HTLV-1 Tax Tug-of-War: Cellular Senescence and Death or Cellular Transformation
Abstract
1. Introduction
Overview of the Molecular Mechanisms Associated with Tax-Induced Cellular Immortalization and/or Transformation
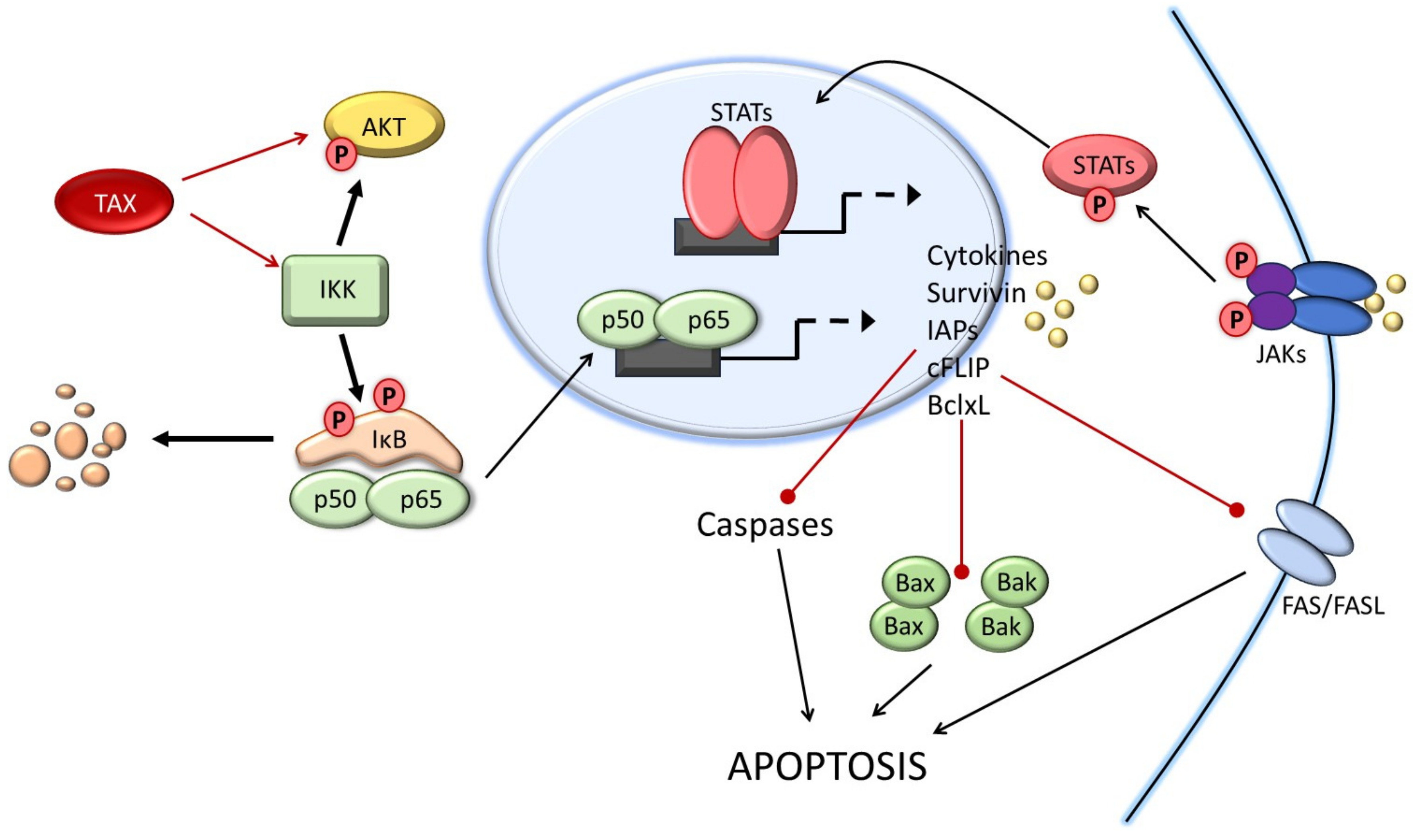
2. HTLV-1 Tax Control of the Apoptosis Cell Death Pathways
2.1. Activation of Apoptotic Pathways by Tax
2.2. Anti-Apoptotic Functions of Tax
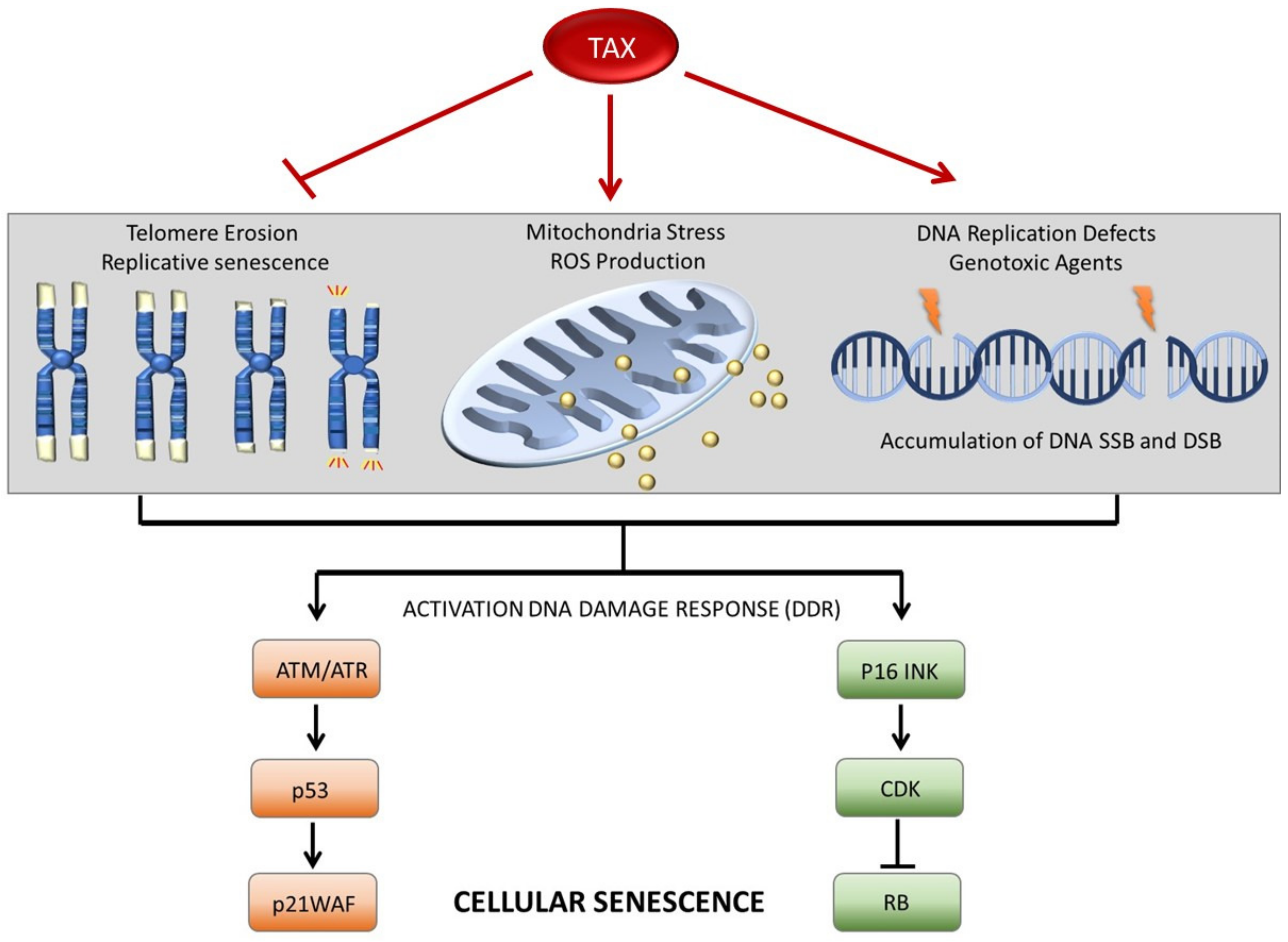
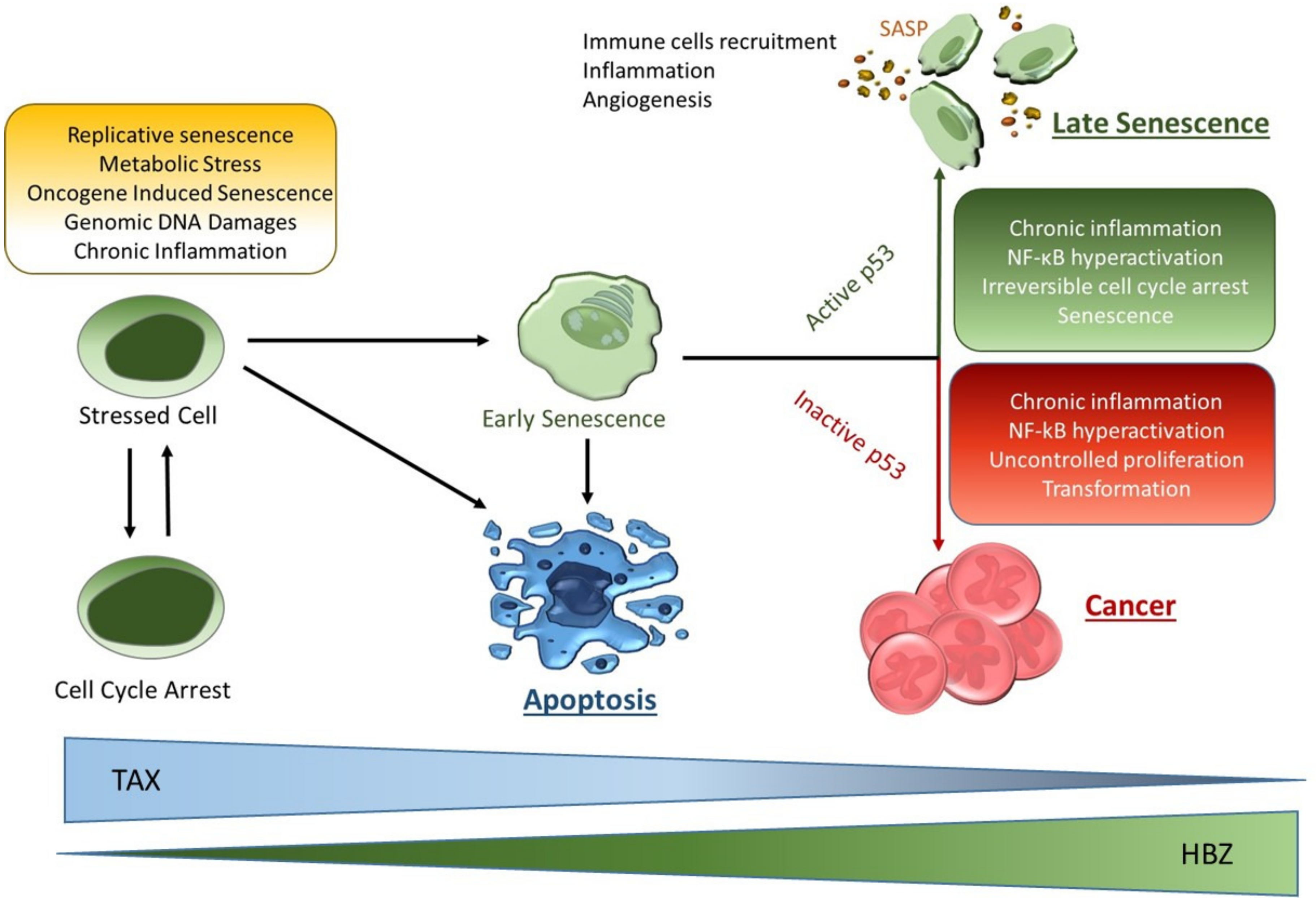
3. Context-Dependent Expression of Tax Induces Senescence or Immortalization
3.1. Tax Overexpression Is Associated with Rapid Stress-Induced Senescence (SIS) in Pre-Transformed p53-Deficient Cells
3.2. Tax Expression in Primary Cells Is Associated with the Reactivation of hTERT and Stability of Telomere Ends
4. Discussion and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Gallo, R.C. The first human retrovirus. Sci. Am. 1986, 255, 88–98. [Google Scholar] [CrossRef]
- Poiesz, B.J.; Ruscetti, F.W.; Gazdar, A.F.; Bunn, P.A.; Minna, J.D.; Gallo, R.C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 1980, 77, 7415–7419. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Miyoshi, I.; Hinuma, Y. A retrovirus from human leukemia cell lines: Its isolation, characterization, and implication in human adult T-cell leukemia (ATL). Princess Takamatsu. Symp. 1982, 12, 285–294. [Google Scholar]
- Blattner, W.A.; Kalyanaraman, V.S.; Robert-Guroff, M.; Lister, T.A.; Galton, D.A.; Sarin, P.S.; Crawford, M.H.; Catovsky, D.; Greaves, M.; Gallo, R.C. The human type-C retrovirus, HTLV, in Blacks from the Caribbean region, and relationship to adult T-cell leukemia/lymphoma. Int. J. Cancer 1982, 30, 257–264. [Google Scholar] [CrossRef]
- Takatsuki, K.; Yamaguchi, K.; Kawano, F.; Hattori, T.; Nishimura, H.; Tsuda, H.; Sanada, I.; Nakada, K.; Itai, Y. Clinical diversity in adult T-cell leukemia-lymphoma. Cancer Res. 1985, 45 (Suppl. 9), 4644s–4645s. [Google Scholar] [PubMed]
- Yamaguchi, K.; Nishimura, H.; Kohrogi, H.; Jono, M.; Miyamoto, Y.; Takatsuki, K. A proposal for smoldering adult T-cell leukemia: A clinicopathologic study of five cases. Blood 1983, 62, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.A. Advances in the treatment of HTLV-1-associated adult T-cell leukemia lymphoma. Curr. Opin. Virol. 2023, 58, 101289. [Google Scholar] [CrossRef]
- Cook, L.B.; Phillips, A.A. How I treat adult T-cell leukemia/lymphoma. Blood 2021, 137, 459–470. [Google Scholar] [CrossRef]
- Cavrois, M.; Leclercq, I.; Gout, O.; Gessain, A.; Wain-Hobson, S.; Wattel, E. Persistent oligoclonal expansion of human T-cell leukemia virus type 1-infected circulating cells in patients with Tropical spastic paraparesis/HTLV-1 associated myelopathy. Oncogene 1998, 17, 77–82. [Google Scholar] [CrossRef]
- Wattel, E.; Vartanian, J.P.; Pannetier, C.; Wain-Hobson, S. Clonal expansion of human T-cell leukemia virus type I-infected cells in asymptomatic and symptomatic carriers without malignancy. J. Virol. 1995, 69, 2863–2868. [Google Scholar] [CrossRef]
- Ohashi, T.; Hanabuchi, S.; Kato, H.; Tateno, H.; Takemura, F.; Tsukahara, T.; Koya, Y.; Hasegawa, A.; Masuda, T.; Kannagi, M. Prevention of adult T-cell leukemia-like lymphoproliferative disease in rats by adoptively transferred T cells from a donor immunized with human T-cell leukemia virus type 1 Tax-coding DNA vaccine. J. Virol. 2000, 74, 9610–9616. [Google Scholar] [CrossRef]
- Ohashi, T.; Nagai, M.; Okada, H.; Takayanagi, R.; Shida, H. Activation and detection of HTLV-I Tax-specific CTLs by epitope expressing single-chain trimers of MHC class I in a rat model. Retrovirology 2008, 5, 90. [Google Scholar] [CrossRef] [PubMed]
- Ciminale, V.; Pavlakis, G.N.; Derse, D.; Cunningham, C.P.; Felber, B.K. Complex splicing in the human T-cell leukemia virus (HTLV) family of retroviruses: Novel mRNAs and proteins produced by HTLV type I. J. Virol. 1992, 66, 1737–1745. [Google Scholar] [CrossRef]
- Seiki, M.; Hikikoshi, A.; Taniguchi, T.; Yoshida, M. Expression of the pX gene of HTLV-I: General splicing mechanism in the HTLV family. Science 1985, 228, 1532–1534. [Google Scholar] [CrossRef]
- Bai, X.T.; Nicot, C. Overview on HTLV-1 p12, p8, p30, p13: Accomplices in persistent infection and viral pathogenesis. Front. Microbiol. 2012, 3, 400. [Google Scholar] [CrossRef]
- Zhao, L.J.; Giam, C.Z. Human T-cell lymphotropic virus type I (HTLV-I) transcriptional activator, Tax, enhances CREB binding to HTLV-I 21-base-pair repeats by protein-protein interaction. Proc. Natl. Acad. Sci. USA 1992, 89, 7070–7074. [Google Scholar] [CrossRef]
- Kwok, R.P.; Laurance, M.E.; Lundblad, J.R.; Goldman, P.S.; Shih, H.; Connor, L.M.; Marriott, S.J.; Goodman, R.H. Control of cAMP-regulated enhancers by the viral transactivator Tax through CREB and the co-activator CBP. Nature 1996, 380, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.P.; Garrus, J.E.; Giebler, H.A.; Stargell, L.A.; Nyborg, J.K. Molecular interactions between the coactivator CBP and the human T-cell leukemia virus Tax protein. J. Mol. Biol. 1998, 281, 395–400. [Google Scholar] [CrossRef][Green Version]
