Survival of Radioresistant Bacteria on Europa’s Surface after Pulse Ejection of Subsurface Ocean Water
Abstract
:1. Introduction
2. Materials and Methods
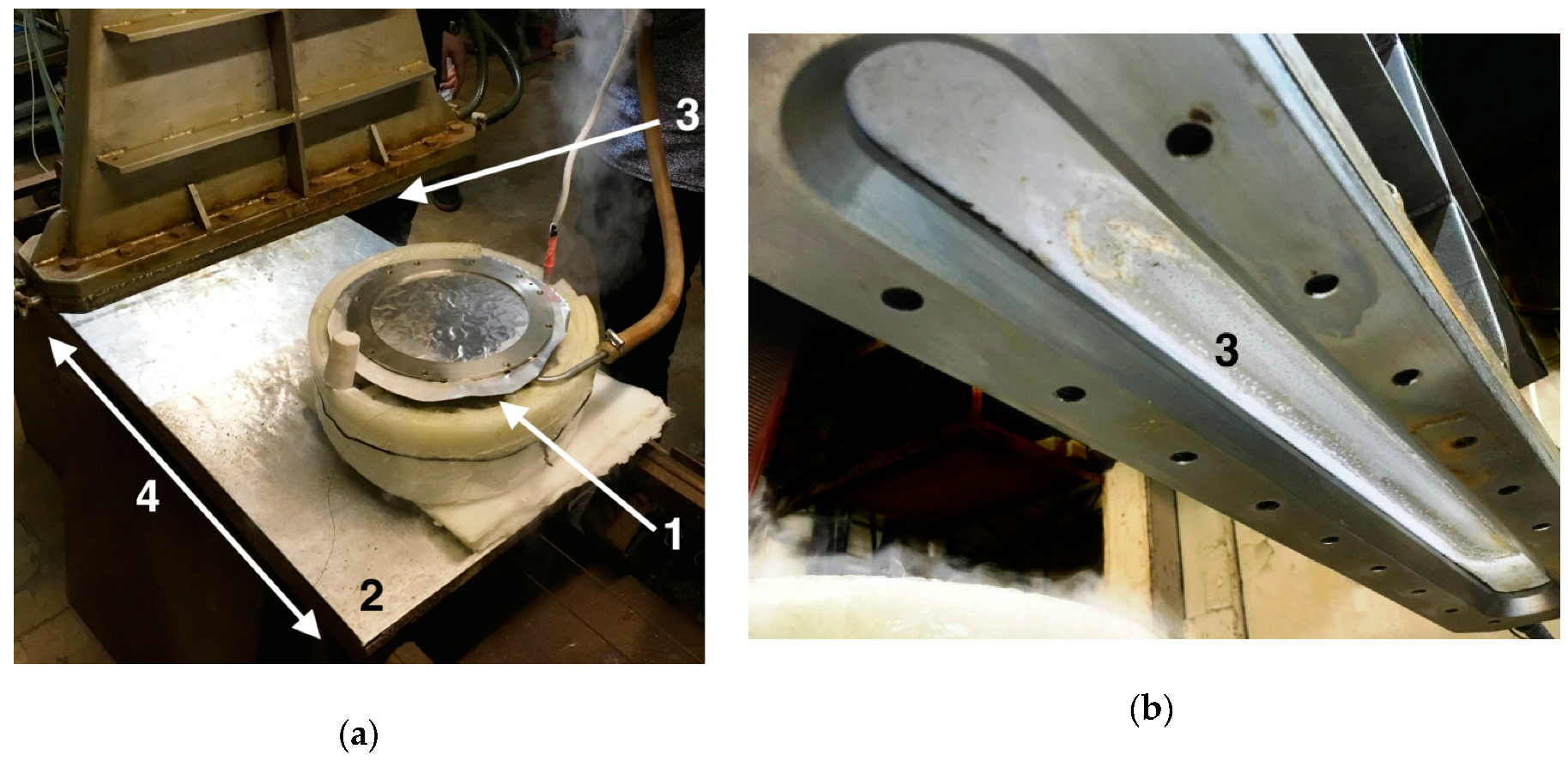
2.1. Vacuum Chamber
2.2. Accelerator
2.3. Samples Preparation and Analysis
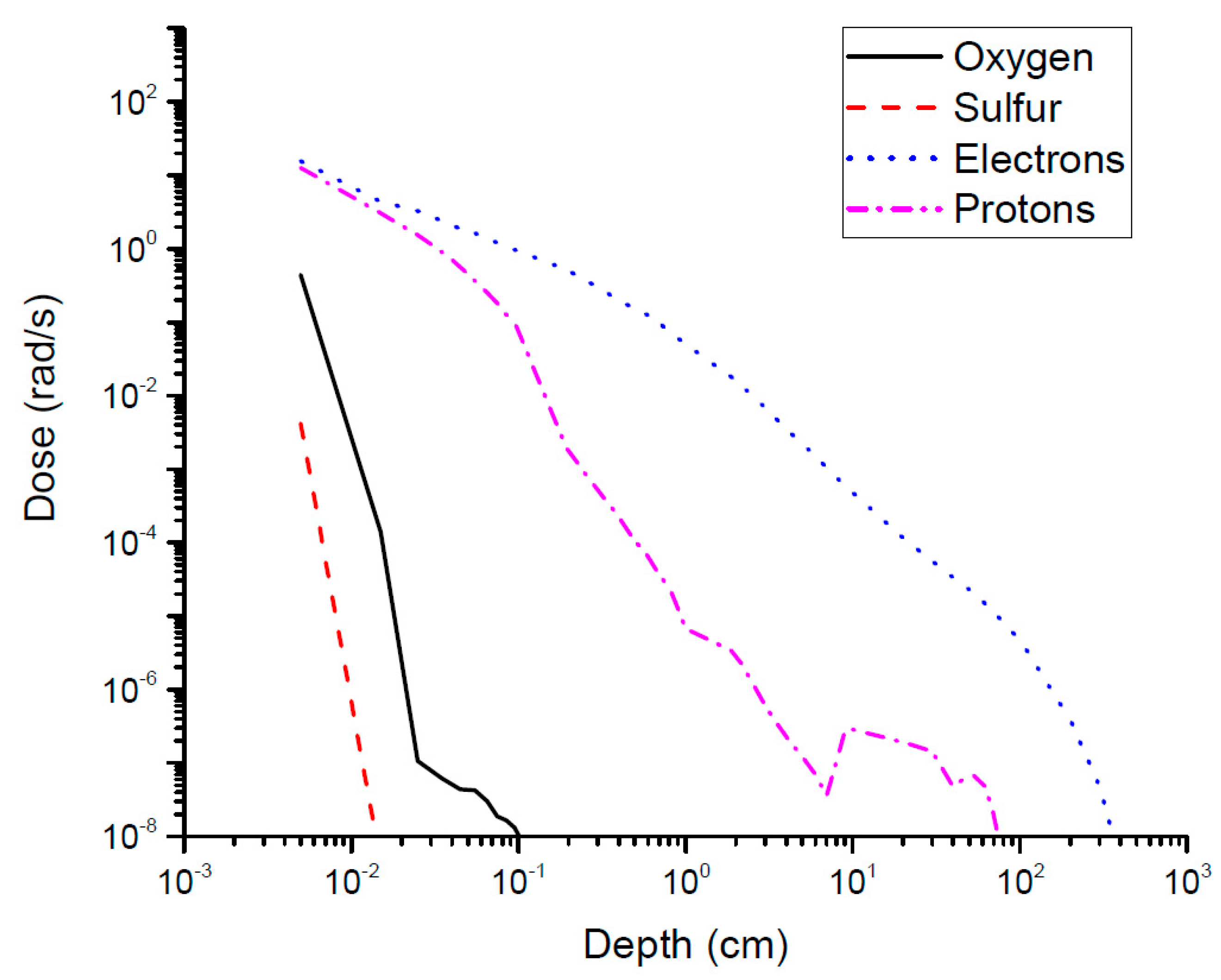
2.4. Calculating Accumulated Dose after Water Release on the Surface of Europa
3. Results and Discussion
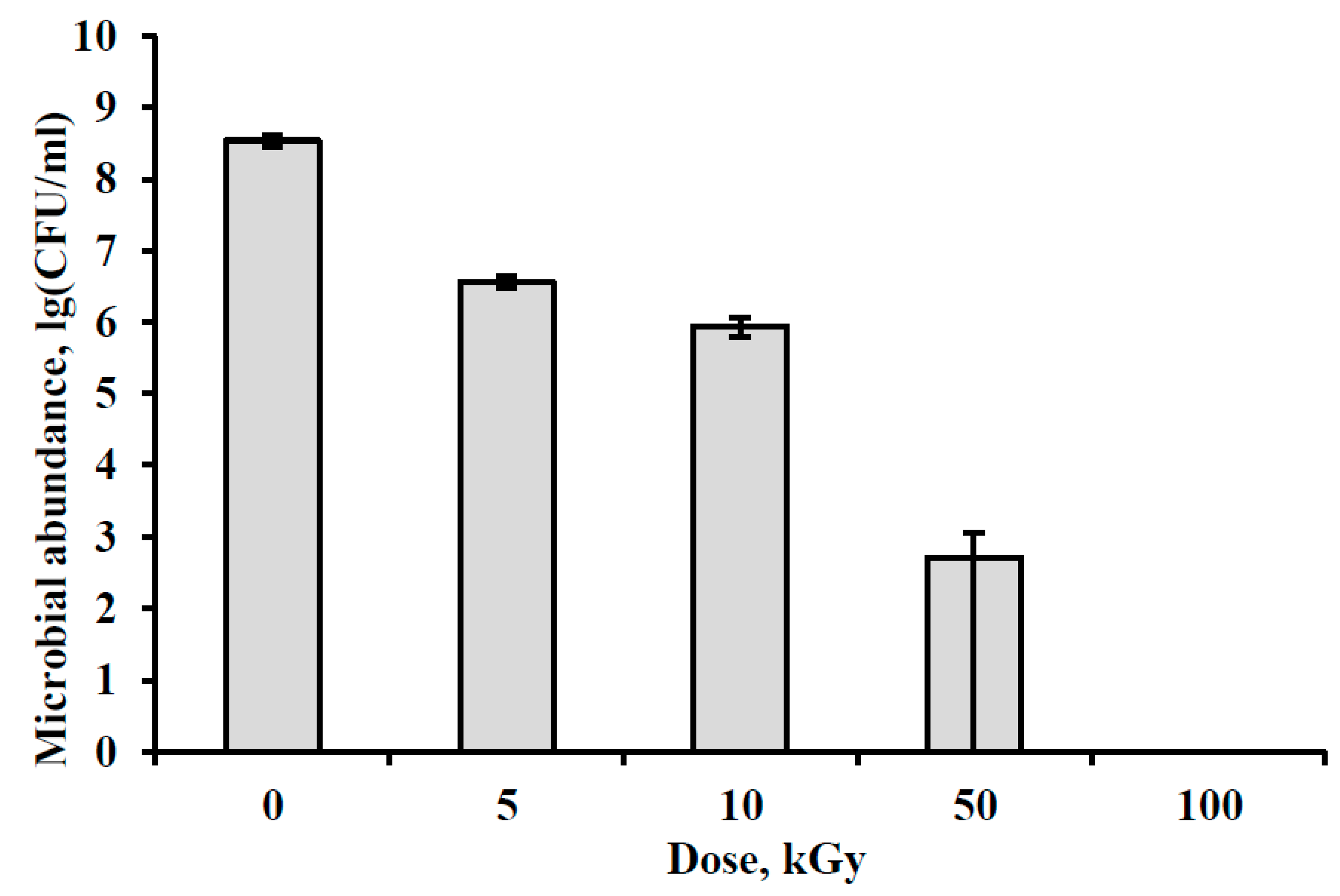
3.1. Bacteria Survival after Irradiation
3.2. Dose Dependence on Depth and Exposure Time
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Chyba, C.F.; Phillips, C.B. Possible ecosystems and the search for life on Europa. Proc. Natl. Acad. Sci. USA 2001, 98, 801–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marion, G.M.; Fritsen, C.H.; Eicken, H.; Payne, M.C. The search for life on Europa: Limiting environmental factors, potential habitats, and Earth analogues. Astrobiology 2003, 3, 785–811. [Google Scholar] [CrossRef] [PubMed]
- Judge, P. A novel strategy to seek biosignatures at Enceladus and Europa. Astrobiology 2017, 17, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, J.; Lindensmith, C.; Deming, J.W.; Fernandez, V.I.; Stocker, R. Microbial morphology and motility as biosignatures for outer planet missions. Astrobiology 2016, 16, 755–774. [Google Scholar] [CrossRef] [PubMed]
- Sparks, W.B.; Hand, K.P.; McGrath, M.A.; Bergeron, E.; Cracraft, M.; Deustua, S.E. Probing for evidence of plumes on Europa with HST/STIS. Astrophys. J. 2016, 829, 121. [Google Scholar] [CrossRef]
