Henipavirus Infections: Lessons from Animal Models
Abstract
:1. Introduction
2. Fruit Bats
| Species | Clinical signs | Gross lesions | Histology | Virus found in | Utilisation of the model | References | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Respiratory | Neurological | Patho-logy | Immu-nology | Drugs assess-ment | Vaccine assess-ment | Epide-miology | ||||||
| Fruit bat | - | - | petechial hemorrhages on urine bladder wall | granulomatous hepatitis, inflammation of bladder epithelium, vasculitis, testicular degeneration | kidney, urine, uterus | - | + | - | - | +++ | [17] | |
| - | - | - | necrosis and hemorrhage of the adrenal gland | urine, rectal swab | [18,19] | |||||||
| Pig | severe non-productive cough | shivering, seizures | lymph nodes hyperplasia, congestion, edema | hyperplasia of BALT, meningitis, encephalitis | lymphoid organs, CNS, lung | + | + | ++ (vet. use) | ++ (vet. use) | +++ | [20,21,22,23,24] | |
| cough, respiratory distress | incoordination (transient) | congestion, hemorrhages | syncytial cells in nasal turbinates and bronchiolar epithelium | tonsils, BALF, lung, nasal turbinates, lymph nodes, swabs | [25] | |||||||
| Horse | - | - | - | meningitis | - | + | - | ++ (vet. use) | ++ (vet. use) | +++ | [26] | |
| increased respiratory rate, frothy nasal discharge | ataxia, head pressing, myoclonic twitches | congestion, edema, hemorrhages in lung | interstitial pneumonia, vasculitis, syncytia in endothelium | brain, lung, lymphoid organs, kidney, bronchial and oral swabs, urine | [5,18,27,28] | |||||||
| Cat | tachypnea, dyspnea | - | congestion ,edema, hemorrhages, enlarged lymph nodes | acute bronchiolitis, necrotizing alveolitis, vasculitis, endothelial syncytia | lung, spleen, kidney, lymph nodes | +/- | - | + | ++ | - | [21,29,30,31] | |
| dyspnea, open mouth breathing | - | hydrothorax, congestion, edema, hemorrhages | inflammation in lung, alveolar wall necrosis, vasculitis, endothelial syncytia | lung, spleen, lymph nodes | [18,32,33,34] | |||||||
| Ferret | dyspnea, cough, serous nasal discharge | depression, tremors, myoclonus, hind limb paresis | edema, hemorrhages, enlarged lymph nodes | necrotizing alveolitis, glomerular necrosis, vasculitis, endothelial syncytia, meningitis | brain, lung, lymphoid organs, adrenal, kidney, testes, uterus, liver, pharyngeal and rectal swabs | + | - | ++ | ++ | - | [35,36,37,38] | |
| - | depression, tremors | lung, lymphoid organs, adrenal, meninges, kidney, liver, testes, oral and rectal swabs, blood, urine | [39] | |||||||||
| Squirrel Monkey | dyspnea, tachypnea | ataxia, coma, seizures | not reported | pulmonar inflammation, mild vasculitis | lung, brain, kidney, spleen | + | + | +/- | +/- | - | [40] | |
| African Green Monkey | severe dyspnea, open-mouth breathing, serosanguineous nasal discharge | muscle twitches, behavioral changes, loss of balance | pleural effusion, congestion and hemorrhage in lungs, hemorrhages on mucosal surface of urinary bladder, edema and hemorrhages of the meninges | endothelial syncytial cells, vasculitis, meningitis, inflammation, fibrinoid necrosis | liver, spleen, kidney, adrenal gland, lung, lymph nodes, pancreas, sex organs, urine, nasal swabs | +++ | + | +++ | +++ | - | [41,42] | |
| nasal discharge, labored breathing | muscle twitches, seizures | pulmonary consolidation, congestion of lungs, enlarged lymph nodes, congested liver, inflammation of gastrointestinal tract, congestion in the brain | alveolar hemorrhages, pulmonary edema and inflammation, alveolitis, fibrinoid necrosis, vasculitis, meningitis | lung, lymph nodes, heart, liver, spleen, kidney, adrenal gland, brain, urinary bladder, sex organs | [43,44] | |||||||