- Harrod, R.; Tang, Y.; Nicot, C.; Lu, H.S.; Vassilev, A.; Nakatani, Y.; Giam, C.Z. An exposed KID-like domain in human T-cell lymphotropic virus type 1 Tax is responsible for the recruitment of coactivators CBP/p300. Mol. Cell. Biol. 1998, 18, 5052–5061. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Lu, H.; Schiltz, R.L.; Pise-Masison, C.A.; Ogryzko, V.V.; Nakatani, Y.; Brady, J.N. PCAF interacts with tax and stimulates tax transactivation in a histone acetyltransferase-independent manner. Mol. Cell. Biol. 1999, 19, 8136–8145. [Google Scholar] [CrossRef]
- Mohanty, S.; Harhaj, E.W. Mechanisms of Oncogenesis by HTLV-1 Tax. Pathogens 2020, 9, 543. [Google Scholar] [CrossRef]
- Nicot, C. HTLV-I Tax-Mediated Inactivation of Cell Cycle Checkpoints and DNA Repair Pathways Contribute to Cellular Transformation: “A Random Mutagenesis Model”. J. Cancer Sci. 2015, 2, 1000009. [Google Scholar]
- Mahgoub, M.; Yasunaga, J.I.; Iwami, S.; Nakaoka, S.; Koizumi, Y.; Shimura, K.; Matsuoka, M. Sporadic on/off switching of HTLV-1 Tax expression is crucial to maintain the whole population of virus-induced leukemic cells. Proc. Natl. Acad. Sci. USA 2018, 115, E1269–E1278. [Google Scholar] [CrossRef]
- Hleihel, R.; Skayneh, H.; de The, H.; Hermine, O.; Bazarbachi, A. Primary cells from patients with adult T cell leukemia/lymphoma depend on HTLV-1 Tax expression for NF-κB activation and survival. Blood Cancer J. 2023, 13, 67. [Google Scholar] [CrossRef]
- Markham, P.D.; Salahuddin, S.Z.; Kalyanaraman, V.S.; Popovic, M.; Sarin, P.; Gallo, R.C. Infection and transformation of fresh human umbilical cord blood cells by multiple sources of human T-cell leukemia-lymphoma virus (HTLV). Int. J. Cancer 1983, 31, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Popovic, M.; Lange-Wantzin, G.; Sarin, P.S.; Mann, D.; Gallo, R.C. Transformation of human umbilical cord blood T cells by human T-cell leukemia/lymphoma virus. Proc. Natl. Acad. Sci. USA 1983, 80, 5402–5406. [Google Scholar] [CrossRef]
- Yamamoto, N.; Okada, M.; Koyanagi, Y.; Kannagi, M.; Hinuma, Y. Transformation of human leukocytes by cocultivation with an adult T cell leukemia virus producer cell line. Science 1982, 217, 737–739. [Google Scholar] [CrossRef]
- Bellon, M.; Baydoun, H.H.; Yao, Y.; Nicot, C. HTLV-I Tax-dependent and -independent events associated with immortalization of human primary T lymphocytes. Blood 2010, 115, 2441–2448. [Google Scholar] [CrossRef] [PubMed]
- Ruben, S.; Poteat, H.; Tan, T.H.; Kawakami, K.; Roeder, R.; Haseltine, W.; Rosen, C.A. Cellular transcription factors and regulation of IL-2 receptor gene expression by HTLV-I tax gene product. Science 1988, 241, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Ballard, D.W.; Bohnlein, E.; Lowenthal, J.W.; Wano, Y.; Franza, B.R.; Greene, W.C. HTLV-I tax induces cellular proteins that activate the kappa B element in the IL-2 receptor alpha gene. Science 1988, 241, 1652–1655. [Google Scholar] [CrossRef]
- Wano, Y.; Feinberg, M.; Hosking, J.B.; Bogerd, H.; Greene, W.C. Stable expression of the tax gene of type I human T-cell leukemia virus in human T cells activates specific cellular genes involved in growth. Proc. Natl. Acad. Sci. USA 1988, 85, 9733–9737. [Google Scholar] [CrossRef] [PubMed]
- Hoyos, B.; Ballard, D.W.; Bohnlein, E.; Siekevitz, M.; Greene, W.C. Kappa B-specific DNA binding proteins: Role in the regulation of human interleukin-2 gene expression. Science 1989, 244, 457–460. [Google Scholar] [CrossRef]
- Bamford, R.N.; Battiata, A.P.; Burton, J.D.; Sharma, H.; Waldmann, T.A. Interleukin (IL) 15/IL-T production by the adult T-cell leukemia cell line HuT-102 is associated with a human T-cell lymphotrophic virus type I region/IL-15 fusion message that lacks many upstream AUGs that normally attenuates IL-15 mRNA translation. Proc. Natl. Acad. Sci. USA 1996, 93, 2897–2902. [Google Scholar] [CrossRef] [PubMed]
- Mariner, J.M.; Lantz, V.; Waldmann, T.A.; Azimi, N. Human T cell lymphotropic virus type I Tax activates IL-15R α gene expression through an NF-κ B site. J. Immunol. 2001, 166, 2602–2609. [Google Scholar] [CrossRef]
- Phillips, K.E.; Herring, B.; Wilson, L.A.; Rickford, M.S.; Zhang, M.; Goldman, C.K.; Tso, J.Y.; Waldmann, T.A. IL-2Ralpha-Directed monoclonal antibodies provide effective therapy in a murine model of adult T-cell leukemia by a mechanism other than blockade of IL-2/IL-2Ralpha interaction. Cancer Res. 2000, 60, 6977–6984. [Google Scholar]
- Watanabe, M.; Nakahata, S.; Hamasaki, M.; Saito, Y.; Kawano, Y.; Hidaka, T.; Yamashita, K.; Umeki, K.; Taki, T.; Taniwaki, M.; et al. Downregulation of CDKN1A in adult T-cell leukemia/lymphoma despite overexpression of CDKN1A in human T-lymphotropic virus 1-infected cell lines. J. Virol. 2010, 84, 6966–6977. [Google Scholar] [CrossRef] [PubMed]