- Sparks, W.B.; Schmidt, B.E.; McGrath, M.A.; Hand, K.P.; Spencer, J.R.; Cracraft, M.; Deustua, S.E. Active cryovolcanism on Europa? Astrophys. J. Lett. 2017, 839, L18. [Google Scholar] [CrossRef]
- Gilichinsky, D. Permafrost as a microbial habitat. In Encyclopedia of Environmental Microbiology; Bitton, G., Ed.; Wiley: New York, NY, USA, 2002; pp. 932–956. ISBN 978-0-471-35450-5. [Google Scholar]
- Gilichinsky, D.A.; Vorobyova, E.A.; Erokhina, L.G.; Fyordorov-Dayvdov, D.G.; Chaikovskaya, N.R. Long-term preservation of microbial ecosystems in permafrost. Adv. Space Res. 1992, 12, 255–263. [Google Scholar] [CrossRef]
- Vorobyova, E.; Soina, V.; Gorlenko, M.; Minkovskaya, N.; Zalinova, N.; Mamukelashvili, A.; Gilichinsky, D.; Rivkina, E.; Vishnivetskaya, T. The deep cold biosphere: Facts and hypothesis. FEMS Microbiol. Rev. 1997, 20, 277–290. [Google Scholar] [CrossRef]
- Paranicas, C.; Cooper, J.F.; Garrett, H.B.; Johnson, R.E.; Sturner, S.J. Europa’s radiation environment and its effects on the surface. In Europa; Pappalardo, R.T., McKinnon, W.B., Khurana, K.K., Eds.; University of Arizona Press: Tucson, AZ, USA, 2009; pp. 529–544. ISBN 9780816528448. [Google Scholar]
- Zolotov, M.Y.; Shock, E.L. Composition and stability of salts on the surface of Europa and their oceanic origin. J. Geophys. Res. Planets 2001, 106, 32815–32827. [Google Scholar] [CrossRef] [Green Version]
- Fanale, F.P.; Li, Y.H.; De Carlo, E.; Farley, C.; Sharma, S.K.; Horton, K.; Granahan, J.C. An experimental estimate of Europa’s “ocean” composition independent of Galileo orbital remote sensing. J. Geophys. Res. Planets 2001, 106, 14595–14600. [Google Scholar] [CrossRef]