| IFNAR KO mouse | - | behavioral troubles, ataxia, pain, paralysis | brain and lung congestion, edema of bladder wall, hemorrhages, necrosis in liver and kidney | vasculitis, inflammation, meningitis, encephalitis, necrotizing alveolitis | brain, lung, spleen, liver | + | +++ | +++ | + | - | [45] | |
| - | brain and lung congestion, hemorrhages | vasculitis, meningitis, encephalitis, gliosis, necrotizing alveolitis | brain, lung, spleen, liver | [45] | ||||||||
| Aged mouse | - | - | - | - | - | +/- | + | + | - | - | - | |
| - | ataxia, muscle tremors | - | encephalitis, meningitis | brain, transiently in lung | [46] | |||||||
| Hamster | labored breathing, serosanguineous nasal exudates | imbalance, muscle twitching, tremor, limb paralysis | edema, hemorrhages, congestion | vasculitis, meningitis, encephalitis, endothelial syncytia | lung, nasal epithelium, CNS, heart, liver, spleen, kidney, bladder, urine | +++ | +/- | ++ | ++ | + | [47,48,49,50,51,52,53,54,55565758] | |
| Hamster | acute respiratory distress, serosanguineous nasal discharge | imbalance, ataxia, muscle twitching, limb paralysis | inflammation and edema of the lungs | inflammation, alveolitis, necrotizing vasculitis, meningitis, encephalitis, hemorhage in the brain, neuronal necrosis, gliosis, endothelial syncytial cells | spleen, kidney, heart, lung, brain, liver | [48,56,58,59] | ||||||
| Guinea pig | - | mild behavioral changes, ataxia | mesenteric edema | vasculitis, lymphoid depletion, endothelial syncytia with fibrinoid necrosis, edema, hemorrhage and ulceration of the urinary bladder, meningitis | heart, kidney, lymph nodes, uterus | -/+ | - | - | - | - | [17] | |
| - | head tilt | congestion, cyanosis, edema | pulmonar fibrinoid necrosis, vasculitis, endothelial syncytia, encephalitis | brain, kidney, urine, uterus, placenta | [34,60,61] | |||||||
3. Farm and Domestic Animals
3.1. Pigs
3.2. Horses
3.3. Cats
3.4. Ferrets
4. Non-Human Primate Models

5. Rodent Models
5.1. Golden Syrian Hamster
5.2. Guinea Pig
5.3. Mice
5.3.1. Aged Mice
5.3.2. Transgenic Mice
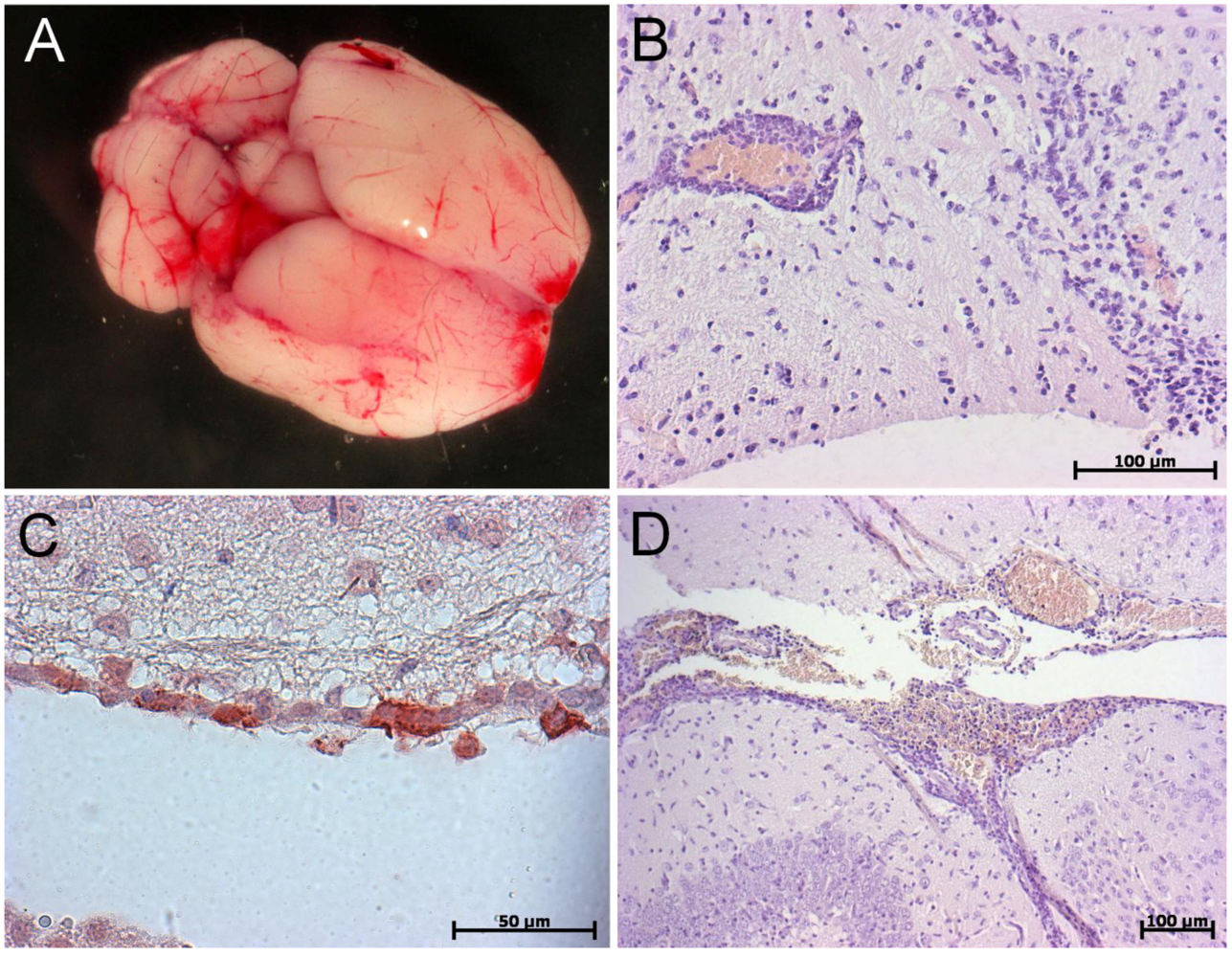
6. Conclusions and Perspectives
Acknowledgments
Conflict of Interest
References
- Eaton, B.T.; Broder, C.C.; Middleton, D.; Wang, L.-F. Hendra and Nipah Viruses: different and dangerous. Nat. Rev. Micro. 2006, 4, 23–35. [Google Scholar] [CrossRef]
- Marsh, G.A.; Wang, L.-F. Hendra and Nipah viruses: Why are they so deadly? Curr. Opin. Virol. 2012, 2, 242–247. [Google Scholar] [CrossRef]
- Field, H.; Kung, N. Henipaviruses — unanswered questions of lethal zoonoses. Curr. Opin. Virol. 2011, 1, 658–661. [Google Scholar] [CrossRef]
- Marsh, G.A.; De Jong, C.; Barr, J.A.; Tachedjian, M.; Smith, C.; Middleton, D.; Yu, M.; Todd, S.; Foord, A.J.; Haring, V.; et al. Cedar virus: A novel henipavirus isolated from australian bats. PLoS Pathogens 2012, 8, e1002836. [Google Scholar] [CrossRef]
- Murray, K.; Rogers, R.; Selvey, L.; Selleck, P.; Hyatt, A.; Gould, A.; Gleeson, L.; Hooper, P.; Westbury, H. A novel morbillivirus pneumonia of horses and its transmission to humans. Emerg. Infect. Dis. 1995, 1, 31–33. [Google Scholar] [CrossRef]
- Chua, K.B.; Bellini, W.J.; Rota, P.A.; Harcourt, B.H.; Tamin, A.; Lam, S.K.; Ksiazek, T.G.; Rollin, P.E.; Zaki, S.R.; Shieh, W.-J.; et al. Nipah virus: A recently emergent deadly paramyxovirus. Science 2000, 288, 1432–1435. [Google Scholar] [CrossRef]