- Haller, K.; Wu, Y.; Derow, E.; Schmitt, I.; Jeang, K.T.; Grassmann, R. Physical interaction of human T-cell leukemia virus type 1 Tax with cyclin-dependent kinase 4 stimulates the phosphorylation of retinoblastoma protein. Mol. Cell. Biol. 2002, 22, 3327–3338. [Google Scholar] [CrossRef]
- Kehn, K.; de la Fuente, C.; Strouss, K.; Berro, R.; Jiang, H.; Brady, J.; Mahieux, R.; Pumfery, A.; Bottazzi, M.E.; Kashanchi, F. The HTLV-I Tax oncoprotein targets the retinoblastoma protein for proteasomal degradation. Oncogene 2005, 24, 525–540. [Google Scholar] [CrossRef]
- Suzuki, T.; Narita, T.; Uchida-Toita, M.; Yoshida, M. Down-regulation of the INK4 family of cyclin-dependent kinase inhibitors by tax protein of HTLV-1 through two distinct mechanisms. Virology 1999, 259, 384–391. [Google Scholar] [CrossRef]
- Migone, T.S.; Lin, J.X.; Cereseto, A.; Mulloy, J.C.; O’Shea, J.J.; Franchini, G.; Leonard, W.J. Constitutively activated Jak-STAT pathway in T cells transformed with HTLV-I. Science 1995, 269, 79–81. [Google Scholar] [CrossRef]
- Takemoto, S.; Mulloy, J.C.; Cereseto, A.; Migone, T.S.; Patel, B.K.; Matsuoka, M.; Yamaguchi, K.; Takatsuki, K.; Kamihira, S.; White, J.D.; et al. Proliferation of adult T cell leukemia/lymphoma cells is associated with the constitutive activation of JAK/STAT proteins. Proc. Natl. Acad. Sci. USA 1997, 94, 13897–13902. [Google Scholar] [CrossRef] [PubMed]
- Tomita, M.; Kawakami, H.; Uchihara, J.N.; Okudaira, T.; Masuda, M.; Matsuda, T.; Tanaka, Y.; Ohshiro, K.; Mori, N. Inhibition of constitutively active Jak-Stat pathway suppresses cell growth of human T-cell leukemia virus type 1-infected T-cell lines and primary adult T-cell leukemia cells. Retrovirology 2006, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Mathews Griner, L.A.; Ju, W.; Duveau, D.Y.; Guha, R.; Petrus, M.N.; Wen, B.; Maeda, M.; Shinn, P.; Ferrer, M.; et al. Selective targeting of JAK/STAT signaling is potentiated by Bcl-xL blockade in IL-2-dependent adult T-cell leukemia. Proc. Natl. Acad. Sci. USA 2015, 112, 12480–12485. [Google Scholar] [CrossRef]
- Bellon, M.; Lu, L.; Nicot, C. Constitutive activation of Pim1 kinase is a therapeutic target for adult T-cell leukemia. Blood 2016, 127, 2439–2450. [Google Scholar] [CrossRef]
- Migone, T.S.; Cacalano, N.A.; Taylor, N.; Yi, T.; Waldmann, T.A.; Johnston, J.A. Recruitment of SH2-containing protein tyrosine phosphatase SHP-1 to the interleukin 2 receptor; loss of SHP-1 expression in human T-lymphotropic virus type I-transformed T cells. Proc. Natl. Acad. Sci. USA 1998, 95, 3845–3850. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, D.; Zhou, C.; Marasco, W.A. Down-regulation of SHP1 and up-regulation of negative regulators of JAK/STAT signaling in HTLV-1 transformed cell lines and freshly transformed human peripheral blood CD4+ T-cells. Leuk. Res. 2004, 28, 71–82. [Google Scholar] [CrossRef]
- Jeong, S.J.; Dasgupta, A.; Jung, K.J.; Um, J.H.; Burke, A.; Park, H.U.; Brady, J.N. PI3K/AKT inhibition induces caspase-dependent apoptosis in HTLV-1-transformed cells. Virology 2008, 370, 264–272. [Google Scholar] [CrossRef]
- Bellon, M.; Nicot, C. Central role of PI3K in transcriptional activation of hTERT in HTLV-I-infected cells. Blood 2008, 112, 2946–2955. [Google Scholar] [CrossRef]
- Pancewicz, J.; Taylor, J.M.; Datta, A.; Baydoun, H.H.; Waldmann, T.A.; Hermine, O.; Nicot, C. Notch signaling contributes to proliferation and tumor formation of human T-cell leukemia virus type 1-associated adult T-cell leukemia. Proc. Natl. Acad. Sci. USA 2010, 107, 16619–16624. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.H.; Bellon, M.; Pancewicz-Wojtkiewicz, J.; Nicot, C. Oncogenic mutations in the FBXW7 gene of adult T-cell leukemia patients. Proc. Natl. Acad. Sci. USA 2016, 113, 6731–6736. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Yamakuchi, M.; Masuda, S.; Tokioka, T.; Yamaoka, S.; Maruyama, I.; Kitajima, I. Phosphoinositide-3 kinase-PKB/Akt pathway activation is involved in fibroblast Rat-1 transformation by human T-cell leukemia virus type I tax. Oncogene 2001, 20, 2514–2526. [Google Scholar] [CrossRef]
- Suzuki, T.; Ohsugi, Y.; Uchida-Toita, M.; Akiyama, T.; Yoshida, M. Tax oncoprotein of HTLV-1 binds to the human homologue of Drosophila discs large tumor suppressor protein, hDLG, and perturbs its function in cell growth control. Oncogene 1999, 18, 5967–5972. [Google Scholar] [CrossRef]
- Okajima, M.; Takahashi, M.; Higuchi, M.; Ohsawa, T.; Yoshida, S.; Yoshida, Y.; Oie, M.; Tanaka, Y.; Gejyo, F.; Fujii, M. Human T-cell leukemia virus type 1 Tax induces an aberrant clustering of the tumor suppressor Scribble through the PDZ domain-binding motif dependent and independent interaction. Virus Genes 2008, 37, 231–240. [Google Scholar] [CrossRef]
