Virulence Studies of Different Sequence Types and Geographical Origins of Streptococcus suis Serotype 2 in a Mouse Model of Infection
Abstract
:1. Introduction
2. Results
2.1. Lack of Correlation between In Vitro Resistance to Phagocytosis and to Bacterial Killing by Different S. suis ST Strains
2.2. Systemic Infection: An Important Variation of Virulence Among Strains Belonging to the Same STs
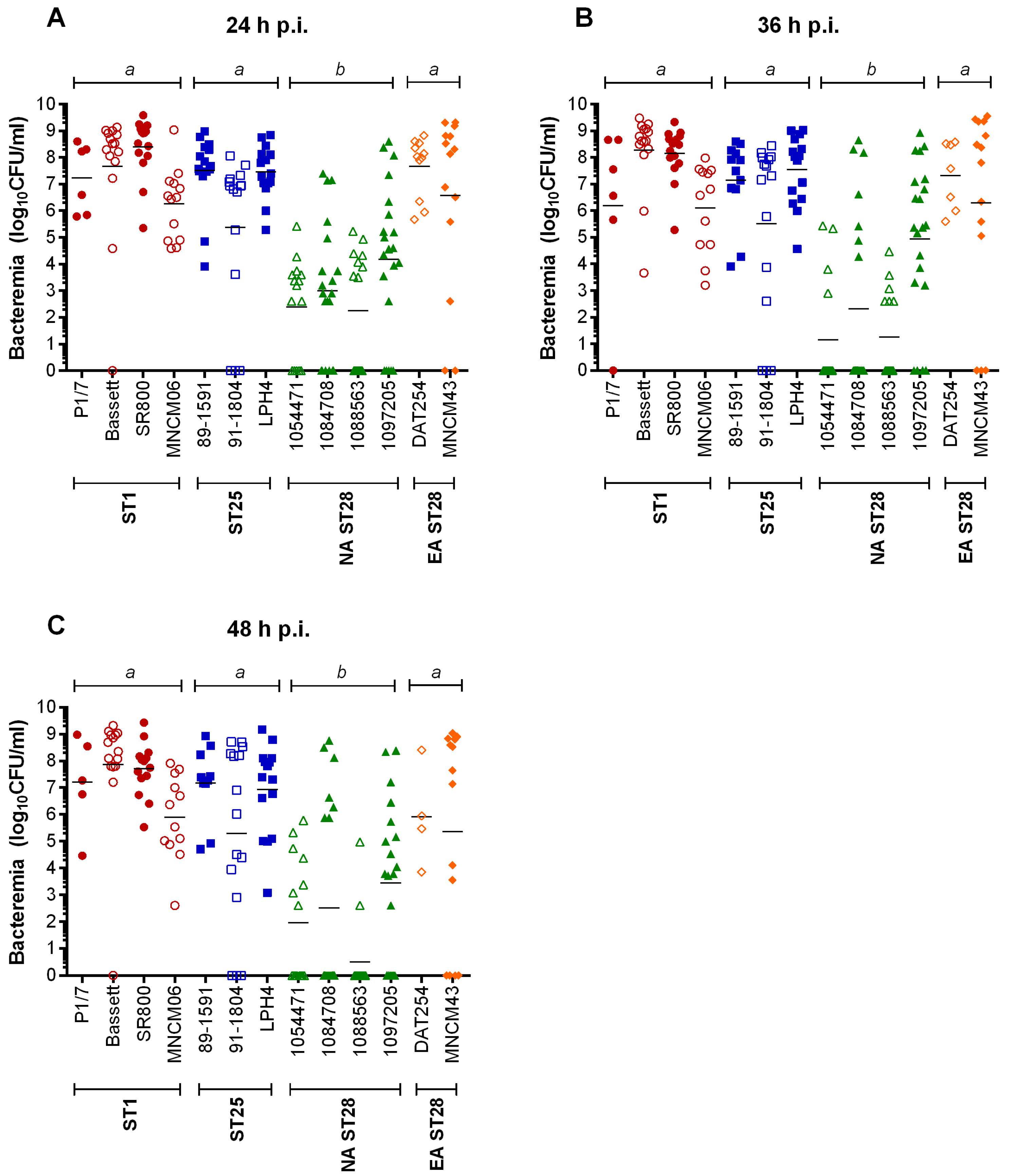
2.3. Bacteremia during the Acute Phase of Systemic Infection: Blood Bacterial Burden is Associated with Mortality Recorded at 72 h p.i. for Most Strains
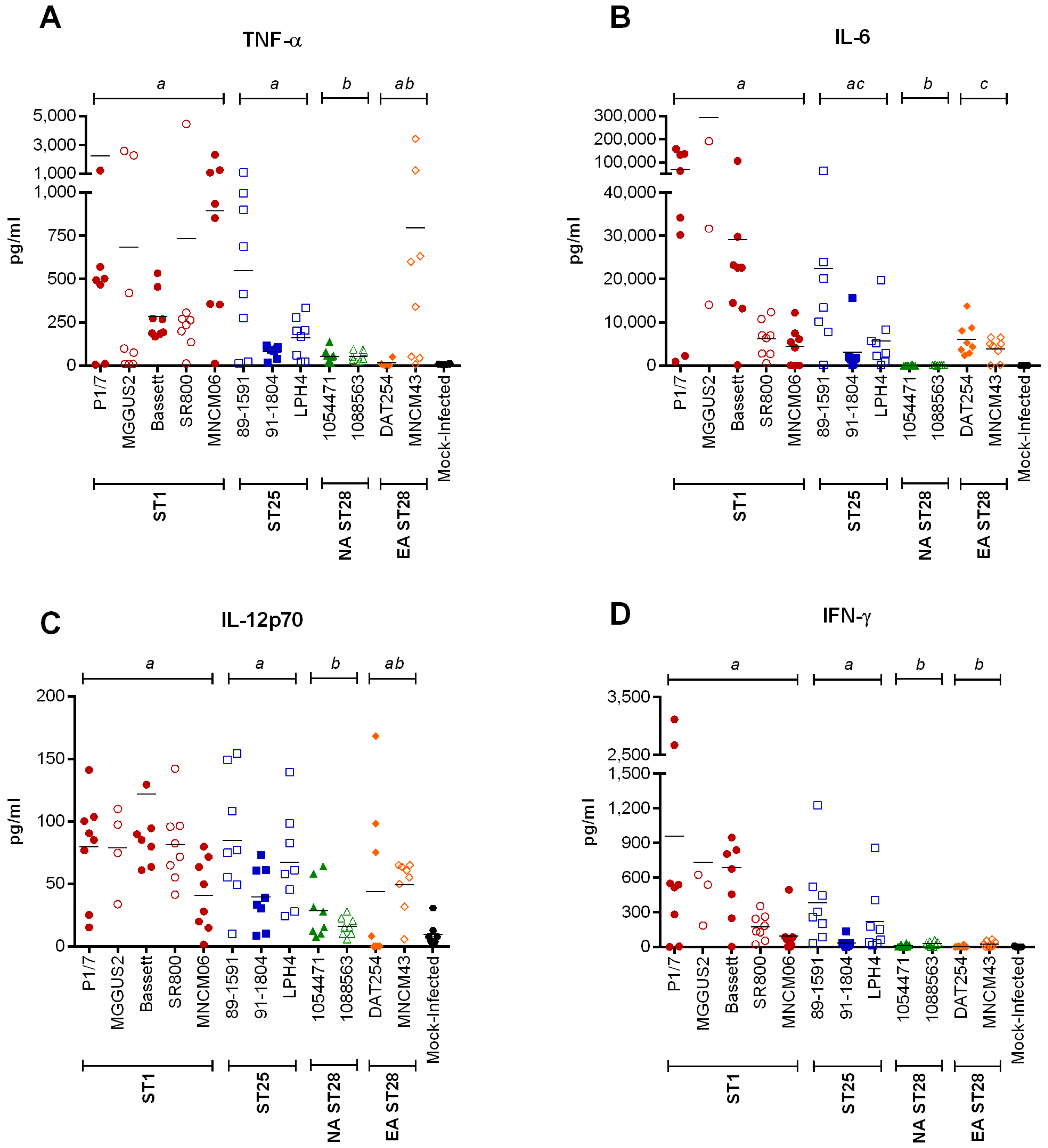
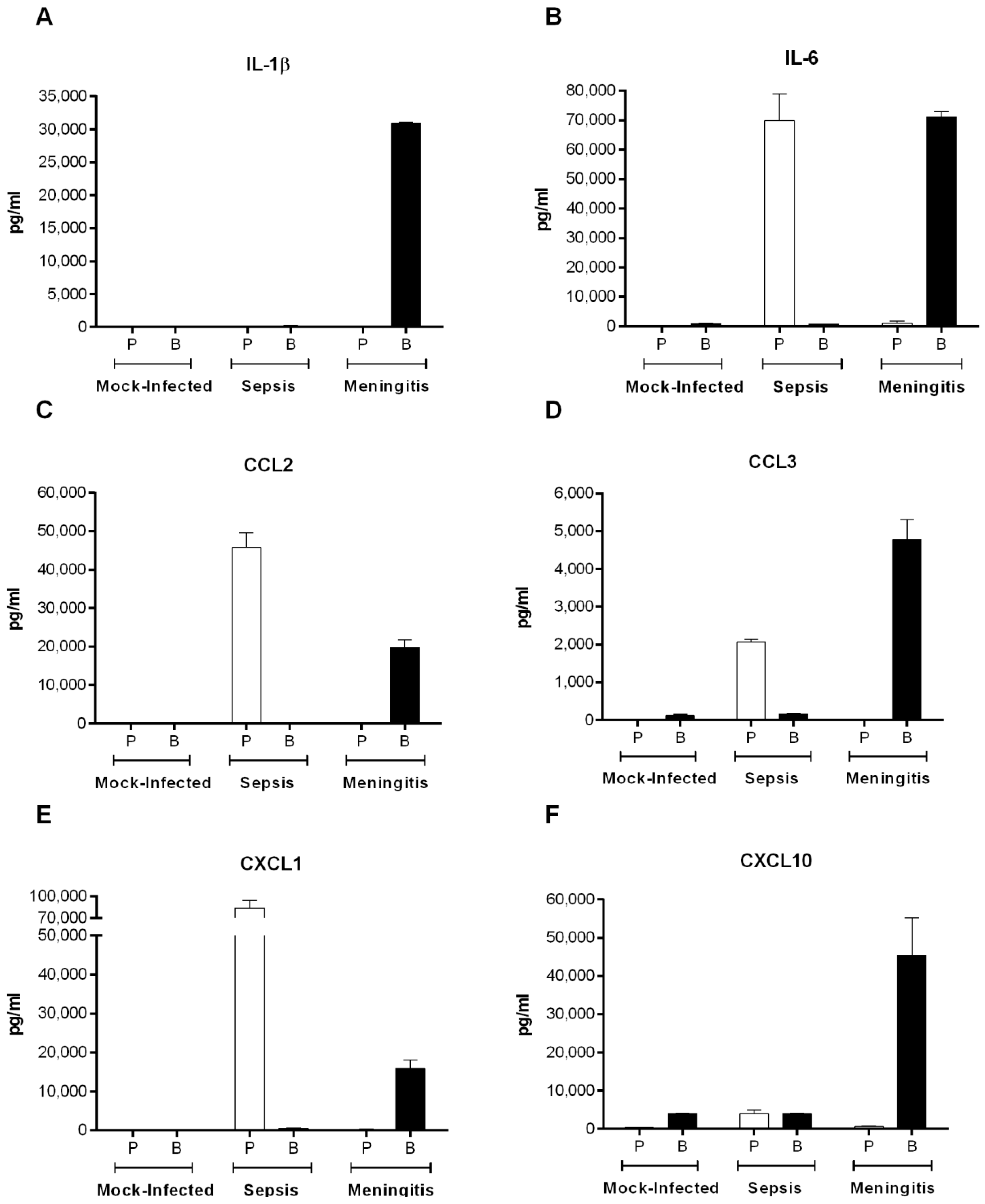
2.4. Inflammation during the Acute Phase of Systemic Infection: Plasma Pro-Inflammatory Mediator Levels Are Associated with Mortality Recorded at 72 h p.i. for Most Strains
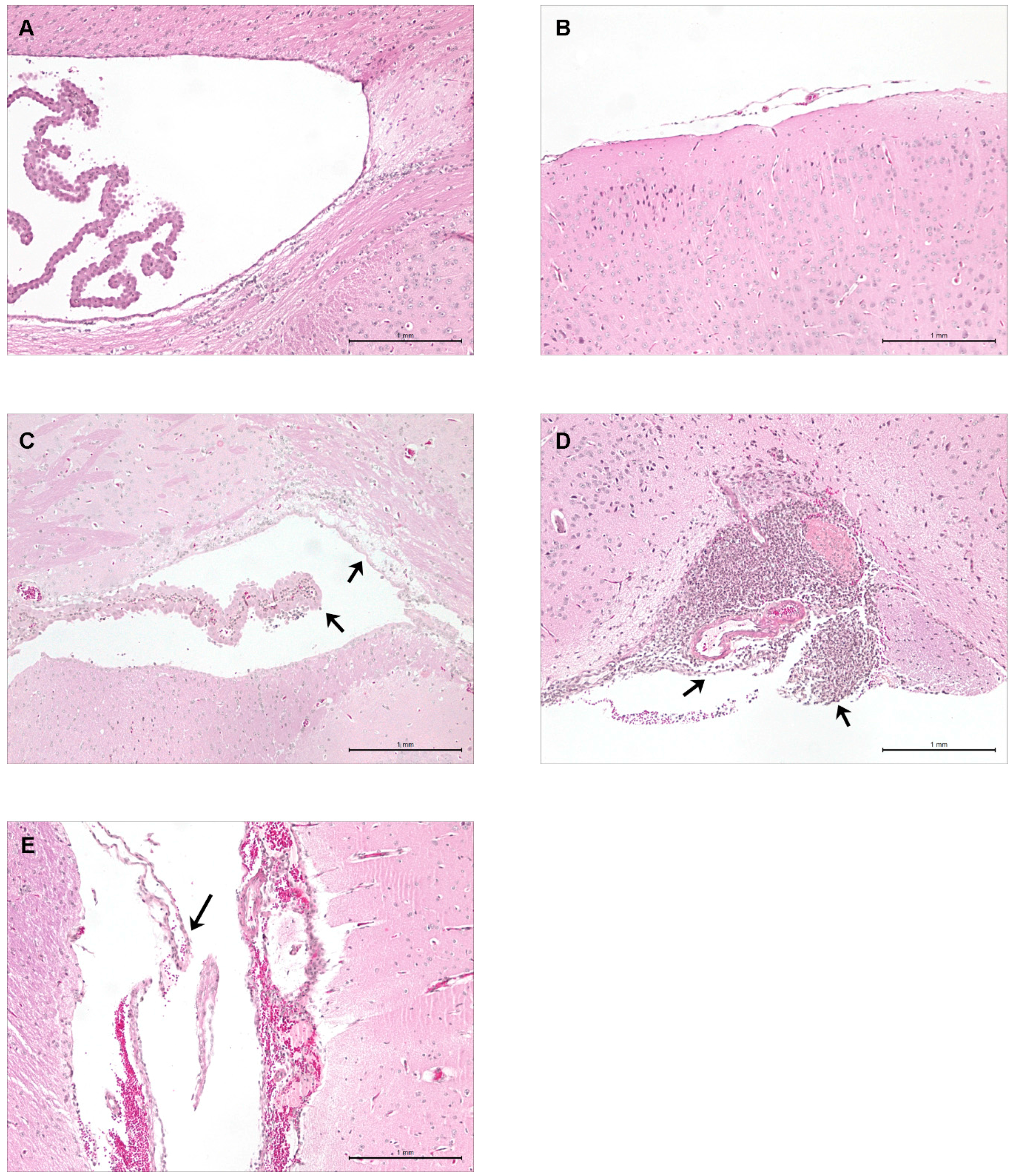
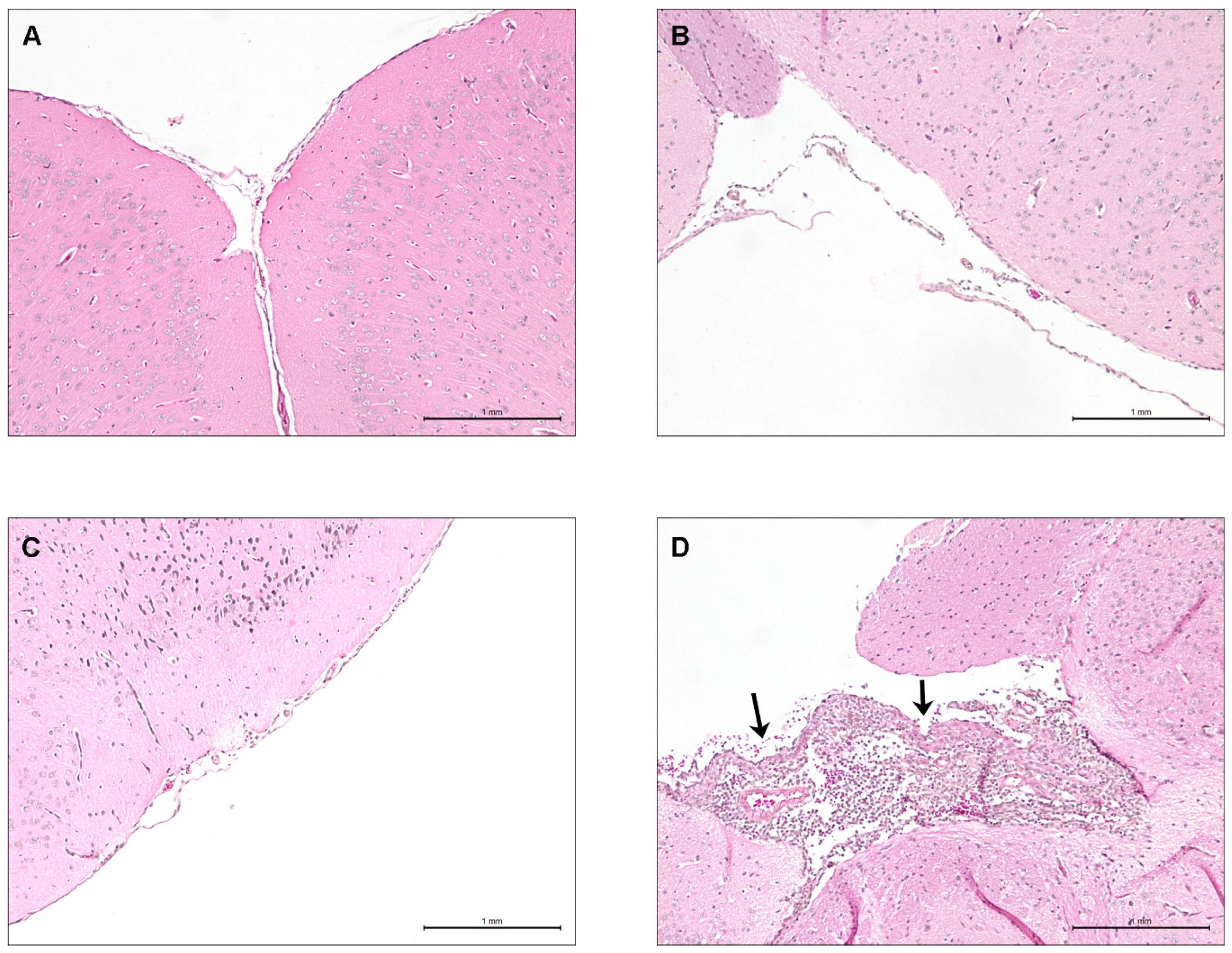
2.5. Hematogenous-Induced Meningitis: The ST1, ST25 and Eurasian ST28 Strains Induce Similar, Yet Higher Rates of Meningitis in Infected Mice than Do the North American ST28 Strains
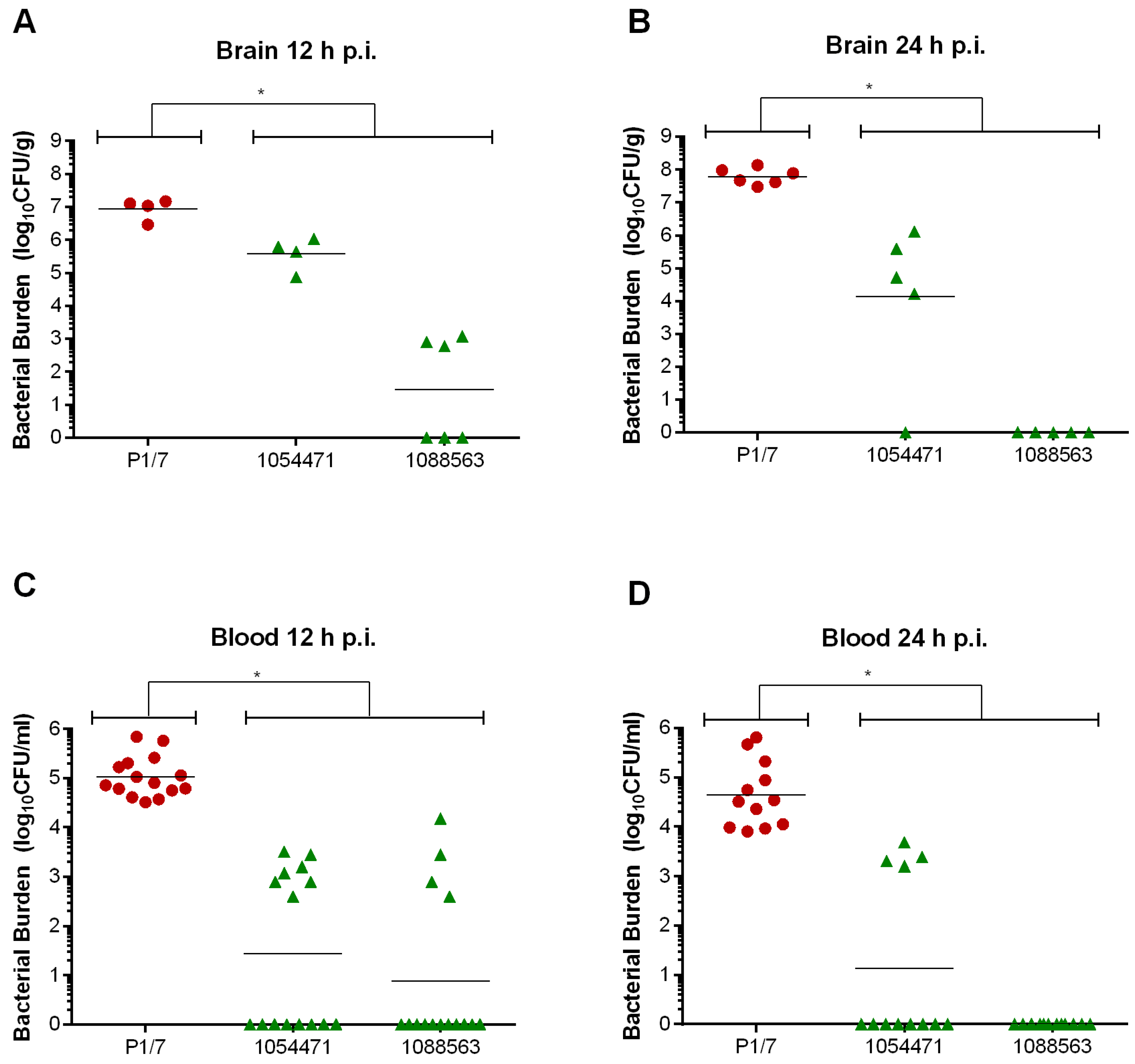
2.6. Brain Bacterial Burden Is Only Present in Infected Mice Following the Development of Meningitis, Regardless of the ST
2.7. Lower Virulence North American ST28 Strains Remain Incapable of Causing Meningitis Following Intracisternal Injection into the Cerebrospinal Fluid
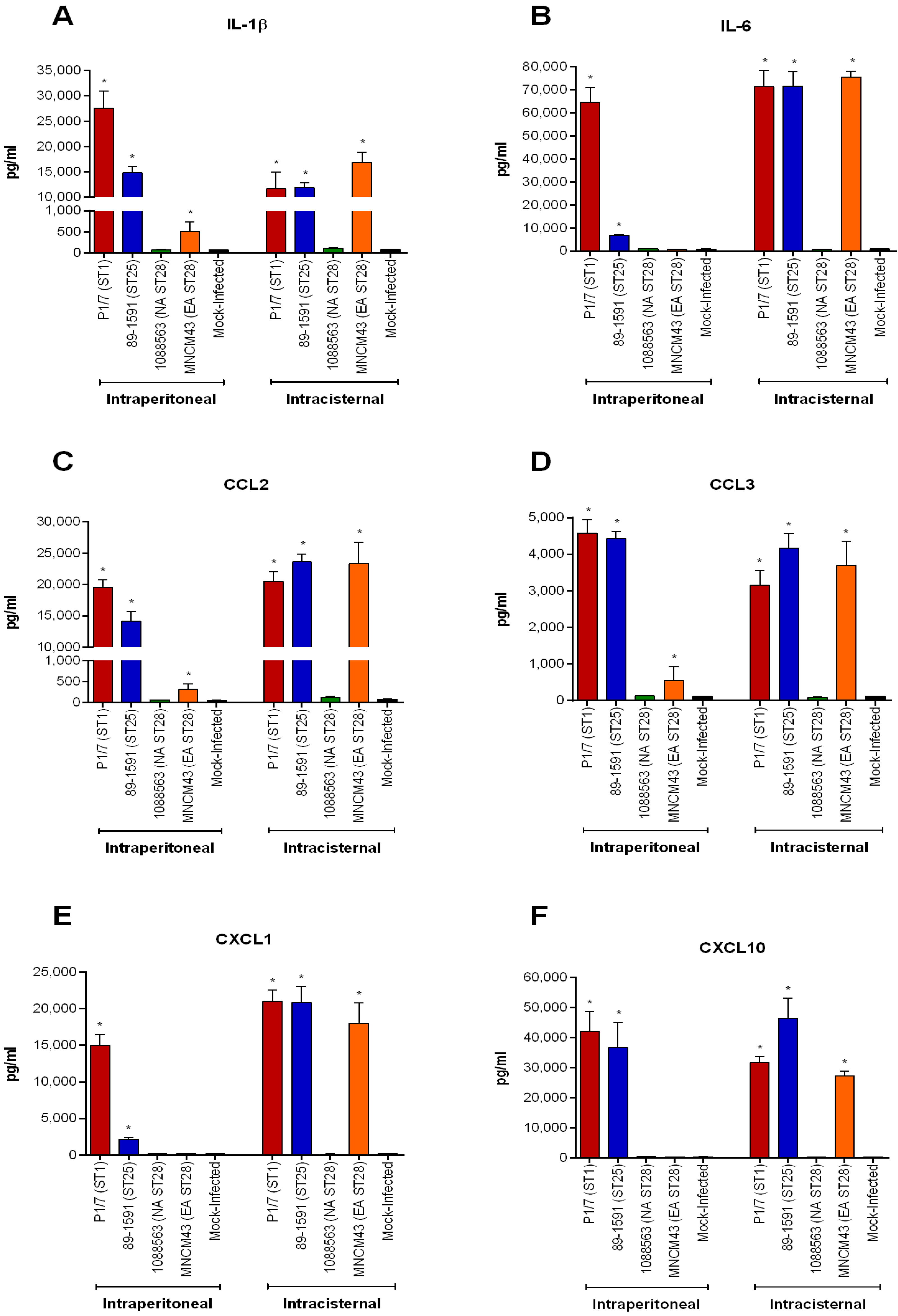
2.8. S. suis Serotype 2-Induced Pro-Inflammatory Brain Response Only Occurs in the Presence of Meningitis
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Growth Conditions
4.2. S. suis MLST
4.3. S. suis Serotype 2 Phagocytosis Assay
4.4. S. suis Serotype 2 Bactericidal Assay
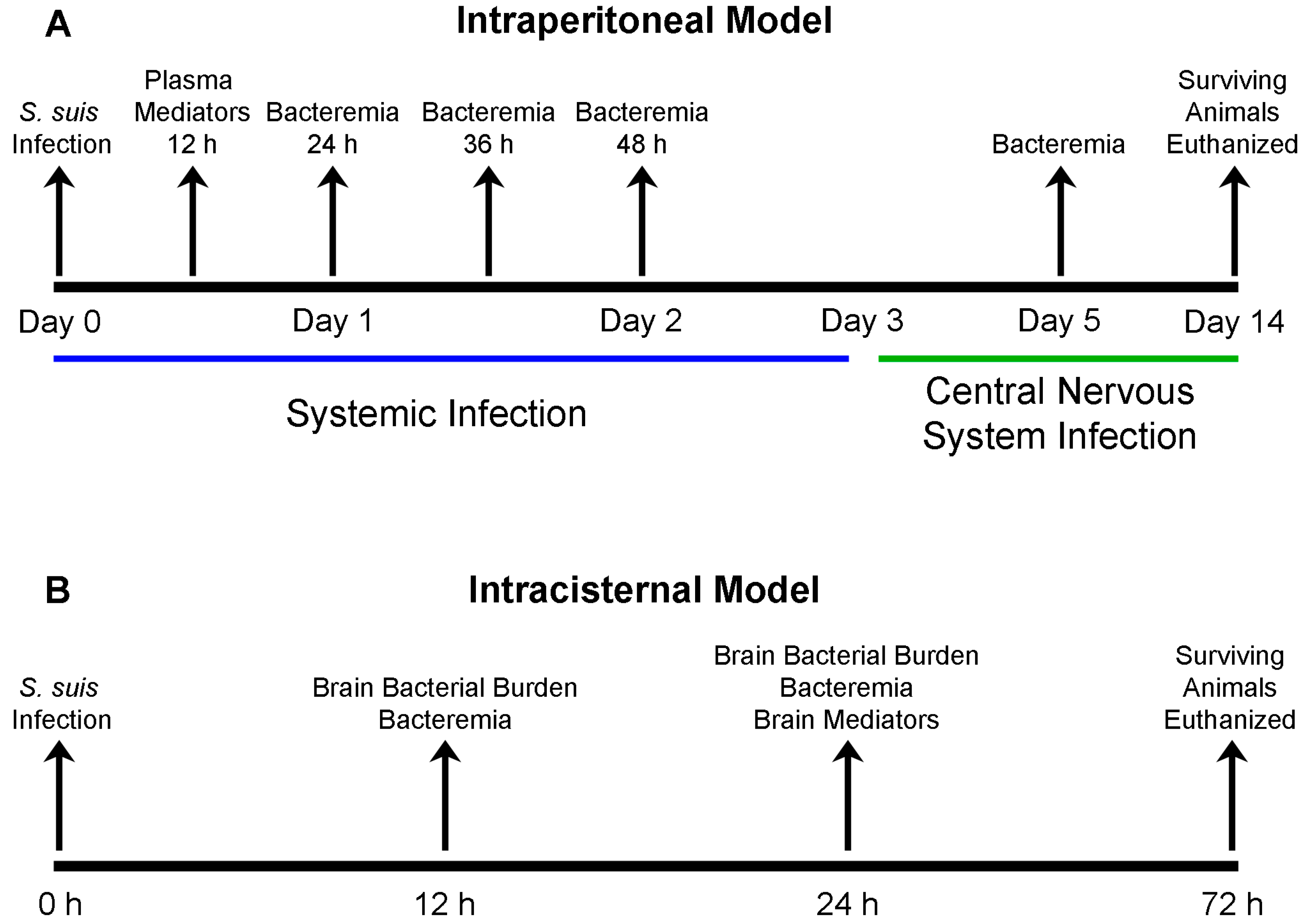
4.5. S. suis Serotype 2 Experimental Mouse Infections
4.6. Measurement of Mouse Blood Bacterial Burden
4.7. Measurement of Mouse Plasma (Systemic) Cytokine and Chemokine Levels
4.8. Mouse Brain Histopathological Studies
4.9. Measurement of Mouse Brain Bacterial Burden
4.10. Evaluation of Systemic and Brain Compartmentalization and Measurement of Mouse Brain Cytokine and Chemokine Levels
4.11. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CCL | C-C motif ligand |
| CFU | Colony forming unit |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| CXCL | C-X-C motif ligand |
| EA | Eurasia |
| ELISA | Enzyme-linked immunosorbent assay |
| HPS | Hematoxylin phloxine saffron |
| IL | Interleukin |
| IFN | Interferon |
| MLST | Multilocus sequence typing |
| MOI | Multiplicity of infection |
| NA | North America |
| p.i. | Post-infection |
| PBS | Phosphate-buffered saline |
| SEM | Standard error of the mean |
| ST | Sequence type |