- Wong, K.T.; Shieh, W.-J.; Kumar, S.; Norain, K.; Abdullah, W.; Guarner, J.; Goldsmith, C.S.; Chua, K.B.; Lam, S.K.; Tan, C.T.; et al. Nipah virus infection. Am. J. Pathol. 2002, 161, 2153–2167. [Google Scholar] [CrossRef]
- Chadha, M.S.; Comer, J.A.; Lowe, L.; Rota, P.A.; Rollin, P.E.; Bellini, W.J.; Ksiazek, T.G.; Mishra, A. Nipah virus-associated encephalitis outbreak, Siliguri, India. Emerg. Infect. Dis. 2006, 12, 235–240. [Google Scholar] [CrossRef]
- Hsu, V.P.; Hossain, M.J.; Parashar, U.D.; Ali, M.M.; Ksiazek, T.G.; Kuzmin, I.; Niezgoda, M.; Rupprecht, C.; Bresee, J.; Breiman, R.F. Nipah virus encephalitis reemergence, Bangladesh. Emerg. Infect. Dis. 2004, 10, 2082–2087. [Google Scholar] [CrossRef]
- Stone, R. Breaking the Chain in Bangladesh. Science 2011, 331, 1128–1131. [Google Scholar] [CrossRef]
- Harcourt, B.H.; Lowe, L.; Tamin, A.; Liu, X.; Bankamp, B.; Bowden, N.; Rollin, P.E.; Comer, J.A.; Ksiazek, T.G.; Hossain, M.J.; et al. Genetic characterization of Nipah virus, Bangladesh, 2004. Emerg. Infect. Dis. 2005, 11, 1594–1597. [Google Scholar] [CrossRef]
- Lo, M.K.; Lowe, L.; Hummel, K.B.; Sazzad, H.M.S.; Gurley, E.S.; Hossain, M.J.; Luby, S.P.; Miller, D.M.; Comer, J.A.; Rollin, P.E.; et al. Characterization of Nipah Virus from Outbreaks in Bangladesh, 2008–2010. Emerg. Infect. Dis. 2012, 18, 248–255. [Google Scholar] [CrossRef]
- Tan, C.T.; Goh, K.J.; Wong, K.T.; Sarji, S.A.; Chua, K.B.; Chew, N.K.; Murugasu, P.; Loh, Y.L.; Chong, H.T.; Tan, K.S.; et al. Relapsed and late-onset Nipah encephalitis. Ann. Neurol. 2002, 51, 703–708. [Google Scholar] [CrossRef]
- Lo, M.K.; Rota, P.A. The emergence of Nipah virus, a highly pathogenic paramyxovirus. J. Clin. Virol. 2008, 43, 396–400. [Google Scholar]
- Luby, S.P.; Gurley, E.S.; Hossain, M.J. Transmission of human infection with Nipah virus. Clin. Infect. Dis. 2009, 49, 1743–1748. [Google Scholar] [CrossRef]
- Drexler, J.F.; Corman, V.M.; Müller, M.A.; Maganga, G.D.; Vallo, P.; Binger, T.; Gloza-Rausch, F.; Rasche, A.; Yordanov, S.; Seebens, A.; et al. Bats host major mammalian paramyxoviruses. Nat. Commun. 2012, 3, 796. [Google Scholar] [CrossRef]
- Middleton, D.J.; Morrissy, C.J.; Van der Heide, B.M.; Russell, G.M.; Braun, M.A.; Westbury, H.A.; Halpin, K.; Daniels, P.W. Experimental Nipah virus infection in pteropid bats (Pteropus poliocephalus). J. Comp. Pathol. 2007, 136, 266–272. [Google Scholar] [CrossRef]
- Williamson, M.; Hooper, P.; Selleck, P.; Gleeson, L.; Daniels, P.; Westbury, H.; Murray, P. Transmission studies of Hendra virus (equine morbilli-virus) in fruit bats, horses and cats. Aust. Vet. J. 1998, 76, 813–818. [Google Scholar] [CrossRef]
- Halpin, K.; Hyatt, A.D.; Fogarty, R.; Middleton, D.; Bingham, J.; Epstein, J.H.; Rahman, S.A.; Hughes, T.; Smith, C.; Field, H.E.; Daszak, P. Pteropid bats are confirmed as the reservoir hosts of henipaviruses: a comprehensive experimental study of virus transmission. Am. J. Trop. Med. Hyg. 2011, 85, 946–951. [Google Scholar] [CrossRef]
- Mohd Nor, M.N.; Gan, C.H.; Ong, B.L. Nipah virus infection of pigs in peninsular Malaysia. Rev. Off. Int. Epizoot. 2000, 19, 160–165. [Google Scholar]
- Middleton, D.J.; Westbury, H.A.; Morrissy, C.J.; Van der Heide, B.M.; Russell, G.M.; Braun, M.A.; Hyatt, A.D. Experimental Nipah virus infection in pigs and cats. J. Comp. Pathol. 2002, 126, 124–136. [Google Scholar] [CrossRef]
- 22 Weingartl, H.M.; Berhane, Y.; Caswell, J.L.; Loosmore, S.; Audonnet, J.-C.; Roth, J.A.; Czub, M. Recombinant Nipah virus vaccines protect pigs against challenge. J. Virol. 2006, 80, 7929–7938. [Google Scholar]