- Orlando, T.M.; McCord, T.B.; Grieves, G.A. The chemical nature of Europa surface material and the relation to a subsurface ocean. Icarus 2005, 177, 528–533. [Google Scholar] [CrossRef]
- Cheptsov, V.S.; Vorobyova, E.A.; Manucharova, N.A.; Gorlenko, M.V.; Pavlov, A.K.; Vdovina, M.A.; Lomasov, V.N.; Bulat, S.A. 100 kGy gamma-affected microbial communities within the ancient Arctic permafrost under simulated Martian conditions. Extremophiles 2017, 21, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Allison, J.; Amako, K.; Apostolakis, J.; Arce, P.; Asai, M.; Aso, T.; Bagli, E.; Bagulya, A.; Banerjee, S.; Barrand, G.; et al. Recent developments in Geant4. Nucl. Instrum. Methods Phys. Res. A 2016, 835, 186–225. [Google Scholar] [CrossRef]
- Agostinelli, S.; Allison, J.; Amako, K.A.; Apostolakis, J.; Araujo, H.; Arce, P.; Asai, M.; Axen, D.; Banerjee, S.; Barrand, G.; et al. GEANT4—A simulation toolkit. Nucl. Instrum. Methods Phys. Res. A 2003, 506, 250–303. [Google Scholar] [CrossRef]
- Ip, W.H.; Williams, D.J.; McEntire, R.W.; Mauk, B.H. Ion sputtering and surface erosion at Europa. Geophys. Res. Lett. 1998, 25, 829–832. [Google Scholar] [CrossRef] [Green Version]
- Musilova, M.; Wright, G.; Ward, J.M.; Dartnell, L.R. Isolation of radiation-resistant bacteria from Mars analog Antarctic Dry Valleys by preselection, and the correlation between radiation and desiccation resistance. Astrobiology 2015, 15, 1076–1090. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.M.; Battista, J.R. Deinococcus radiodurans—The consummate survivor. Nat. Rev. Microbiol. 2005, 3, 882–892. [Google Scholar] [CrossRef]
