 |
| VIT 2 IDE 1,5 | 8.7 | 4.4 | 5.0 | 2.0 | 1.5 |
| VIT 2 IDE2 | 8.7 | 4.4 | 5.0 | 2.0 | 2.0 |
| VIT 3 IDE1 | 8.7 | 4.4 | 4.0 | 3.0 | 1.0 |
| VIT 3 IDE1,5 | 8.7 | 4.4 | 4.0 | 3.0 | 1.5 |
| VIT 3 IDE2 | 8.7 | 4.4 | 4.0 | 3.0 | 2.0 |
| VIT 3 IDE2,5 | 8.7 | 4.4 | 4.0 | 3.0 | 2.5 |
| VIT 3 IDE3 | 8.7 | 4.4 | 4.0 | 3.0 | 3.0 |
| VIT 3 IDE3,5 | 8.7 | 4.4 | 4.0 | 3.0 | 3.5 |
| VIT 3,5 IDE1 | 8.7 | 4.4 | 3.5 | 3.5 | 1.0 |
| VIT 3,5 IDE1,5 | 8.7 | 4.4 | 3.5 | 3.5 | 1.5 |
| VIT 3,5 IDE2 | 8.7 | 4.4 | 3.5 | 3.5 | 2.0 |
| VIT 3,5 IDE2,5 | 8.7 | 4.4 | 3.5 | 3.5 | 2.5 |
| VIT 3,5 IDE3 | 8.7 | 4.4 | 3.5 | 3.5 | 3.0 |
| VIT 3,5 IDE3,5 | 8.7 | 4.4 | 3.5 | 3.5 | 3.5 |
| VIT 3,5 IDE4 | 8.7 | 4.4 | 3.5 | 3.5 | 4.0 |
| VIT 4 IDE1 | 8.7 | 4.4 | 3.0 | 4.0 | 1.0 |
| VIT 4 IDE1,5 | 8.7 | 4.4 | 3.0 | 4.0 | 1.5 |
| VIT 4 IDE2 | 8.7 | 4.4 | 3.0 | 4.0 | 2.0 |
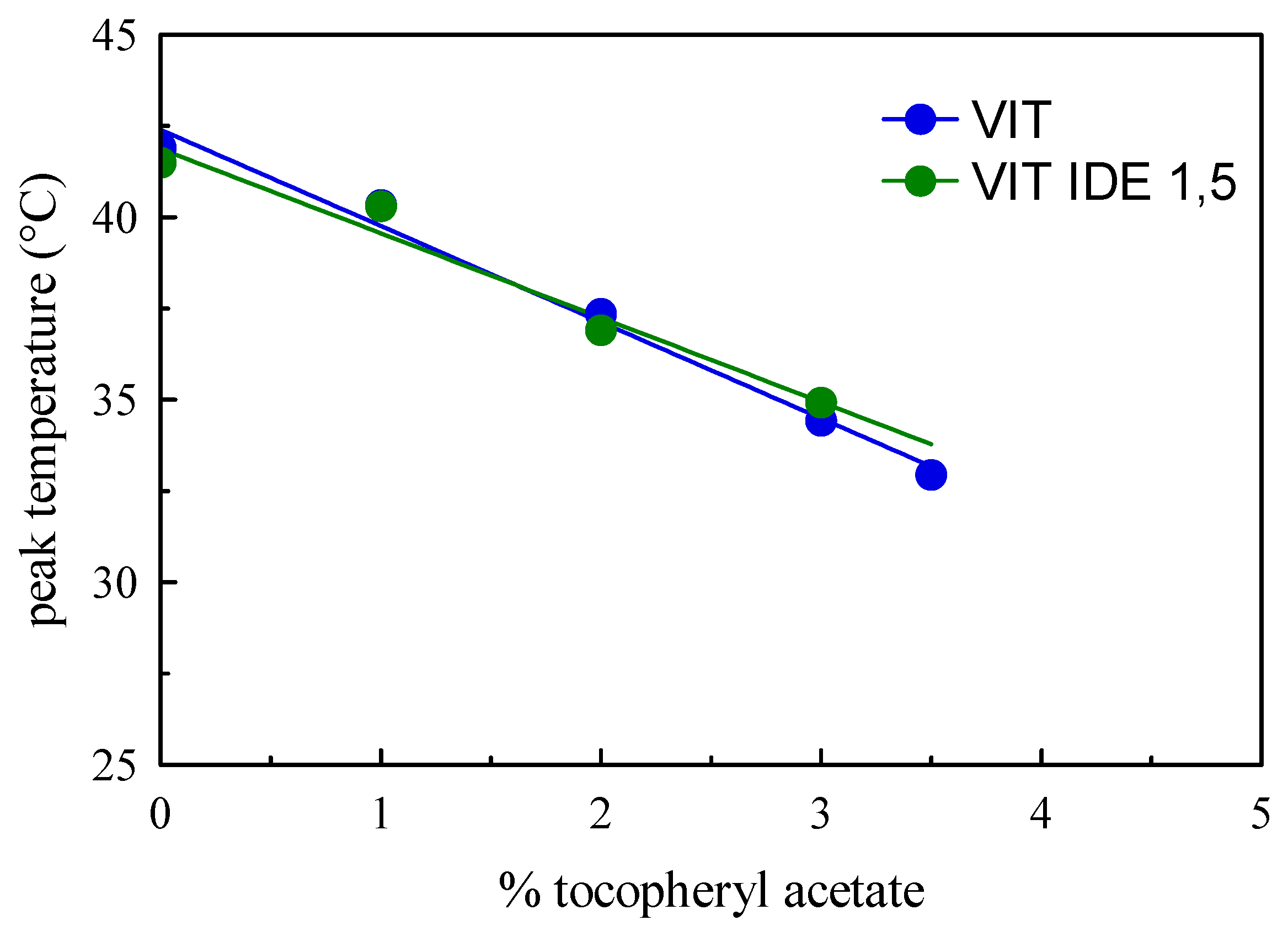
| Sample | Size ± S.D. (nm) | PDI ± S.D. | Ζeta ± S.D. (mV) | PIT (°C) |