| THA | Todd-Hewitt broth agar |
| THB | Todd-Hewitt broth |
| TNF | Tumor necrosis factor |
References
- Fittipaldi, N.; Segura, M.; Grenier, D.; Gottschalk, M. Virulence factors involved in the pathogenesis of the infection caused by the swine pathogen and zoonotic agent Streptococcus suis. Future Microbiol. 2012, 7, 259–279. [Google Scholar] [CrossRef] [PubMed]
- Goyette-Desjardins, G.; Auger, J.P.; Xu, J.; Segura, M.; Gottschalk, M. Streptococcus suis, an important pig pathogen and emerging zoonotic agent—An update on the worldwide distribution based on serotyping and sequence typing. Emerg. Microbes. Infect. 2014, 3, e45. [Google Scholar] [CrossRef] [PubMed]
- Okura, M.; Lachance, C.; Osaki, M.; Sekizaki, T.; Maruyama, F.; Nozawa, T.; Nakagawa, I.; Hamada, S.; Rossignol, C.; Gottschalk, M.; et al. Development of a two-step multiplex PCR assay for typing of capsular polysaccharide synthesis gene clusters of Streptococcus suis. J. Clin. Microbiol. 2014, 52, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Berthelot-Hérault, F.; Gottschalk, M.; Morvan, H.; Kobisch, M. Dilemma of virulence of Streptococcus suis: Canadian isolate 89-1591 characterized as a virulent strain using a standardized experimental model in pigs. Can. J. Vet. Res. 2005, 69, 236–240. [Google Scholar] [PubMed]
- Martinez, G.; Pestana de Castro, A.F.; Ribeiro Pagnani, K.J.; Nakazato, G.; Dias da Silveira, W.; Gottschalk, M. Clonal distribution of an atypical MRP+, EF*, and suilysin+ phenotype of virulent Streptococcus suis serotype 2 strains in Brazil. Can. J. Vet. Res. 2003, 67, 52–55. [Google Scholar] [PubMed]
- Takamatsu, D.; Osaki, M.; Tharavichitkul, P.; Takai, S.; Sekizaki, T. Allelic variation and prevalence of serum opacity factor among the Streptococcus suis population. J. Med. Microbiol. 2008, 57, 488–494. [Google Scholar] [CrossRef] [PubMed]
- King, S.J.; Heath, P.J.; Luque, I.; Tarradas, C.; Dowson, C.G.; Whatmore, A.M. Distribution and genetic diversity of suilysin in Streptococcus suis isolated from different diseases of pigs and characterization of the genetic basis of suilysin absence. Infect. Immun. 2001, 69, 7572–7582. [Google Scholar] [CrossRef] [PubMed]
- Chatellier, S.; Gottschalk, M.; Higgins, R.; Brousseau, R.; Harel, J. Relatedness of Streptococcus suis serotype 2 isolates from different geographic origins as evaluated by molecular fingerprinting and phenotyping. J. Clin. Microbiol. 1999, 37, 362–366. [Google Scholar] [PubMed]
- De Greeff, A.; Wisselink, H.J.; de Bree, F.M.; Schultsz, C.; Baums, C.G.; Thi, H.N.; Stockhofe-Zurwieden, N.; Smith, H.E. Genetic diversity of Streptococcus suis isolates as determined by comparative genome hybridization. BMC Microbiol. 2011, 11, 161. [Google Scholar] [CrossRef] [PubMed]