- Cheptsov, V.; Vorobyova, E.; Belov, A.; Pavlov, A.; Tsurkov, D.; Lomasov, V.; Bulat, S. Survivability of soil and permafrost microbial communities after irradiation with accelerated electrons under simulated Martian and open space conditions. Geosciences 2018, 8, 298. [Google Scholar] [CrossRef]
- Cheptsov, V.S.; Vorobyova, E.A.; Osipov, G.A.; Manucharova, N.A.; Polyanskaya, L.M.; Gorlenko, M.V.; Pavlov, A.K.; Rosanova, M.S.; Lomasov, V.N. Microbial activity in Martian analog soils after ionizing radiation: Implications for the preservation of subsurface life on Mars. AIMS Microbiol. 2018, 4, 541–562. [Google Scholar] [CrossRef]
- Richmond, R.; Sridhar, R.; Daly, M.J. Physicochemical survival pattern for the radiophile Deinococcus radiodurans: A polyextremophile model for life on Mars. Proc. SPIE 1999, 3755, 210–222. [Google Scholar] [CrossRef]
- Dartnell, L.R.; Hunter, S.J.; Lovell, K.V.; Coates, A.J.; Ward, J.M. Low-temperature ionizing radiation resistance of Deinococcus radiodurans and Antarctic Dry Valley bacteria. Astrobiology 2010, 10, 717–732. [Google Scholar] [CrossRef] [PubMed]
- Zakharov, Y.A.; Nevostruev, V.A. Radiolysis of solid inorganic salts with oxygen-containing anions. Russ. Chem. Rev. 1968, 37, 61–73. [Google Scholar] [CrossRef]
- Shuryak, I.; Matrosova, V.Y.; Gaidamakova, E.K.; Grichenko, O.; Klimenkova, P.; Volpe, R.P.; Daly, M.J. Microbial cells can cooperate to resist high-level chronic ionizing radiation. PLoS ONE 2017, 12, e0189261. [Google Scholar] [CrossRef] [PubMed]
- Zawierucha, K.; Ostrowska, M.; Kolicka, M. Applicability of cryoconite consortia of microorganisms and glacier-dwelling animals in astrobiological studies. Contemp. Trends Geosci. 2017, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Tranter, M.; Fountain, A.G.; Fritsen, C.H.; Berry Lyons, W.; Priscu, J.C.; Statham, P.J.; Welch, K.A. Extreme hydrochemical conditions in natural microcosms entombed within Antarctic ice. Hydrol. Process. 2004, 18, 379–387. [Google Scholar] [CrossRef]