|---|---|---|---|---|
| SLN | 35.5 ± 0.9 | 0.22 ± 0.01 | −7.52 | 78 |
| VIT 1 | 28.8 ± 0.9 | 0.23 ± 0.03 | −6.89 | 85 |
| VIT 2 | 28.0 ± 0.6 | 0.23 ± 0.05 | −7.34 | 78 |
| VIT 3 | 26.0 ± 0.5 | 0.18 ± 0.01 | −8.14 | 82 |
| VIT 3,5 | 26.8 ± 0.8 | 0.22 ± 0.01 | −7.66 | 85 |
| VIT 4 | 38.6 ± 0.4 | 0.55 ± 0.02 | −8.39 | 72 |
| SLN IDE1 | 36.4 ± 0.3 | 0.26 ± 0.01 | −8.43 | 78 |
| VIT 1 IDE1 | 26.8 ± 1.3 | 0.18 ± 0.04 | −7.91 | 78 |
| VIT 1 IDE1,5 | 24.1 ± 0.3 | 0.16 ± 0.04 | −6.54 | 65 |
| VIT 1 IDE2 | N.D. a | N.D. a | N.D. a | 62 |
| VIT 2 IDE1 | 25.3 ± 0.6 | 0.17 ± 0.03 | −7.01 | 75 |
| VIT 2 IDE 1,5 | 23.2 ± 0.1 | 0.11 ± 0.01 | −7.55 | 68 |
| VIT 2 IDE2 | N.D. a | N.D. a | N.D. a | 63 |
| VIT 3 IDE1 | 25.3 ± 0.8 | 0.16 ± 0.02 | −8.69 | 75 |
| VIT 3 IDE1,5 | 23.3 ± 0.2 | 0.15 ± 0.01 | −7.31 | 69 |
| VIT 3 IDE2 | 23.6 ± 0.5 | 0.19 ± 0.08 | −8.33 | 67 |
| VIT 3 IDE2,5 | 24.5 ± 0.3 | 0.24 ± 0.01 | −7.49 | 62 |
| VIT 3 IDE3 | 27.8 ± 0.5 | 0.20 ± 0.01 | −7.78 | 60 |