- Makokha, G.N.; Takahashi, M.; Higuchi, M.; Saito, S.; Tanaka, Y.; Fujii, M. Human T-cell leukemia virus type 1 Tax protein interacts with and mislocalizes the PDZ domain protein MAGI-1. Cancer Sci. 2013, 104, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Cherian, M.A.; Baydoun, H.H.; Al-Saleem, J.; Shkriabai, N.; Kvaratskhelia, M.; Green, P.; Ratner, L. Akt Pathway Activation by Human T-cell Leukemia Virus Type 1 Tax Oncoprotein. J. Biol. Chem. 2015, 290, 26270–26281. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Saito, M.; Taniura, N.; Okuwa, T.; Ohara, Y. Activation of the PI3K-Akt pathway by human T cell leukemia virus type 1 (HTLV-1) oncoprotein Tax increases Bcl3 expression, which is associated with enhanced growth of HTLV-1-infected T cells. Virology 2010, 403, 173–180. [Google Scholar] [CrossRef]
- Cheng, W.; Zheng, T.; Wang, Y.; Cai, K.; Wu, W.; Zhao, T.; Xu, R. Activation of Notch1 signaling by HTLV-1 Tax promotes proliferation of adult T-cell leukemia cells. Biochem. Biophys. Res. Commun. 2019, 512, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.P.; Irvine, J.; Blyth, K.; Cameron, E.R.; Onions, D.E.; Campbell, M.E. Tumours derived from HTLV-I tax transgenic mice are characterized by enhanced levels of apoptosis and oncogene expression. J. Pathol. 1998, 186, 209–214. [Google Scholar] [CrossRef]
- Nicot, C.; Harrod, R. Distinct p300-responsive mechanisms promote caspase-dependent apoptosis by human T-cell lymphotropic virus type 1 Tax protein. Mol. Cell. Biol. 2000, 20, 8580–8589. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takahashi, M.; Higuchi, M.; Makokha, G.N.; Matsuki, H.; Yoshita, M.; Tanaka, Y.; Fujii, M. HTLV-1 Tax oncoprotein stimulates ROS production and apoptosis in T cells by interacting with USP10. Blood 2013, 122, 715–725. [Google Scholar] [CrossRef]
- Arainga, M.; Takeda, E.; Aida, Y. Identification of bovine leukemia virus tax function associated with host cell transcription, signaling, stress response and immune response pathway by microarray-based gene expression analysis. BMC Genom. 2012, 13, 121. [Google Scholar] [CrossRef] [PubMed]
- Kao, S.Y.; Lemoine, F.J.; Mariott, S.J. HTLV-1 Tax protein sensitizes cells to apoptotic cell death induced by DNA damaging agents. Oncogene 2000, 19, 2240–2248. [Google Scholar] [CrossRef]
- Kao, S.Y.; Lemoine, F.J.; Marriott, S.J. p53-independent induction of apoptosis by the HTLV-I tax protein following UV irradiation. Virology 2001, 291, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Chlichlia, K.; Busslinger, M.; Peter, M.E.; Walczak, H.; Krammer, P.H.; Schirrmacher, V.; Khazaie, K. ICE-proteases mediate HTLV-I Tax-induced apoptotic T-cell death. Oncogene 1997, 14, 2265–2272. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zachar, V.; Zdravkovic, M.; Guo, M.; Ebbesen, P.; Liu, X. Role of the Fas/Fas ligand pathway in apoptotic cell death induced by the human T cell lymphotropic virus type I Tax transactivator. J. Gen. Virol. 1997, 78 Pt 12, 3277–3285. [Google Scholar] [CrossRef]
- Saggioro, D.; Barp, S.; Chieco-Bianchi, L. Block of a mitochondrial-mediated apoptotic pathway in Tax-expressing murine fibroblasts. Exp. Cell Res. 2001, 269, 245–255. [Google Scholar] [CrossRef]
- El Hajj, H.; El-Sabban, M.; Hasegawa, H.; Zaatari, G.; Ablain, J.; Saab, S.T.; Janin, A.; Mahfouz, R.; Nasr, R.; Kfoury, Y.; et al. Therapy-induced selective loss of leukemia-initiating activity in murine adult T cell leukemia. J. Exp. Med. 2010, 207, 2785–2792. [Google Scholar] [CrossRef] [PubMed]
- McGuire, K.L.; Curtiss, V.E.; Larson, E.L.; Haseltine, W.A. Influence of human T-cell leukemia virus type I tax and rex on interleukin-2 gene expression. J. Virol. 1993, 67, 1590–1599. [Google Scholar] [CrossRef]
- Azimi, N.; Brown, K.; Bamford, R.N.; Tagaya, Y.; Siebenlist, U.; Waldmann, T.A. Human T cell lymphotropic virus type I Tax protein trans-activates interleukin 15 gene transcription through an NF-κB site. Proc. Natl. Acad. Sci. USA 1998, 95, 2452–2457. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, O.; Kaisho, T.; Tanabe, M.; Hirano, T. Transcriptional activation of the interleukin-6 gene by HTLV-1 p40tax through an NF-κ B-like binding site. Immunol. Lett. 1993, 37, 159–165. [Google Scholar] [CrossRef]
- Mori, N.; Shirakawa, F.; Abe, M.; Kamo, Y.; Koyama, Y.; Murakami, S.; Shimizu, H.; Yamamoto, K.; Oda, S.; Eto, S. Human T-cell leukemia virus type I tax transactivates the interleukin-6 gene in human rheumatoid synovial cells. J. Rheumatol. 1995, 22, 2049–2054. [Google Scholar] [PubMed]
- Mori, N.; Prager, D. Transactivation of the interleukin-1alpha promoter by human T-cell leukemia virus type I and type II Tax proteins. Blood 1996, 87, 3410–3417. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, S.G.; Aichele, G.; Wirth, T.; Czernilofsky, A.P.; Nordheim, A.; Dittmer, J. Regulation of the human interleukin-5 promoter by Ets transcription factors. Ets1 and Ets2, but not Elf-1, cooperate with GATA3 and HTLV-I Tax1. J. Biol. Chem. 1999, 274, 12910–12916. [Google Scholar] [CrossRef]
- Li-Weber, M.; Giaisi, M.; Chlichlia, K.; Khazaie, K.; Krammer, P.H. Human T cell leukemia virus type I Tax enhances IL-4 gene expression in T cells. Eur. J. Immunol. 2001, 31, 2623–2632. [Google Scholar] [CrossRef]