- Luey, C.K.; Chu, Y.W.; Cheung, T.K.; Law, C.C.; Chu, M.Y.; Cheung, D.T.; Kam, K.M. Rapid pulsed-field gel electrophoresis protocol for subtyping of Streptococcus suis serotype 2. J. Microbiol. Methods 2007, 68, 648–650. [Google Scholar] [CrossRef] [PubMed]
- Marois, C.; Le Devendec, L.; Gottschalk, M.; Kobisch, M. Molecular characterization of Streptococcus suis strains by 16S–23S intergenic spacer polymerase chain reaction and restriction fragment length polymorphism analysis. Can. J. Vet. Res. 2006, 70, 94–104. [Google Scholar] [PubMed]
- Rehm, T.; Baums, C.G.; Strommenger, B.; Beyerbach, M.; Valentin-Weigand, P.; Goethe, R. Amplified fragment length polymorphism of Streptococcus suis strains correlates with their profile of virulence-associated genes and clinical background. J. Med. Microbiol. 2007, 56, 102–109. [Google Scholar] [CrossRef] [PubMed]
- King, S.J.; Leigh, J.A.; Heath, P.J.; Luque, I.; Tarradas, C.; Dowson, C.G.; Whatmore, A.M. Development of a multilocus sequence typing scheme for the pig pathogen Streptococcus suis: Identification of virulent clones and potential capsular serotype exchange. J. Clin. Microbiol. 2002, 40, 3671–3680. [Google Scholar] [CrossRef] [PubMed]
- Kerdsin, A.; Dejsirilert, S.; Puangpatra, P.; Sripakdee, S.; Chumla, K.; Boonkerd, N.; Polwichai, P.; Tanimura, S.; Takeuchi, D.; Nakayama, T.; et al. Genotypic profile of Streptococcus suis serotype 2 and clinical features of infection in humans, Thailand. Emerg. Infect. Dis. 2011, 17, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Schultsz, C.; Jansen, E.; Keijzers, W.; Rothkamp, A.; Duim, B.; Wagenaar, J.A.; van der Ende, A. Differences in the population structure of invasive Streptococcus suis strains isolated from pigs and from humans in The Netherlands. PLoS ONE 2012, 7, e33854. [Google Scholar] [CrossRef] [PubMed]
- Lachance, C.; Gottschalk, M.; Gerber, P.P.; Lemire, P.; Xu, J.; Segura, M. Exacerbated type II interferon response drives hypervirulence and toxic shock by an emergent epidemic strain of Streptococcus suis. Infect. Immun. 2013, 81, 1928–1939. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Lan, R.; Zheng, X.; Cui, Z.; Liu, Z.; Bai, X.; Ji, S.; Gottschalk, M.; Xu, J. Comparative genomic hybridization identifies virulence differences in Streptococcus suis. PLoS ONE 2014, 9, e87866. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Zheng, H.; Zhang, J.; Jing, H.; Wang, L.; Xiong, Y.; Wang, W.; Zhou, Z.; Sun, Q.; Luo, X.; et al. Clinical, experimental, and genomic differences between intermediately pathogenic, highly pathogenic, and epidemic Streptococcus suis. J. Infect. Dis. 2009, 199, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Fittipaldi, N.; Xu, J.; Lacouture, S.; Tharavichitkul, P.; Osaki, M.; Sekizaki, T.; Takamatsu, D.; Gottschalk, M. Lineage and virulence of Streptococcus suis serotype 2 isolates from North America. Emerg. Infect. Dis. 2011, 17, 2239–2244. [Google Scholar] [CrossRef] [PubMed]