- Weingartl, H.; Czub, S.; Copps, J.; Berhane, Y.; Middleton, D.; Marszal, P.; Gren, J.; Smith, G.; Ganske, S.; Manning, L.; Czub, M. Invasion of the Central Nervous System in a Porcine Host by Nipah Virus. J. Virol. 2005, 79, 7528–7534. [Google Scholar] [CrossRef]
- Berhane, Y.; Weingartl, H.M.; Lopez, J.; Neufeld, J.; Czub, S.; Embury-Hyatt, C.; Goolia, M.; Copps, J.; Czub, M. Bacterial infections in pigs experimentally infected with Nipah virus. Transbound. Emerg. Dis. 2008, 55, 165–174. [Google Scholar] [CrossRef]
- Li, M.; Embury-Hyatt, C.; Weingartl, H.M. Experimental inoculation study indicates swine as a potential host for Hendra virus. Vet. Res. 2010, 41, 33. [Google Scholar] [CrossRef]
- Hooper, P.; Zaki, S.; Daniels, P.; Middleton, D. Comparative pathology of the diseases caused by Hendra and Nipah viruses. Microbes Infect. 2001, 3, 315–322. [Google Scholar] [CrossRef]
- Marsh, G.; Haining, J.; Hancock, T.; Robinson, R.; Foord, A.; Barr, J.; Riddell, S.; Heine, H.; White, J.; Crameri, G.; et al. Experimental Infection of Horses with Hendra Virus/Australia/Horse/2008/Redlands. Emerg. Infect. Dis. 2011, 17. [Google Scholar]
- Rogers, R.J.; Douglas, I.C.; Baldock, F.C.; Glanville, R.J.; Seppanen, K.T.; Gleeson, L.J.; Selleck, P.N.; Dunn, K.J. Investigation of a second focus of equine morbillivirus infection in coastal Queensland. Aust. Vet. J. 1996, 74, 243–244. [Google Scholar] [CrossRef]
- Mungall, B.A.; Middleton, D.; Crameri, G.; Bingham, J.; Halpin, K.; Russell, G.; Green, D.; McEachern, J.; Pritchard, L.I.; Eaton, B.T.; et al. Feline model of acute nipah virus infection and protection with a soluble glycoprotein-based subunit vaccine. J. Virol. 2006, 80, 12293–12302. [Google Scholar]
- Mungall, B.A.; Middleton, D.; Crameri, G.; Halpin, K.; Bingham, J.; Eaton, B.T.; Broder, C.C. Vertical transmission and fetal replication of nipah virus in an experimentally infected cat. J. Infect. Dis. 2007, 196, 812–816. [Google Scholar] [CrossRef]
- McEachern, J.A.; Bingham, J.; Crameri, G.; Green, D.J.; Hancock, T.J.; Middleton, D.; Feng, Y.-R.; Broder, C.C.; Wang, L.-F.; et al. A recombinant subunit vaccine formulation protects against lethal Nipah virus challenge in cats. Vaccine 2008, 26, 3842–3852. [Google Scholar] [CrossRef]
- Westbury, H.A.; Hooper, P.T.; Selleck, P.W.; Murray, P.K. Equine morbillivirus pneumonia: susceptibility of laboratory animals to the virus. Aust. Vet. J. 1995, 72, 278–279. [Google Scholar]
- Westbury, H.A.; Hooper, P.T.; Brouwer, S.L.; Selleck, P.W. Susceptibility of cats to equine morbillivirus. Aust. Vet. J. 1996, 74, 132–134. [Google Scholar] [CrossRef]
- Hooper, P.T.; Westbury, H.A.; Russell, G.M. The lesions of experimental equine morbillivirus disease in cats and guinea pigs. Vet. Pathol. 1997, 34, 323–329. [Google Scholar] [CrossRef]
- Clayton, B.A.; Middleton, D.; Bergfeld, J.; Haining, J.; Arkinstall, R.; Wang, L.; Marsh, G.A. Transmission routes for nipah virus from Malaysia and Bangladesh. Emerg. Infect. Dis. 2012, 18, 1983–1993. [Google Scholar] [CrossRef]
- Bossart, K.N.; Bingham, J.; Middleton, D. Targeted Strategies for Henipavirus Therapeutics. Open Virol. J. 2007, 1, 14–25. [Google Scholar]
- Pallister, J.; Middleton, D.; Crameri, G.; Yamada, M.; Klein, R.; Hancock, T.J.; Foord, A.; Shiell, B.; Michalski, W.; Broder, C.C.; et al. Chloroquine administration does not prevent nipah virus infection and disease in ferrets. J. Virol. 2009, 83, 11979–11982. [Google Scholar] [CrossRef]
- Bossart, K.N.; Zhu, Z.; Middleton, D.; Klippel, J.; Crameri, G.; Bingham, J.; McEachern, J.A.; Green, D.; Hancock, T.J.; Chan, Y.-P.; et al. A neutralizing human monoclonal antibody protects against lethal disease in a new ferret model of acute nipah virus infection. PLoS Pathog. 2009, 5, e1000642. [Google Scholar] [CrossRef]