- Chung, H.K.; Young, H.A.; Goon, P.K.; Heidecker, G.; Princler, G.L.; Shimozato, O.; Taylor, G.P.; Bangham, C.R.; Derse, D. Activation of interleukin-13 expression in T cells from HTLV-1-infected individuals and in chronically infected cell lines. Blood 2003, 102, 4130–4136. [Google Scholar] [CrossRef]
- Dodon, M.D.; Li, Z.; Hamaia, S.; Gazzolo, L. Tax protein of human T-cell leukaemia virus type 1 induces interleukin 17 gene expression in T cells. J. Gen. Virol. 2004, 85, 1921–1932. [Google Scholar] [CrossRef]
- Ahuja, J.; Lepoutre, V.; Wigdahl, B.; Khan, Z.K.; Jain, P. Induction of pro-inflammatory cytokines by human T-cell leukemia virus type-1 Tax protein as determined by multiplexed cytokine protein array analyses of human dendritic cells. Biomed. Pharmacother. 2007, 61, 201–208. [Google Scholar] [CrossRef]
- Chen, J.; Petrus, M.; Bryant, B.R.; Phuc Nguyen, V.; Stamer, M.; Goldman, C.K.; Bamford, R.; Morris, J.C.; Janik, J.E.; Waldmann, T.A. Induction of the IL-9 gene by HTLV-I Tax stimulates the spontaneous proliferation of primary adult T-cell leukemia cells by a paracrine mechanism. Blood 2008, 111, 5163–5172. [Google Scholar] [CrossRef]
- Mizuguchi, M.; Asao, H.; Hara, T.; Higuchi, M.; Fujii, M.; Nakamura, M. Transcriptional activation of the interleukin-21 gene and its receptor gene by human T-cell leukemia virus type 1 Tax in human T-cells. J. Biol. Chem. 2009, 284, 25501–25511. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, S.; Yamamoto, N.; Dewan, M.Z.; Takahashi, Y.; Yamashita, A.; Yoshida, T.; Nowell, M.A.; Richards, P.J.; Jones, S.A.; Yamamoto, N. Human T-cell leukemia virus type-I Tax induces expression of interleukin-6 receptor (IL-6R): Shedding of soluble IL-6R and activation of STAT3 signaling. Int. J. Cancer 2006, 119, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Lavorgna, A.; Matsuoka, M.; Harhaj, E.W. A critical role for IL-17RB signaling in HTLV-1 tax-induced NF-κB activation and T-cell transformation. PLoS Pathog. 2014, 10, e1004418. [Google Scholar] [CrossRef]
- Nimer, S.D.; Gasson, J.C.; Hu, K.; Smalberg, I.; Williams, J.L.; Chen, I.S.; Rosenblatt, J.D. Activation of the GM-CSF promoter by HTLV-I and -II tax proteins. Oncogene 1989, 4, 671–676. [Google Scholar]
- Sharma, V.; May, C.C. Human T-cell lymphotrophic virus type-I tax gene induces secretion of human macrophage inflammatory protein-1α. Biochem. Biophys. Res. Commun. 1999, 262, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Lorey, S.L. Autocrine role of macrophage inflammatory protein-1 β in human T-cell lymphotropic virus type-I tax-transfected Jurkat T-cells. Biochem. Biophys. Res. Commun. 2001, 287, 910–913. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, Y.; Sugita, S.; Yamamoto, K.; Imanishi, D.; Kohno, T.; Tomonaga, M.; Matsuyama, T. Human T cell leukemia virus type-I Tax activates human macrophage inflammatory protein-3 α/CCL20 gene transcription via the NF-κ B pathway. Int. Immunol. 2002, 14, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Ueda, A.; Ikeda, S.; Yamasaki, Y.; Yamada, Y.; Tomonaga, M.; Morikawa, S.; Geleziunas, R.; Yoshimura, T.; Yamamoto, N. Human T-cell leukemia virus type I tax activates transcription of the human monocyte chemoattractant protein-1 gene through two nuclear factor-kappaB sites. Cancer Res. 2000, 60, 4939–4945. [Google Scholar]
- Mori, N.; Krensky, A.M.; Ohshima, K.; Tomita, M.; Matsuda, T.; Ohta, T.; Yamada, Y.; Tomonaga, M.; Ikeda, S.; Yamamoto, N. Elevated expression of CCL5/RANTES in adult T-cell leukemia cells: Possible transactivation of the CCL5 gene by human T-cell leukemia virus type I tax. Int. J. Cancer 2004, 111, 548–557. [Google Scholar] [CrossRef]
- Saito, M.; Sejima, H.; Naito, T.; Ushirogawa, H.; Matsuzaki, T.; Matsuura, E.; Tanaka, Y.; Nakamura, T.; Takashima, H. The CC chemokine ligand (CCL) 1, upregulated by the viral transactivator Tax, can be downregulated by minocycline: Possible implications for long-term treatment of HTLV-1-associated myelopathy/tropical spastic paraparesis. Virol. J. 2017, 14, 234. [Google Scholar] [CrossRef]
- Hieshima, K.; Nagakubo, D.; Nakayama, T.; Shirakawa, A.K.; Jin, Z.; Yoshie, O. Tax-inducible production of CC chemokine ligand 22 by human T cell leukemia virus type 1 (HTLV-1)-infected T cells promotes preferential transmission of HTLV-1 to CCR4-expressing CD4+ T cells. J. Immunol. 2008, 180, 931–939. [Google Scholar] [CrossRef]
- Jin, Z.; Nagakubo, D.; Shirakawa, A.K.; Nakayama, T.; Shigeta, A.; Hieshima, K.; Yamada, Y.; Yoshie, O. CXCR7 is inducible by HTLV-1 Tax and promotes growth and survival of HTLV-1-infected T cells. Int. J. Cancer 2009, 125, 2229–2235. [Google Scholar] [CrossRef]
- Nagakubo, D.; Jin, Z.; Hieshima, K.; Nakayama, T.; Shirakawa, A.K.; Tanaka, Y.; Hasegawa, H.; Hayashi, T.; Tsukasaki, K.; Yamada, Y.; et al. Expression of CCR9 in HTLV-1+ T cells and ATL cells expressing Tax. Int. J. Cancer 2007, 120, 1591–1597. [Google Scholar] [CrossRef]
- Harhaj, E.W.; Giam, C.Z. NF-κB signaling mechanisms in HTLV-1-induced adult T-cell leukemia/lymphoma. FEBS J. 2018, 285, 3324–3336. [Google Scholar] [CrossRef]