- Baumstark-Khan, C.; Facius, R. Life under conditions of ionizing radiation. In Astrobiology: The Quest for the Conditions of Life; Horneck, G., Baumstark-Khan, C., Eds.; Springer: Berlin, Germany, 2002; pp. 261–284. ISBN 978-3-642-59381-9. [Google Scholar] [CrossRef]
- Rummel, J.D.; Beaty, D.W.; Jones, M.A.; Bakermans, C.; Barlow, N.G.; Boston, P.J.; Chevrier, V.F.; Clark, B.C.; de Vera, J.-P.P.; Gough, R.V.; et al. A new analysis of Mars “special regions”: Findings of the second MEPAG Special Regions Science Analysis Group (SR-SAG2). Astrobiology 2014, 14, 887–968. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlov, A.; Cheptsov, V.; Tsurkov, D.; Lomasov, V.; Frolov, D.; Vasiliev, G. Survival of Radioresistant Bacteria on Europa’s Surface after Pulse Ejection of Subsurface Ocean Water. Geosciences 2019, 9, 9. https://doi.org/10.3390/geosciences9010009
Pavlov A, Cheptsov V, Tsurkov D, Lomasov V, Frolov D, Vasiliev G. Survival of Radioresistant Bacteria on Europa’s Surface after Pulse Ejection of Subsurface Ocean Water. Geosciences. 2019; 9(1):9. https://doi.org/10.3390/geosciences9010009
Chicago/Turabian StylePavlov, Anatoly, Vladimir Cheptsov, Denis Tsurkov, Vladimir Lomasov, Dmitry Frolov, and Gennady Vasiliev. 2019. "Survival of Radioresistant Bacteria on Europa’s Surface after Pulse Ejection of Subsurface Ocean Water" Geosciences 9, no. 1: 9. https://doi.org/10.3390/geosciences9010009