- Pallister, J.; Middleton, D.; Wang, L.-F.; Klein, R.; Haining, J.; Robinson, R.; Yamada, M.; White, J.; Payne, J.; Feng, Y.-R.; et al. A recombinant Hendra virus G glycoprotein-based subunit vaccine protects ferrets from lethal Hendra virus challenge. Vaccine 2011, 29, 5623–5630. [Google Scholar] [CrossRef]
- Marianneau, P. Experimental infection of squirrel monkeys with Nipah virus. Emerg. Infect. Dis. 2010, 16. [Google Scholar]
- Geisbert, T.W.; Daddario-DiCaprio, K.M.; Hickey, A.C.; Smith, M.A.; Chan, Y.-P.; Wang, L.-F.; Mattapallil, J.J.; Geisbert, J.B.; Bossart, K.N.; Broder, C.C. Development of an Acute and Highly Pathogenic Nonhuman Primate Model of Nipah Virus Infection. PLoS One 2010, 5, e10690. [Google Scholar] [CrossRef]
- Bossart, K.N.; Rockx, B.; Feldmann, F.; Brining, D.; Scott, D.; Lacasse, R.; Geisbert, J.B.; Feng, Y.-R.; Chan, Y.-P.; Hickey, A.C.; et al. A hendra virus g glycoprotein subunit vaccine protects african green monkeys from nipah virus challenge. Sci. Transl. Med. 2012, 4, 46ra107. [Google Scholar] [CrossRef]
- Rockx, B.; Bossart, K.N.; Feldmann, F.; Geisbert, J.B.; Hickey, A.C.; Brining, D.; Callison, J.; Safronetz, D.; Marzi, A.; Kercher, L.; et al. A novel model of lethal hendra virus infection in african green monkeys and the effectiveness of ribavirin treatment. J. Virol. 2010, 84, 9831–9839. [Google Scholar] [CrossRef]
- Bossart, K.N.; Geisbert, T.W.; Feldmann, H.; Zhu, Z.; Feldmann, F.; Geisbert, J.B.; Yan, L.; Feng, Y.-R.; Brining, D.; et al. A neutralizing human monoclonal antibody protects african green monkeys from hendra virus challenge. Sci. Transl. Med. 2011, 3, 105ra103. [Google Scholar] [CrossRef]
- Dhondt, K.P.; Mathieu, C.; Chalons, M.; Reynaud, J.M.; Vallve, A.; Raoul, H.; Horvat, B. Type I interferon signaling protects mice from lethal Henipavirus infection. J. Infect. Dis. 2012, 207, 142–151. [Google Scholar]
- Dups, J.; Middleton, D.; Yamada, M.; Monaghan, P.; Long, F.; Robinson, R.; Marsh, G.A.; Wang, L.-F. A new model for hendra virus encephalitis in the mouse. PLoS One 2012, 7, e40308. [Google Scholar]
- Wong, K.T.; Grosjean, I.; Brisson, C.; Blanquier, B.; Fevre-Montange, M.; Bernard, A.; Loth, P.; Georges-Courbot, M.-C.; Chevallier, M.; Akaoka, H.; et al. A golden hamster model for human acute Nipah virus infection. Am. J. Pathol. 2003, 163, 2127–2137. [Google Scholar] [CrossRef]
- Rockx, B.; Brining, D.; Kramer, J.; Callison, J.; Ebihara, H.; Mansfield, K.; Feldmann, H. Clinical outcome of henipavirus infection in hamsters is determined by the route and dose of infection. J. Virol. 2011, 85, 7658–7671. [Google Scholar] [CrossRef]
- De Wit, E.; Bushmaker, T.; Scott, D.; Feldmann, H.; Munster, V.J. Nipah virus transmission in a hamster model. PLoS Negl. Trop. Dis. 2011, 5, e1432. [Google Scholar] [CrossRef]
- Debuysscher, B.L.; De Wit, E.; Munster, V.J.; Scott, D.; Feldmann, H.; Prescott, J. Comparison of the pathogenicity of nipah virus isolates from bangladesh and malaysia in the Syrian hamster. PLoS Negl. Trop. Dis. 2013, 7, e2024. [Google Scholar] [CrossRef]
- Yoneda, M.; Guillaume, V.; Sato, H.; Fujita, K.; Georges-Courbot, M.-C.; Ikeda, F.; Omi, M.; Muto-Terao, Y.; Wild, T.F.; Kai, C. The Nonstructural proteins of nipah virus play a key role in pathogenicity in experimentally infected animals. PLoS One 2010, 5, e12709. [Google Scholar] [CrossRef]