- Mizuguchi, M.; Sasaki, Y.; Hara, T.; Higuchi, M.; Tanaka, Y.; Funato, N.; Tanaka, N.; Fujii, M.; Nakamura, M. Induction of Cell Death in Growing Human T-Cells and Cell Survival in Resting Cells in Response to the Human T-Cell Leukemia Virus Type 1 Tax. PLoS ONE 2016, 11, e0148217. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tsukahara, T.; Kannagi, M.; Ohashi, T.; Kato, H.; Arai, M.; Nunez, G.; Iwanaga, Y.; Yamamoto, N.; Ohtani, K.; Nakamura, M.; et al. Induction of Bcl-x(L) expression by human T-cell leukemia virus type 1 Tax through NF-kappaB in apoptosis-resistant T-cell transfectants with Tax. J. Virol. 1999, 73, 7981–7987. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, H.; Tomita, M.; Matsuda, T.; Ohta, T.; Tanaka, Y.; Fujii, M.; Hatano, M.; Tokuhisa, T.; Mori, N. Transcriptional activation of survivin through the NF-κB pathway by human T-cell leukemia virus type I tax. Int. J. Cancer 2005, 115, 967–974. [Google Scholar] [CrossRef]
- Macaire, H.; Riquet, A.; Moncollin, V.; Biemont-Trescol, M.C.; Duc Dodon, M.; Hermine, O.; Debaud, A.L.; Mahieux, R.; Mesnard, J.M.; Pierre, M.; et al. Tax protein-induced expression of antiapoptotic Bfl-1 protein contributes to survival of human T-cell leukemia virus type 1 (HTLV-1)-infected T-cells. J. Biol. Chem. 2012, 287, 21357–21370. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.H.; Bellon, M.; Nicot, C. FBXW7: A critical tumor suppressor of human cancers. Mol. Cancer 2018, 17, 115. [Google Scholar] [CrossRef]
- Choi, Y.B.; Harhaj, E.W. HTLV-1 tax stabilizes MCL-1 via TRAF6-dependent K63-linked polyubiquitination to promote cell survival and transformation. PLoS Pathog. 2014, 10, e1004458. [Google Scholar] [CrossRef]
- Yamada, T.; Yamaoka, S.; Goto, T.; Nakai, M.; Tsujimoto, Y.; Hatanaka, M. The human T-cell leukemia virus type I Tax protein induces apoptosis which is blocked by the Bcl-2 protein. J. Virol. 1994, 68, 3374–3379. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, J.; Shi, J.; Zhang, Y.; Liu, S.; Liu, Y.; Zheng, D. Human T-cell leukemia virus type 1 Tax-deregulated autophagy pathway and c-FLIP expression contribute to resistance against death receptor-mediated apoptosis. J. Virol. 2014, 88, 2786–2798. [Google Scholar] [CrossRef] [PubMed]
- Bellon, M.; Yuan, Y.; Nicot, C. Transcription Independent Stimulation of Telomerase Enzymatic Activity by HTLV-I Tax Through Stimulation of IKK. J. Cancer Sci. 2021, 8, 1000024. [Google Scholar]
- Muhleisen, A.; Giaisi, M.; Kohler, R.; Krammer, P.H.; Li-Weber, M. Tax contributes apoptosis resistance to HTLV-1-infected T cells via suppression of Bid and Bim expression. Cell Death Dis. 2014, 5, e1575. [Google Scholar] [CrossRef] [PubMed]
- Pise-Masison, C.A.; Choi, K.S.; Radonovich, M.; Dittmer, J.; Kim, S.J.; Brady, J.N. Inhibition of p53 transactivation function by the human T-cell lymphotropic virus type 1 Tax protein. J. Virol. 1998, 72, 1165–1170. [Google Scholar] [CrossRef]
- Mulloy, J.C.; Kislyakova, T.; Cereseto, A.; Casareto, L.; LoMonico, A.; Fullen, J.; Lorenzi, M.V.; Cara, A.; Nicot, C.; Giam, C.; et al. Human T-cell lymphotropic/leukemia virus type 1 Tax abrogates p53-induced cell cycle arrest and apoptosis through its CREB/ATF functional domain. J. Virol. 1998, 72, 8852–8860. [Google Scholar] [CrossRef] [PubMed]
- Kaida, A.; Ariumi, Y.; Ueda, Y.; Lin, J.Y.; Hijikata, M.; Ikawa, S.; Shimotohno, K. Functional impairment of p73 and p51, the p53-related proteins, by the human T-cell leukemia virus type 1 Tax oncoprotein. Oncogene 2000, 19, 827–830. [Google Scholar] [CrossRef] [PubMed]
- Pise-Masison, C.A.; Mahieux, R.; Jiang, H.; Ashcroft, M.; Radonovich, M.; Duvall, J.; Guillerm, C.; Brady, J.N. Inactivation of p53 by human T-cell lymphotropic virus type 1 Tax requires activation of the NF-κB pathway and is dependent on p53 phosphorylation. Mol. Cell. Biol. 2000, 20, 3377–3386. [Google Scholar] [CrossRef]
- Trevisan, R.; Daprai, L.; Paloschi, L.; Vajente, N.; Chieco-Bianchi, L.; Saggioro, D. Antiapoptotic effect of human T-cell leukemia virus type 1 tax protein correlates with its creb transcriptional activity. Exp. Cell Res. 2006, 312, 1390–1400. [Google Scholar] [CrossRef]
- Courtois-Cox, S.; Jones, S.L.; Cichowski, K. Many roads lead to oncogene-induced senescence. Oncogene 2008, 27, 2801–2809. [Google Scholar] [CrossRef]
- Xiao, S.; Qin, D.M.; Hou, X.Y.; Tian, L.L.; Yu, Y.P.; Zhang, R.; Lyu, H.; Guo, D.; Chen, X.Z.; Zhou, C.F.; et al. Cellular senescence: A double-edged sword in cancer therapy. Front. Oncol. 2023, 13, 1189015. [Google Scholar] [CrossRef]
- Kao, S.Y.; Marriott, S.J. Disruption of nucleotide excision repair by the human T-cell leukemia virus type 1 Tax protein. J. Virol. 1999, 73, 4299–4304. [Google Scholar] [CrossRef]
- Lemoine, F.J.; Kao, S.Y.; Marriott, S.J. Suppression of DNA repair by HTLV type 1 Tax correlates with Tax trans-activation of proliferating cell nuclear antigen gene expression. AIDS Res. Hum. Retroviruses 2000, 16, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Philpott, S.M.; Buehring, G.C. Defective DNA repair in cells with human T-cell leukemia/bovine leukemia viruses: Role of tax gene. J. Natl. Cancer Inst. 1999, 91, 933–942. [Google Scholar] [CrossRef]