- Willenburg, K.S.; Sentochnik, D.E.; Zadoks, R.N. Human Streptococcus suis meningitis in the United States. N. Engl. J. Med. 2006, 354, 1325. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, D.; Wongsawan, K.; Osaki, M.; Nishino, H.; Ishiji, T.; Tharavichitkul, P.; Khantawa, B.; Fongcom, A.; Takai, S.; Sekizaki, T. Streptococcus suis in humans, Thailand. Emerg. Infect. Dis. 2008, 14, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Wada, A.; Ikebe, T.; Ohnishi, M.; Mita, K.; Endo, M.; Matsuo, H.; Asatuma, Y.; Kuramoto, S.; Sekiguichi, H.; et al. Characteristics of Streptococcus suis isolated from patients in Japan. Jpn. J. Infect. Dis. 2006, 59, 397–399. [Google Scholar] [PubMed]
- Athey, T.B.; Auger, J.P.; Teatero, S.; Dumesnil, A.; Takamatsu, D.; Wasserscheid, J.; Dewar, K.; Gottschalk, M.; Fittipaldi, N. Complex population structure and virulence differences among serotype 2 Streptococcus suis strains belonging to sequence type 28. PLoS ONE 2015, 10, e0137760. [Google Scholar] [CrossRef] [PubMed]
- Athey, T.B.; Teatero, S.; Takamatsu, D.; Wasserscheid, J.; Dewar, K.; Gottschalk, M.; Fittipaldi, N. Population structure and antimicrobial resistance profiles of Streptococcus suis serotype 2 sequence type 25 strains. PLoS ONE 2016, 11, e0150908. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, M. Streptococcosis. In Diseases of Swine, 10th ed.; Zimmerman, J.J., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Eds.; Wiley-Blackwell Publishing: Ames, IA, USA, 2012; pp. 841–855. [Google Scholar]
- Dominguez-Punaro, M.C.; Segura, M.; Plante, M.M.; Lacouture, S.; Rivest, S.; Gottschalk, M. Streptococcus suis serotype 2, an important swine and human pathogen, induces strong systemic and cerebral inflammatory responses in a mouse model of infection. J. Immunol. 2007, 179, 1842–1854. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Punaro Mde, L.; Segura, M.; Radzioch, D.; Rivest, S.; Gottschalk, M. Comparison of the susceptibilities of C57BL/6 and A/J mouse strains to Streptococcus suis serotype 2 infection. Infect. Immun. 2008, 76, 3901–3910. [Google Scholar] [CrossRef] [PubMed]
- Lachance, C.; Segura, M.; Gerber, P.P.; Xu, J.; Gottschalk, M. Toll-Like receptor 2-independent host innate immune response against an epidemic strain of Streptococcus suis that causes a toxic shock-like syndrome in humans. PLoS ONE 2013, 8, e65031. [Google Scholar] [CrossRef] [PubMed]