- Mathieu, C.; Guillaume, V.; Volchkova, V.A.; Pohl, C.; Jacquot, F.; Looi, R.Y.; Wong, K.T.; Legras-Lachuer, C.; Volchkov, V.E.; Lachuer, J.; et al. Nonstructural Nipah virus C protein regulates both the early host proinflammatory response and viral virulence. J. Virol. 2012. In Press.. [Google Scholar]
- Mathieu, C.; Guillaume, V.; Sabine, A.; Ong, K.C.; Wong, K.T.; Legras-Lachuer, C.; Horvat, B. Lethal Nipah virus infection induces rapid overexpression of CXCL10. PLoS One 2012, 7, e32157. [Google Scholar]
- Guillaume, V.; Contamin, H.; Loth, P.; Georges-Courbot, M.-C.; Lefeuvre, A.; Marianneau, P.; Chua, K.B.; Lam, S.K.; Buckland, R.; Deubel, V.; et al. Nipah virus: Vaccination and passive protection studies in a hamster model. J. Virol. 2004, 78, 834–840. [Google Scholar] [CrossRef]
- Guillaume, V.; Contamin, H.; Loth, P.; Grosjean, I.; Georges-Courbot, M.C.; Deubel, V.; Buckland, R.; Wild, T.F. Antibody prophylaxis and therapy against Nipah virus infection in hamsters. J. Virol. 2006, 80, 1972–1978. [Google Scholar] [CrossRef]
- Freiberg, A.N.; Worthy, M.N.; Lee, B.; Holbrook, M.R. Combined chloroquine and ribavirin treatment does not prevent death in a hamster model of Nipah and Hendra virus infection. J. Gen. Virol. 2010, 91, 765–772. [Google Scholar] [CrossRef]
- Georges-Courbot, M.C.; Contamin, H.; Faure, C.; Loth, P.; Baize, S.; Leyssen, P.; Neyts, J.; Deubel, V. Poly(I)-poly(C12U) but not ribavirin prevents death in a hamster model of Nipah virus infection. Antimicrob. Agents Chemother. 2006, 50, 1768–1772. [Google Scholar] [CrossRef]
- Ploquin, A.; Szécsi, J.; Mathieu, C.; Guillaume, V.; Barateau, V.; Ong, K.C.; Wong, K.T.; Cosset, F.-L.; Horvat, B.; Salvetti, A. Protection against henipavirus infection by use of recombinant adeno-associated virus-vector vaccines. J. Infect. Dis. 2013, 207, 469–478. [Google Scholar] [CrossRef]
- Guillaume, V.; Wong, K.T.; Looi, R.Y.; Georges-Courbot, M.-C.; Barrot, L.; Buckland, R.; Wild, T.F.; Horvat, B. Acute Hendra virus infection: Analysis of the pathogenesis and passive antibody protection in the hamster model. Virology 2009, 387, 459–465. [Google Scholar] [CrossRef]
- Williamson, M.M.; Hooper, P.T.; Selleck, P.W.; Westbury, H.A.; Slocombe, R.F.S. A guinea-pig model of hendra virus encephalitis. J. Comp. Pathol. 2001, 124, 273–279. [Google Scholar] [CrossRef]
- Williamson, M.M.; Hooper, P.T.; Selleck, P.W.; Westbury, H.A.; Slocombe, R.F. Experimental hendra virus infectionin pregnant guinea-pigs and fruit Bats (Pteropus poliocephalus). J. Comp. Pathol. 2000, 122, 201–207. [Google Scholar] [CrossRef]
- Calisher, C.H.; Childs, J.E.; Field, H.E.; Holmes, K.V.; Schountz, T. Bats: Important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 2006, 19, 531–545. [Google Scholar] [CrossRef]
- Wong, S.; Lau, S.; Woo, P.; Yuen, K.-Y. Bats as a continuing source of emerging infections in humans. Rev. Med. Virol. 2007, 17, 67–91. [Google Scholar] [CrossRef]
- Young, P.L.; Halpin, K.; Selleck, P.W.; Field, H.; Gravel, J.L.; Kelly, M.A.; Mackenzie, J.S. Serologic evidence for the presence in Pteropus bats of a paramyxovirus related to equine morbillivirus. Emerg. Infect. Dis. 1996, 2, 239–240. [Google Scholar] [CrossRef]
- Halpin, K.; Young, P.L.; Field, H.E.; Mackenzie, J.S. Isolation of Hendra virus from pteropid bats: a natural reservoir of Hendra virus. J. Comp. Pathol. 2000, 81, 1927–1932. [Google Scholar]
- Yob, J.M.; Field, H.; Rashdi, A.M.; Morrissy, C.; Van der Heide, B.; Rota, P.; Bin Adzhar, A.; White, J.; Daniels, P.; Jamaluddin, A.; et al. Nipah virus infection in bats (order Chiroptera) in peninsular Malaysia. Emerg. Infect. Dis. 2001, 7, 439–441. [Google Scholar]