- Baydoun, H.H.; Bai, X.T.; Shelton, S.; Nicot, C. HTLV-I tax increases genetic instability by inducing DNA double strand breaks during DNA replication and switching repair to NHEJ. PLoS ONE 2012, 7, e42226. [Google Scholar] [CrossRef] [PubMed]
- Haoudi, A.; Semmes, O.J. The HTLV-1 tax oncoprotein attenuates DNA damage induced G1 arrest and enhances apoptosis in p53 null cells. Virology 2003, 305, 229–239. [Google Scholar] [CrossRef]
- Chandhasin, C.; Ducu, R.I.; Berkovich, E.; Kastan, M.B.; Marriott, S.J. Human T-cell leukemia virus type 1 tax attenuates the ATM-mediated cellular DNA damage response. J. Virol. 2008, 82, 6952–6961. [Google Scholar] [CrossRef] [PubMed]
- Durkin, S.S.; Guo, X.; Fryrear, K.A.; Mihaylova, V.T.; Gupta, S.K.; Belgnaoui, S.M.; Haoudi, A.; Kupfer, G.M.; Semmes, O.J. HTLV-1 Tax oncoprotein subverts the cellular DNA damage response via binding to DNA-dependent protein kinase. J. Biol. Chem. 2008, 283, 36311–36320. [Google Scholar] [CrossRef] [PubMed]
- Chaib-Mezrag, H.; Lemacon, D.; Fontaine, H.; Bellon, M.; Bai, X.T.; Drac, M.; Coquelle, A.; Nicot, C. Tax impairs DNA replication forks and increases DNA breaks in specific oncogenic genome regions. Mol. Cancer 2014, 13, 205. [Google Scholar] [CrossRef]
- He, Y.; Pasupala, N.; Zhi, H.; Dorjbal, B.; Hussain, I.; Shih, H.M.; Bhattacharyya, S.; Biswas, R.; Miljkovic, M.; Semmes, O.J.; et al. NF-kappaB-induced R-loop accumulation and DNA damage select for nucleotide excision repair deficiencies in adult T cell leukemia. Proc. Natl. Acad. Sci. USA 2021, 118, 2005568118. [Google Scholar] [CrossRef]
- Gan, W.; Guan, Z.; Liu, J.; Gui, T.; Shen, K.; Manley, J.L.; Li, X. R-loop-mediated genomic instability is caused by impairment of replication fork progression. Genes Dev. 2011, 25, 2041–2056. [Google Scholar] [CrossRef] [PubMed]
- Shudofsky, A.M.D.; Giam, C.Z. Cells of adult T-cell leukemia evade HTLV-1 Tax/NF-κB hyperactivation-induced senescence. Blood Adv. 2019, 3, 564–569. [Google Scholar] [CrossRef]
- Ho, Y.K.; Zhi, H.; DeBiaso, D.; Philip, S.; Shih, H.M.; Giam, C.Z. HTLV-1 tax-induced rapid senescence is driven by the transcriptional activity of NF-κB and depends on chronically activated IKKα and p65/RelA. J. Virol. 2012, 86, 9474–9483. [Google Scholar] [CrossRef] [PubMed]
- Zhi, H.; Yang, L.; Kuo, Y.L.; Ho, Y.K.; Shih, H.M.; Giam, C.Z. NF-kappaB hyper-activation by HTLV-1 tax induces cellular senescence, but can be alleviated by the viral anti-sense protein HBZ. PLoS Pathog. 2011, 7, e1002025. [Google Scholar] [CrossRef] [PubMed]
- Uchida, N.; Otsuka, T.; Arima, F.; Shigematsu, H.; Fukuyama, T.; Maeda, M.; Sugio, Y.; Itoh, Y.; Niho, Y. Correlation of telomerase activity with development and progression of adult T-cell leukemia. Leuk. Res. 1999, 23, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Kubuki, Y.; Suzuki, M.; Sasaki, H.; Toyama, T.; Yamashita, K.; Maeda, K.; Ido, A.; Matsuoka, H.; Okayama, A.; Nakanishi, T.; et al. Telomerase activity and telomere length as prognostic factors of adult T-cell leukemia. Leuk. Lymphoma 2005, 46, 393–399. [Google Scholar] [CrossRef]
- Sinha-Datta, U.; Horikawa, I.; Michishita, E.; Datta, A.; Sigler-Nicot, J.C.; Brown, M.; Kazanji, M.; Barrett, J.C.; Nicot, C. Transcriptional activation of hTERT through the NF-κB pathway in HTLV-I-transformed cells. Blood 2004, 104, 2523–2531. [Google Scholar] [CrossRef] [PubMed]
- Furuta, R.; Yasunaga, J.I.; Miura, M.; Sugata, K.; Saito, A.; Akari, H.; Ueno, T.; Takenouchi, N.; Fujisawa, J.I.; Koh, K.R.; et al. Human T-cell leukemia virus type 1 infects multiple lineage hematopoietic cells in vivo. PLoS Pathog. 2017, 13, e1006722. [Google Scholar] [CrossRef] [PubMed]
- Marcais, A.; Suarez, F.; Sibon, D.; Frenzel, L.; Hermine, O.; Bazarbachi, A. Therapeutic options for adult T-cell leukemia/lymphoma. Curr. Oncol. Rep. 2013, 15, 457–464. [Google Scholar] [CrossRef] [PubMed]
- El Hajj, H.; Khalil, B.; Ghandour, B.; Nasr, R.; Shahine, S.; Ghantous, A.; Abdel-Samad, R.; Sinjab, A.; Hasegawa, H.; Jabbour, M.; et al. Preclinical efficacy of the synthetic retinoid ST1926 for treating adult T-cell leukemia/lymphoma. Blood 2014, 124, 2072–2080. [Google Scholar] [CrossRef] [PubMed]
- Dassouki, Z.; Sahin, U.; El Hajj, H.; Jollivet, F.; Kfoury, Y.; Lallemand-Breitenbach, V.; Hermine, O.; de The, H.; Bazarbachi, A. ATL response to arsenic/interferon therapy is triggered by SUMO/PML/RNF4-dependent Tax degradation. Blood 2015, 125, 474–482. [Google Scholar] [CrossRef]
- Kozako, T.; Arima, N.; Toji, S.; Masamoto, I.; Akimoto, M.; Hamada, H.; Che, X.F.; Fujiwara, H.; Matsushita, K.; Tokunaga, M.; et al. Reduced frequency, diversity, and function of human T cell leukemia virus type 1-specific CD8+ T cell in adult T cell leukemia patients. J. Immunol. 2006, 177, 5718–5726. [Google Scholar] [CrossRef]