- Salasia, S.I.O.; Lammler, C.; Herrmann, G. Properties of a Streptococcus suis isolate of serotype 2 and two capsular mutants. Vet. Microbiol. 1995, 45, 151–156. [Google Scholar] [CrossRef]
- Dominguez-Punaro, M.C.; Koedel, U.; Hoegen, T.; Demel, C.; Klein, M.; Gottschalk, M. Severe cochlear inflammation and vestibular syndrome in an experimental model of Streptococcus suis infection in mice. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2391–2400. [Google Scholar] [CrossRef] [PubMed]
- Koedel, U.; Rupprecht, T.; Angele, B.; Heesemann, J.; Wagner, H.; Pfister, H.W.; Kirschning, C.J. MyD88 is required for mounting a robust host immune response to Streptococcus pneumoniae in the CNS. Brain 2004, 127, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Lecours, M.P.; Segura, M.; Fittipaldi, N.; Rivest, S.; Gottschalk, M. Immune receptors involved in Streptococcus suis recognition by dendritic cells. PLoS ONE 2012, 7, e44746. [Google Scholar] [CrossRef] [PubMed]
- Lecours, M.P.; Fittipaldi, N.; Takamatsu, D.; Okura, M.; Segura, M.; Goyette-Desjardins, G.; van Calsteren, M.R.; Gottschalk, M. Sialylation of Streptococcus suis serotype 2 is essential for capsule expression but is not responsible for the main capsular epitope. Microbes Infect. Inst. Pasteur 2012, 14, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Chabot-Roy, G.; Willson, P.; Segura, M.; Lacouture, S.; Gottschalk, M. Phagocytosis and killing of Streptococcus suis by porcine neutrophils. Microb. Pathog. 2006, 41, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Auger, J.P.; Segura, M.; Fittipaldi, N.; Takamatsu, D.; Okura, M.; Gottschalk, M. Role of the capsular polysaccharide as a virulence factor for Streptococcus suis serotype 14. Can. J. Vet. Res. 2015, 79, 141–146. [Google Scholar] [PubMed]
- Brazeau, C.; Gottschalk, M.; Vincelette, S.; Martineau-Doizé, B. in vitro phagocytosis and survival of Streptococcus suis capsular type 2 inside murine macrophages. Microbiology 1996, 142, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Seitz, M.; Beineke, A.; Singpiel, A.; Willenborg, J.; Dutow, P.; Goethe, R.; Valentin-Weigand, P.; Klos, A.; Baums, C.G. Role of capsule and suilysin in mucosal infection of complement-deficient mice with Streptococcus suis. Infect. Immun. 2014, 82, 2460–2471. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Cao, M.; Shi, J.; Zhang, H.; Hu, D.; Zhu, J.; Zhang, X.; Geng, M.; Zheng, F.; Pan, X.; et al. Attenuation of Streptococcus suis virulence by the alteration of bacterial surface architecture. Sci. Rep. 2012, 2, 710. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Li, M.; Wang, J.; Wang, C.; Hu, D.; Zheng, F.; Pan, X.; Tan, Y.; Zhao, Y.; Hu, L.; et al. Isolation and characterization of a native avirulent strain of Streptococcus suis serotype 2: A perspective for vaccine development. Sci. Rep. 2015, 5, 9835. [Google Scholar] [CrossRef] [PubMed]