- Yadav, P.D.; Raut, C.G.; Shete, A.M.; Mishra, A.C.; Towner, J.S.; Nichol, S.T.; Mourya, D.T. Detection of Nipah virus RNA in fruit bat (Pteropus giganteus) from India. Am. J. Trop. Med. Hyg. 2012, 87, 576–578. [Google Scholar] [CrossRef]
- Olson, J.G.; Rupprecht, C.; Rollin, P.E.; An, U.S.; Niezgoda, M.; Clemins, T.; Walston, J.; Ksiazek, T.G. Antibodies to Nipah-like virus in bats (Pteropus lylei), Cambodia. Emerg. Infect. Dis. 2002, 8, 987–988. [Google Scholar] [CrossRef]
- Reynes, J.-M.; Counor, D.; Ong, S.; Faure, C.; Seng, V.; Molia, S.; Walston, J.; Georges-Courbot, M.C.; Deubel, V.; Sarthou, J.-L. Nipah virus in Lyle’s flying foxes, Cambodia. Emerg. Infect. Dis. 2005, 11, 1042–1047. [Google Scholar] [CrossRef]
- Sendow, I.; Field, H.E.; Curran, J.; Darminto; Morrissy, C.; Meehan, G.; Buick, T.; Daniels, P. Henipavirus in Pteropus vampyrus bats, Indonesia. Emerg. Infect. Dis. 2006, 12, 711–712. [Google Scholar] [CrossRef]
- Wacharapluesadee, S.; Boongird, K.; Wanghongsa, S.; Ratanasetyuth, N.; Supavonwong, P.; Saengsen, D.; Gongal, G.N.; Hemachudha, T. A longitudinal study of the prevalence of Nipah virus in Pteropus lylei bats in Thailand: evidence for seasonal preference in disease transmission. Vector Borne Zoonotic Dis. 2010, 10, 183–190. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Hickey, A.C.; Zhang, Y.; Li, Y.; Wu, Y.; Zhang, H.; Yuan, J.; Han, Z.; McEachern, J.; et al. Antibodies to Nipah or Nipah-like viruses in bats, China. Emerg. Infect. Dis. 2008, 14, 1974–1976. [Google Scholar] [CrossRef]
- Clayton, B.A.; Wang, L.F.; Marsh, G.A. Henipaviruses: an updated review focusing on the pteropid reservoir and features of transmission. Zoonoses Public Health 2013, 60, 69–83. [Google Scholar] [CrossRef]
- Chua, K.B.; Koh, C.L.; Hooi, P.S.; Wee, K.F.; Khong, J.H.; Chua, B.H.; Chan, Y.P.; Lim, M.E.; Lam, S.K. Isolation of Nipah virus from Malaysian Island flying-foxes. Microbes Infect. 2002, 4, 145–151. [Google Scholar] [CrossRef]
- Sohayati, A.R.; Hassan, L.; Sharifah, S.H.; Lazarus, K.; Zaini, C.M.; Epstein, J.H.; Shamsyul Naim, N.; Field, H.E.; Arshad, S.S.; Abdul Aziz, J.; et al. Evidence for Nipah virus recrudescence and serological patterns of captive Pteropus vampyrus. Epidemiol. Infect. 2011, 1–10. [Google Scholar]
- Bossart, K. Functional studies of host-specific ephrin-B ligands as Henipavirus receptors. Virology 2008, 372, 357–371. [Google Scholar] [CrossRef]
- Negrete, O.A.; Chu, D.; Aguilar, H.C.; Lee, B. Single amino acid changes in the nipah and hendra virus attachment glycoproteins distinguish EphrinB2 from EphrinB3 usage. J. Virol. 2007, 81, 10804–10814. [Google Scholar] [CrossRef]
- Field, H.; Young, P.; Yob, J.M.; Mills, J.; Hall, L.; Mackenzie, J. The natural history of Hendra and Nipah viruses. Microbes Infect. 2001, 3, 307–314. [Google Scholar] [CrossRef]
- Chua, K.B. Nipah virus outbreak in Malaysia. J. Clin. Virol. 2003, 26, 265–275. [Google Scholar] [CrossRef]
- Epstein, J.H.; Abdul Rahman, S.; Zambriski, J.A.; Halpin, K.; Meehan, G.; Jamaluddin, A.A.; Hassan, S.S.; Field, H.E.; Hyatt, A.D.; Daszak, P. Feral cats and risk for Nipah virus transmission. Emerg. Infect. Dis. 2006, 12, 1178–1179. [Google Scholar] [CrossRef]
- Mills, J.N.; Alim, A.N. M.; Bunning, M.L.; Lee, O.B.; Wagoner, K.D.; Amman, B.R.; Stockton, P.C.; Ksiazek, T.G. Nipah virus infection in dogs, Malaysia, 1999. Emerg. Infect. Dis. 2009, 15, 950–952. [Google Scholar] [CrossRef]
- Anonymous. Hendra virus, equine - Australia (21): (QL) canine. ProMed Mail. 2011. Archive Number: 20110802.2324. Available online: http://www.promedmail.org/ (access on 4 April 2013).