- Sabouri, A.H.; Usuku, K.; Hayashi, D.; Izumo, S.; Ohara, Y.; Osame, M.; Saito, M. Impaired function of human T-lymphotropic virus type 1 (HTLV-1)-specific CD8+ T cells in HTLV-1-associated neurologic disease. Blood 2008, 112, 2411–2420. [Google Scholar] [CrossRef]
- Marino-Merlo, F.; Grelli, S.; Mastino, A.; Lai, M.; Ferrari, P.; Nicolini, A.; Pistello, M.; Macchi, B. Human T-Cell Leukemia Virus Type 1 Oncogenesis between Active Expression and Latency: A Possible Source for the Development of Therapeutic Targets. Int. J. Mol. Sci. 2023, 24, 14807. [Google Scholar] [CrossRef]
- Ohashi, T.; Hanabuchi, S.; Suzuki, R.; Kato, H.; Masuda, T.; Kannagi, M. Correlation of major histocompatibility complex class I downregulation with resistance of human T-cell leukemia virus type 1-infected T cells to cytotoxic T-lymphocyte killing in a rat model. J. Virol. 2002, 76, 7010–7019. [Google Scholar] [CrossRef]
- Thomson, B.J. Viruses and apoptosis. Int. J. Exp. Pathol. 2001, 82, 65–76. [Google Scholar] [CrossRef]
- Cassens, S.; Ulrich, U.; Beimling, P.; Simon, D. Inhibition of human T cell leukaemia virus type I long terminal repeat expression by DNA methylation: Implications for latency. J. Gen. Virol. 1994, 75 Pt 11, 3255–3259. [Google Scholar] [CrossRef] [PubMed]
- Nicot, C.; Dundr, M.; Johnson, J.M.; Fullen, J.R.; Alonzo, N.; Fukumoto, R.; Princler, G.L.; Derse, D.; Misteli, T.; Franchini, G. HTLV-1-encoded p30II is a post-transcriptional negative regulator of viral replication. Nat. Med. 2004, 10, 197–201. [Google Scholar] [CrossRef]
- Ko, N.L.; Taylor, J.M.; Bellon, M.; Bai, X.T.; Shevtsov, S.P.; Dundr, M.; Nicot, C. PA28γ is a novel corepressor of HTLV-1 replication and controls viral latency. Blood 2013, 121, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Andresen, V.; Pise-Masison, C.A.; Sinha-Datta, U.; Bellon, M.; Valeri, V.; Washington Parks, R.; Cecchinato, V.; Fukumoto, R.; Nicot, C.; Franchini, G. Suppression of HTLV-1 replication by Tax-mediated rerouting of the p13 viral protein to nuclear speckles. Blood 2011, 118, 1549–1559. [Google Scholar] [CrossRef]
- Akkouche, A.; Moodad, S.; Hleihel, R.; Skayneh, H.; Chambeyron, S.; El Hajj, H.; Bazarbachi, A. In vivo antagonistic role of the Human T-Cell Leukemia Virus Type 1 regulatory proteins Tax and HBZ. PLoS Pathog. 2021, 17, e1009219. [Google Scholar] [CrossRef] [PubMed]
- Lemasson, I.; Lewis, M.R.; Polakowski, N.; Hivin, P.; Cavanagh, M.H.; Thebault, S.; Barbeau, B.; Nyborg, J.K.; Mesnard, J.M. Human T-cell leukemia virus type 1 (HTLV-1) bZIP protein interacts with the cellular transcription factor CREB to inhibit HTLV-1 transcription. J. Virol. 2007, 81, 1543–1553. [Google Scholar] [CrossRef]
- Wiley, C.D. Bubble Bubble, Senescent Cells Are a Cauldron of Tumor Trouble. Cancer Res. 2020, 80, 3193–3194. [Google Scholar] [CrossRef]
- Wiley, C.D. Role of Senescent Renal Cells in Pathophysiology of Diabetic Kidney Disease. Curr. Diab. Rep. 2020, 20, 33. [Google Scholar] [CrossRef]
| Effects of Tax | Mechanism of Activation | |
|---|---|---|
| Cytokines | ||
| IL-1α | activated | NF-κB |
| IL-2 | activated | NF-κB/NF-AT |
| IL-4 | activated | NF-κB/NF-AT/NF-IL6 |
| IL-6 | activated | NF-κB |
| IL-8 | activated | NF-κB |
| IL-9 | activated | NF-κB/IRF4 |
| IL-10 | activated | NF-κB |
| IL-12 | activated | NF-κB |
| IL-13 | activated | NF-AT/GATA3 |
| IL-15 | activated | NF-κB |
| IL-17 | activated | CREB/ATF |
| IL-21 | activated | NF-κB/AP1 |
| Cytokine Receptors | ||
| IL-2R | activated | NF-κB/NF-AT |
| IL-6R | activated | ? |
| IL-15R | activated | NF-κB/IRF-4 |
| IL-17R | activated | ? |
| IL-21R | activated | AP1/IRF |
| Chemokines/Ligands | ||
| IFN-γ | activated | IL-12 |
| TNF-α | activated | NF-κB |
| GM-CSF | activated | NF-κB/CREB/ATF/NF-IL6 |
| MIP-1α/CCL3 | activated | NF-κB |
| MIP-1β/CCL4 | activated | NF-κB |
| MIP-3 | activated | NF-κB |
| MCP-1 | activated | NF-κB |
| CCL5/RANTES | activated | NF-κB |
| CCL1 | activated | ? |
| CCL22 | activated | ? |
| CXCR7 | activated | NF-κB |
| CCR9 | activated | ? |
| Apoptosis Regulators | ||
| Bcl-xL | activated | NF-κB/CREB/ATF |
| Survivin | activated | NF-κB |
| Bfl-1 | activated | NF-κB/cJun/JunD |
| MCL-1 | activated | TRAF6 |
| c-FLIP | activated | NF-κB |
| Bim | suppressed | HIF-1α |
| Bid | suppressed | HIF-1α |
| Bax | suppressed | TP53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellon, M.; Nicot, C. HTLV-1 Tax Tug-of-War: Cellular Senescence and Death or Cellular Transformation. Pathogens 2024, 13, 87. https://doi.org/10.3390/pathogens13010087
Bellon M, Nicot C. HTLV-1 Tax Tug-of-War: Cellular Senescence and Death or Cellular Transformation. Pathogens. 2024; 13(1):87. https://doi.org/10.3390/pathogens13010087
Chicago/Turabian StyleBellon, Marcia, and Christophe Nicot. 2024. "HTLV-1 Tax Tug-of-War: Cellular Senescence and Death or Cellular Transformation" Pathogens 13, no. 1: 87. https://doi.org/10.3390/pathogens13010087
APA StyleBellon, M., & Nicot, C. (2024). HTLV-1 Tax Tug-of-War: Cellular Senescence and Death or Cellular Transformation. Pathogens, 13(1), 87. https://doi.org/10.3390/pathogens13010087