| VIT 3 IDE3,5 | N.D. a | N.D. a | N.D. a | 57 |
| VIT 3,5 IDE1 | 25.3 ± 0.2 | 0.17 ± 0.01 | −6.98 | 70 |
| VIT 3,5 IDE1,5 | 22.1 ± 0.1 | 0.16 ± 0.04 | −6.71 | 66 |
| VIT 3,5 IDE2 | 23.5 ± 0.4 | 0.18 ± 0.06 | −7.86 | 64 |
| VIT 3,5 IDE2,5 | 25.4 ± 0.3 | 0.19 ± 0.03 | −8.76 | 63 |
| VIT 3,5 IDE3 | 28.8 ± 0.2 | 0.18 ± 0.01 | −8.43 | 62 |
| VIT 3,5 IDE3,5 | 32.4 ± 0.4 | 0.16 ± 0.01 | −7.53 | 60 |
| VIT 3,5 IDE4 | 36.7 ± 0.5 | 0.15 ± 0.01 | −6.99 | 59 |
| VIT 4 IDE1 | 25.7 ± 1.5 | 0.13 ± 0.03 | −8.43 | 70 |
| VIT 4 IDE1,5 | 22.2 ± 0.2 | 0.12 ± 0.01 | −7.42 | 64 |
| VIT 4 IDE2 | N.D. a | N.D. a | N.D. a | 58 |
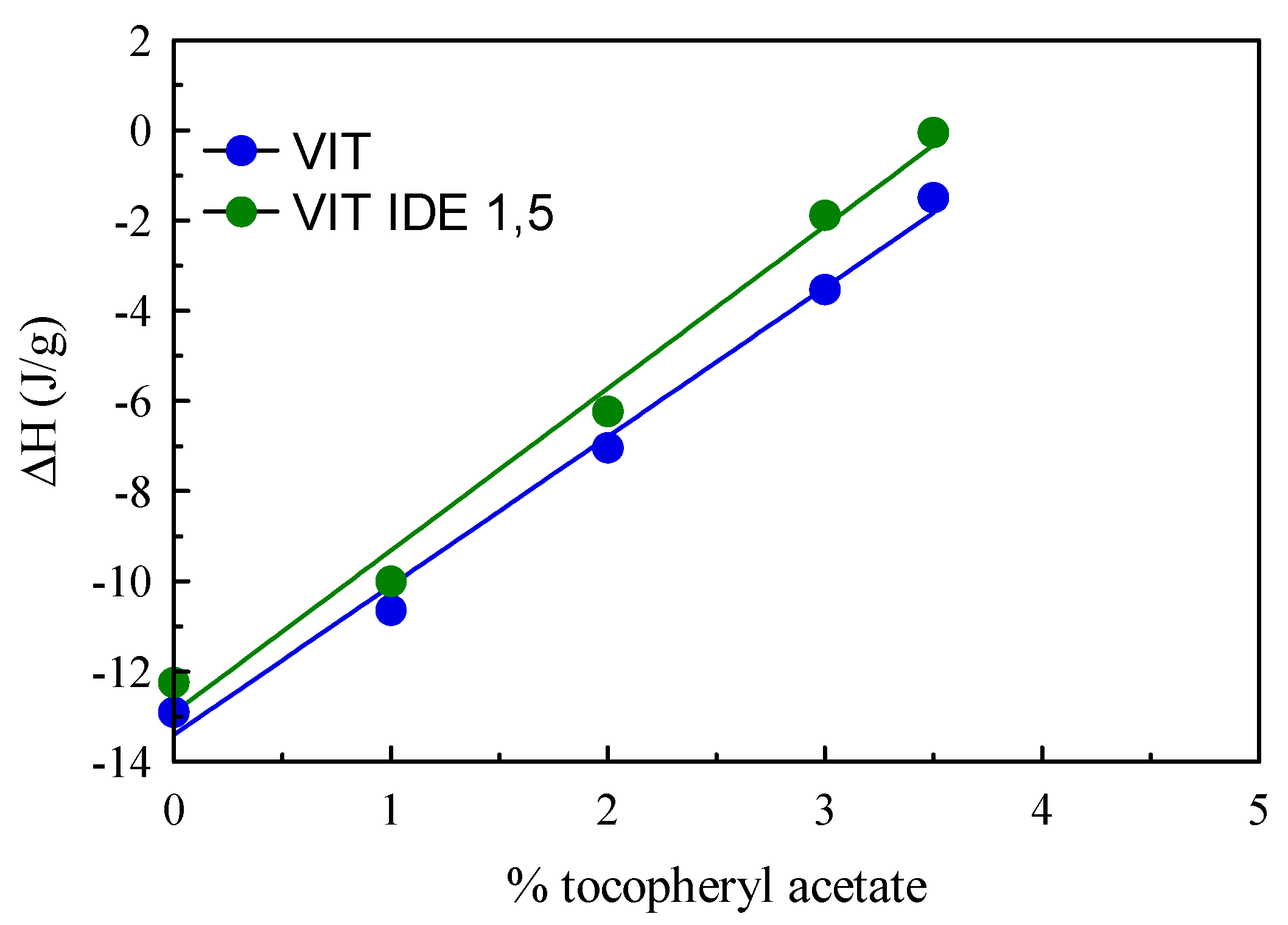
| Sample | ΔH (J/g) | RI (%) |
|---|---|---|
| Cetyl palmitate | −247.00 | 100.00 |
| SLN | −12.90 | 74.61 |
| VIT 1 | −10.64 | 71.79 |
| VIT 2 | −7.04 | 5.00 |
| VIT 3 | −3.53 | 35.72 |
| VIT 3,5 | −1.49 | 17.23 |
| VIT 4 | - | - |
| SLN IDE | 12.24 | 70.79 |
| VIT 1 IDE1,5 | 10.00 | 67.47 |
| VIT 2 IDE1,5 | 6.23 | 50.44 |
| VIT 3 IDE1,5 | 1.88 | 19.02 |
| VIT 3,5 IDE1,5 | 0.05 | 0.58 |
| VIT 4 IDE1,5 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarpietro, M.G.; Torrisi, C.; Pignatello, R.; Castelli, F.; Montenegro, L. Assessment of the Technological Properties of Idebenone and Tocopheryl Acetate Co-Loaded Lipid Nanoparticles. Appl. Sci. 2021, 11, 3553. https://doi.org/10.3390/app11083553
Sarpietro MG, Torrisi C, Pignatello R, Castelli F, Montenegro L. Assessment of the Technological Properties of Idebenone and Tocopheryl Acetate Co-Loaded Lipid Nanoparticles. Applied Sciences. 2021; 11(8):3553. https://doi.org/10.3390/app11083553
Chicago/Turabian StyleSarpietro, Maria Grazia, Cristina Torrisi, Rosario Pignatello, Francesco Castelli, and Lucia Montenegro. 2021. "Assessment of the Technological Properties of Idebenone and Tocopheryl Acetate Co-Loaded Lipid Nanoparticles" Applied Sciences 11, no. 8: 3553. https://doi.org/10.3390/app11083553