- Hayman, D.T.S.; Wang, L.-F.; Barr, J.; Baker, K.S.; Suu-Ire, R.; Broder, C.C.; Cunningham, A.A.; Wood, J.L.N. Antibodies to henipavirus or henipa-like viruses in domestic pigs in ghana, west Africa. PLoS One 2011, 6, e25256. [Google Scholar] [CrossRef]
- Mathieu, C.; Pohl, C.; Szecsi, J.; Trajkovic-Bodennec, S.; Devergnas, S.; Raoul, H.; Cosset, F.-L.; Gerlier, D.; Wild, T.F.; Horvat, B. Nipah Virus uses leukocytes for efficient dissemination within a host. J. Virol. 2011, 18. [Google Scholar]
- Stachowiak, B.; Weingartl, H.M. Nipah virus infects specific subsets of porcine peripheral blood mononuclear cells. PLoS One 2012, 7, e30855. [Google Scholar] [CrossRef]
- Field, H.; Schaaf, K.; Kung, N.; Simon, C.; Waltisbuhl, D.; Hobert, H.; Moore, F.; Middleton, D.; Crook, A.; Smith, G.; Daniels, P.; et al. Hendra virus outbreak with novel clinical features, Australia. Emerg. Infect. Dis. 2010, 16, 338–340. [Google Scholar] [CrossRef]
- Westbury, H.A. Hendra virus disease in horses. Rev. Off. Int. Epizoot. 2000, 19, 151–159. [Google Scholar]
- Parashar, U.D.; Sunn, L.M.; Ong, F.; Mounts, A.W.; Arif, M.T.; Ksiazek, T.G.; Kamaluddin, M.A.; Mustafa, A.N.; Kaur, H.; Ding, L.M.; et al. Case-control study of risk factors for human infection with a new zoonotic paramyxovirus, Nipah virus, during a 1998-1999 outbreak of severe encephalitis in Malaysia. J. Infect. Dis. 2000, 181, 1755–1759. [Google Scholar] [CrossRef]
- Munster, V.J.; Prescott, J.B.; Bushmaker, T.; Long, D.; Rosenke, R.; Thomas, T.; Scott, D.; Fischer, E.R.; Feldmann, H.; Wit, E. de Rapid Nipah virus entry into the central nervous system of hamsters via the olfactory route. Sci. Rep. 2012, 2, 736. [Google Scholar]
- Lo, M.K.; Miller, D.; Aljofan, M.; Mungall, B.A.; Rollin, P.E.; Bellini, W.J.; Rota, P.A. Characterization of the antiviral and inflammatory responses against Nipah virus in endothelial cells and neurons. Virology 2010, 404, 78–88. [Google Scholar] [CrossRef]
- Torres-Velez, F.J.; Shieh, W.-J.; Rollin, P.E.; Morken, T.; Brown, C.; Ksiazek, T.G.; Zaki, S.R. Histopathologic and immunohistochemical characterization of Nipah virus infection in the guinea pig. Vet. Pathol 2008, 45, 576–585. [Google Scholar] [CrossRef]
- Blackman, M.A.; Woodland, D.L. The narrowing of the CD8 T cell repertoire in old age. Curr. Opin. Immunol. 2011, 23, 537–542. [Google Scholar] [CrossRef]
- Frasca, D.; Blomberg, B.B. Aging impairs murine B cell differentiation and function in primary and secondary lymphoid tissues. Aging Dis. 2011, 2, 361–373. [Google Scholar]
- Kovacs, E.J.; Palmer, J.L.; Fortin, C.F.; Fülöp, T., Jr; Goldstein, D.R.; Linton, P.-J. Aging and innate immunity in the mouse: impact of intrinsic and extrinsic factors. Trends Immunol. 2009, 30, 319–324. [Google Scholar] [CrossRef]
- Müller, U.; Steinhoff, U.; Reis, L.F.; Hemmi, S.; Pavlovic, J.; Zinkernagel, R.M.; Aguet, M. Functional role of type I and type II interferons in antiviral defense. Science 1994, 264, 1918–1921. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dhondt, K.P.; Horvat, B. Henipavirus Infections: Lessons from Animal Models. Pathogens 2013, 2, 264-287. https://doi.org/10.3390/pathogens2020264
Dhondt KP, Horvat B. Henipavirus Infections: Lessons from Animal Models. Pathogens. 2013; 2(2):264-287. https://doi.org/10.3390/pathogens2020264
Chicago/Turabian StyleDhondt, Kévin P., and Branka Horvat. 2013. "Henipavirus Infections: Lessons from Animal Models" Pathogens 2, no. 2: 264-287. https://doi.org/10.3390/pathogens2020264