- De Buhr, N.; Neumann, A.; Jerjomiceva, N.; von Kockritz-Blickwede, M.; Baums, C.G. Streptococcus suis DNase SsnA contributes to degradation of neutrophil extracellular traps (NETs) and evasion of NET-mediated antimicrobial activity. Microbiology 2014, 160, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, M.; Xu, J.; Calzas, C.; Segura, M. Streptococcus suis: a new emerging or an old neglected zoonotic pathogen? Future Microbiol. 2010, 5, 371–391. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S. Pathogenesis of bacterial meningitis: From bacteraemia to neuronal injury. Nat. Rev. Neurosci. 2003, 4, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Dando, S.J.; Mackay-Sim, A.; Norton, R.; Currie, B.J.; St John, J.A.; Ekberg, J.A.; Batzloff, M.; Ulett, G.C.; Beacham, I.R. Pathogens penetrating the central nervous system: infection pathways and the cellular and molecular mechanisms of invasion. Clin. Microbiol. Rev. 2014, 27, 691–726. [Google Scholar] [CrossRef] [PubMed]
- Koedel, U.; Angele, B.; Rupprecht, T.; Wagner, H.; Roggenkamp, A.; Pfister, H.W.; Kirschning, C.J. Toll-like receptor 2 participates in mediation of immune response in experimental pneumococcal meningitis. J. Immunol. 2003, 170, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Scheld, W.M.; Park, T.S.; Dacey, R.G.; Winn, H.R.; Jane, J.A.; Sande, M.A. Clearance of bacteria from cerebrospinal fluid to blood in experimental meningitis. Infect. Immun. 1979, 24, 102–105. [Google Scholar] [PubMed]
- Ransohoff, R.M.; Brown, M.A. Innate immunity in the central nervous system. J. Clin. Invest. 2012, 122, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Farina, C.; Aloisi, F.; Meinl, E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007, 28, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Busque, P.; Higgins, R.; Caya, F.; Quessy, S. Immunization of pigs against Streptococcus suis serotype 2 infection using a live avirulent strain. Can. J. Vet. Res. 1997, 61, 275–279. [Google Scholar] [PubMed]
- Liechti, F.D.; Grandgirard, D.; Leib, S.L. Bacterial meningitis: Insights into pathogenesis and evaluation of new treatment options: A perspective from experimental studies. Future Microbiol. 2015, 10, 1195–1213. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Punaro Mde, L.; Segura, M.; Contreras, I.; Lachance, C.; Houde, M.; Lecours, M.P.; Olivier, M.; Gottschalk, M. In vitro characterization of the microglial inflammatory response to Streptococcus suis, an important emerging zoonotic agent of meningitis. Infect. Immun. 2010, 78, 5074–5085. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Punaro, M.C.; Segura, M.; Lachance, C.; Rivest, S.; Xu, J.; Houde, M.; Gottschalk, M. Toll-like receptor 2 is partially involved in the activation of murine astrocytes by Streptococcus suis, an important zoonotic agent of meningitis. J. Neuroimmunol. 2011, 234, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Segura, M.; Vadeboncoeur, N.; Gottschalk, M. CD14-dependent and -independent cytokine and chemokine production by human THP-1 monocytes stimulated by Streptococcus suis capsular type 2. Clin. Exp. Immunol. 2002, 127, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.; Koedel, U.; Winkler, F.; Kieseier, B.C.; Fontana, A.; Kopf, M.; Hartung, H.P.; Pfister, H.W. Lack of IL-6 augments inflammatory response but decreases vascular permeability in bacterial meningitis. Brain 2003, 126, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Barichello, T.; Generoso, J.S.; Simoes, L.R.; Goularte, J.A.; Petronilho, F.; Saigal, P.; Badawy, M.; Quevedo, J. Role of microglial activation in the pathophysiology of bacterial meningitis. Mol. Neurobiol. 2016, 53, 1770–1781. [Google Scholar] [CrossRef] [PubMed]
- Waage, A.; Halstensen, A.; Espevik, T.; Brandtzaeg, P. Compartmentalization of TNF and IL-6 in meningitis and septic shock. Mediat. Inflamm. 1993, 2, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, M.; Higgins, R.; Boudreau, M. Use of polyvalent coagglutination reagents for serotyping of Streptococcus suis. J. Clin. Microbiol. 1993, 31, 2192–2194. [Google Scholar] [PubMed]
- Lecours, M.P.; Gottschalk, M.; Houde, M.; Lemire, P.; Fittipaldi, N.; Segura, M. Critical role for Streptococcus suis cell wall modifications and suilysin in resistance to complement-dependent killing by dendritic cells. J. Infect. Dis. 2011, 204, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Slater, J.D.; Allen, A.G.; May, J.P.; Bolitho, S.; Lindsay, H.; Maskell, D.J. Mutagenesis of Streptococcus equi and Streptococcus suis by transposon Tn917. Vet. Microbiol. 2003, 93, 197–206. [Google Scholar] [CrossRef]
- Lopreto, C.; Lopardo, H.A.; Bardi, M.C.; Gottschalk, M. Primary Streptococcus suis meningitis: First case in humans described in Latin America. Enferm. Infecc. Microbiol. Clin. 2005, 23, 110. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, M.; Higgins, R.; Jacques, M.; Dubreuil, D. Production and characterization of two Streptococcus suis capsular type 2 mutants. Vet. Microbiol. 1992, 30, 59–71. [Google Scholar] [CrossRef]
- Trottier, S.; Higgins, R.; Brochu, G.; Gottschalk, M. A case of human endocarditis due to Streptococcus suis in North America. Rev. Infect. Dis. 1991, 13, 1251–1252. [Google Scholar] [CrossRef] [PubMed]
- S. suis Multilocus Sequence Typing Database. Available online: http://ssuis.mlst.net (accessed on 11 July 2016).
- Li, W.; Ye, C.; Jing, H.; Cui, Z.; Bai, X.; Jin, D.; Zheng, H.; Zhao, A.; Xu, Y.; Gottschalk, M.; et al. Streptococcus suis outbreak investigation using multiple-locus variable tandem repeat number analysis. Microbiol. Immunol. 2010, 54, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, D.; Nishino, H.; Ishiji, T.; Ishii, J.; Osaki, M.; Fittipaldi, N.; Gottschalk, M.; Tharavichitkul, P.; Takai, S.; Sekizaki, T. Genetic organization and preferential distribution of putative pilus gene clusters in Streptococcus suis. Vet. Microbiol. 2009, 138, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Segura, M.A.; Cleroux, P.; Gottschalk, M. Streptococcus suis and group B Streptococcus differ in their interactions with murine macrophages. FEMS Immunol. Med. Microbiol. 1998, 21, 189–195. [Google Scholar] [CrossRef] [PubMed]

| Strain | ST | Origin | Phagocytosis (CFU/mL ± SEM) 1 | Bacterial Killing (% ± SEM) 2 | Mouse Mortality 72 h p.i. (%) 3 |
|---|---|---|---|---|---|
| P1/7 | 1 | EA | 2 ± 0.7 ×104 | 0 ± 0 | 67 |
| ΔcpsF | - | - | 2 ± 0.5 × 105 a | 64 ± 5 b | - |
| MGGUS2 | 1 | NA | 2 ± 0.9 × 104 | 0 ± 0 | 80 |
| Bassett | 1 | NA | 2 ± 1 × 104 | 0 ± 0 | 7 |
| SR800 | 1 | SA | 3 ± 0.9 × 104 | 0 ± 0 | 0 |
| MNCM06 | 1 | EA | 2 ± 0.9 × 104 | 0 ± 0 | 20 |
| 89-1591 | 25 | NA | 8 ± 5 × 104 | 20 ± 5 | 47 |
| 91-1804 | 25 | NA | 1 ± 0.4 × 104 | 34 ± 5 | 0 |
| LPH4 | 25 | EA | 6 ± 2 × 104 | 2 ± 2 | 7 |
| 1054471 | 28 | NA | 3 ± 0.5 × 103 | 33 ± 6 | 0 |
| 1084708 | 28 | NA | 8 ± 2 × 103 | 27 ± 3 | 0 |
| 1088563 | 28 | NA | 6 ± 0.3 × 103 | 30 ± 3 | 0 |
| 1097205 | 28 | NA | 8 ± 1 × 103 | 27 ± 2 | 10 |
| DAT254 | 28 | EA | 6 ± 0.9 × 103 | 3 ± 2 | 73 |
| MNCM43 | 28 | EA | 5 ± 3 × 103 | 21 ± 3 | 33 |
| Strain | ST | Origin | Meningitis (%) 1 | Brain Burden (CFU/g) 2 |
|---|---|---|---|---|
| P1/7 | 1 | EA | 31 | 6 × 107 |
| MGGUS2 | 1 | NA | 20 | 2 × 107 |
| Bassett | 1 | NA | 14 | ND |
| SR800 | 1 | SA | 27 | ND |
| MNCM06 | 1 | EA | 8 | ND |
| 89-1591 | 25 | NA | 20 | 2 × 105 |
| 91-1804 | 25 | NA | 27 | ND |
| LPH4 | 25 | EA | 36 | 2 × 106 |
| 1054471 | 28 | NA | 0 | 0 |
| 1084708 | 28 | NA | 20 | 2 × 105 |
| 1088563 | 28 | NA | 0 | 0 |
| 1097205 | 28 | NA | 11 | 9 × 105 |
| DAT254 | 28 | EA | 33 | 2 × 106 |
| MNCM43 | 28 | EA | 30 | 3 × 106 |
| Strain | Sequence Type (ST) | Country | Clinical Feature | Reference |
|---|---|---|---|---|
| P1/7 | 1 | United Kingdom | Pig, meningitis | [58] |
| MGGUS2 | 1 | United States | Pig, meningitis | [19] |
| Bassett | 1 | United States | Human, meningitis | [20] |
| SR800 | 1 | Argentina | Human, meningitis | [59] |
| MNCM06 | 1 | Thailand | Human, meningitis | [21] |
| 89-1591 | 25 | Canada | Pig, sepsis/meningitis | [60] |
| 91-1804 | 25 | Canada | Human, endocarditis | [61] |
| LPH4 | 25 | Thailand | Human, sepsis | [21] |
| 1054471 | 28 | Canada | Pig, meningitis | [19] |
| 1084708 | 28 | Canada | Pig, sepsis | [19] |
| 1088563 | 28 | Canada | Pig, meningitis | [19] |
| 1097205 | 28 | Canada | Pig, meningitis | [19] |
| DAT254 | 28 | Japan | Pig, meningitis | [6] |
| MNCM43 | 28 | Thailand | Human, endocarditis | [21] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auger, J.-P.; Fittipaldi, N.; Benoit-Biancamano, M.-O.; Segura, M.; Gottschalk, M. Virulence Studies of Different Sequence Types and Geographical Origins of Streptococcus suis Serotype 2 in a Mouse Model of Infection. Pathogens 2016, 5, 48. https://doi.org/10.3390/pathogens5030048
Auger J-P, Fittipaldi N, Benoit-Biancamano M-O, Segura M, Gottschalk M. Virulence Studies of Different Sequence Types and Geographical Origins of Streptococcus suis Serotype 2 in a Mouse Model of Infection. Pathogens. 2016; 5(3):48. https://doi.org/10.3390/pathogens5030048
Chicago/Turabian StyleAuger, Jean-Philippe, Nahuel Fittipaldi, Marie-Odile Benoit-Biancamano, Mariela Segura, and Marcelo Gottschalk. 2016. "Virulence Studies of Different Sequence Types and Geographical Origins of Streptococcus suis Serotype 2 in a Mouse Model of Infection" Pathogens 5, no. 3: 48. https://doi.org/10.3390/pathogens5030048







