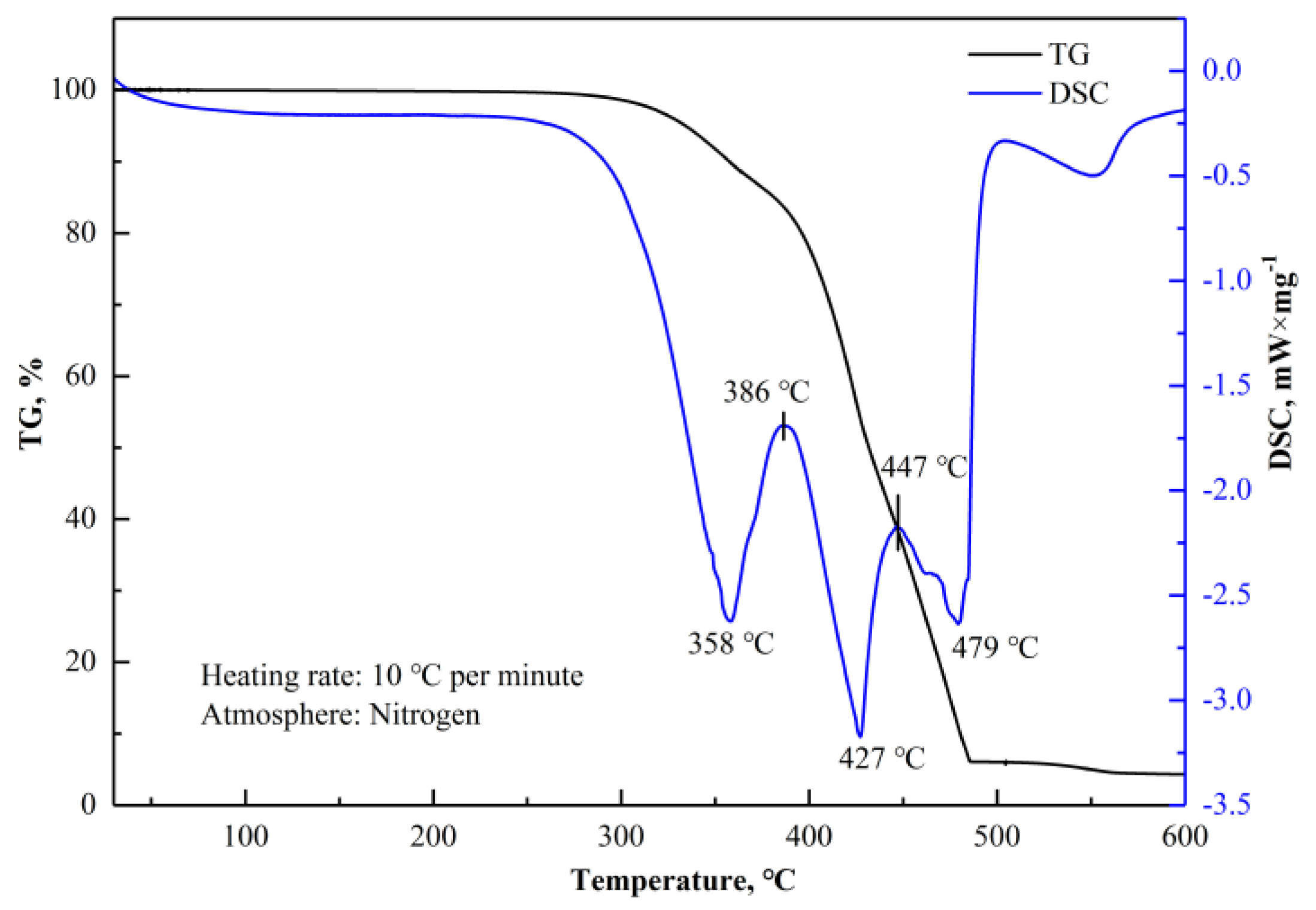
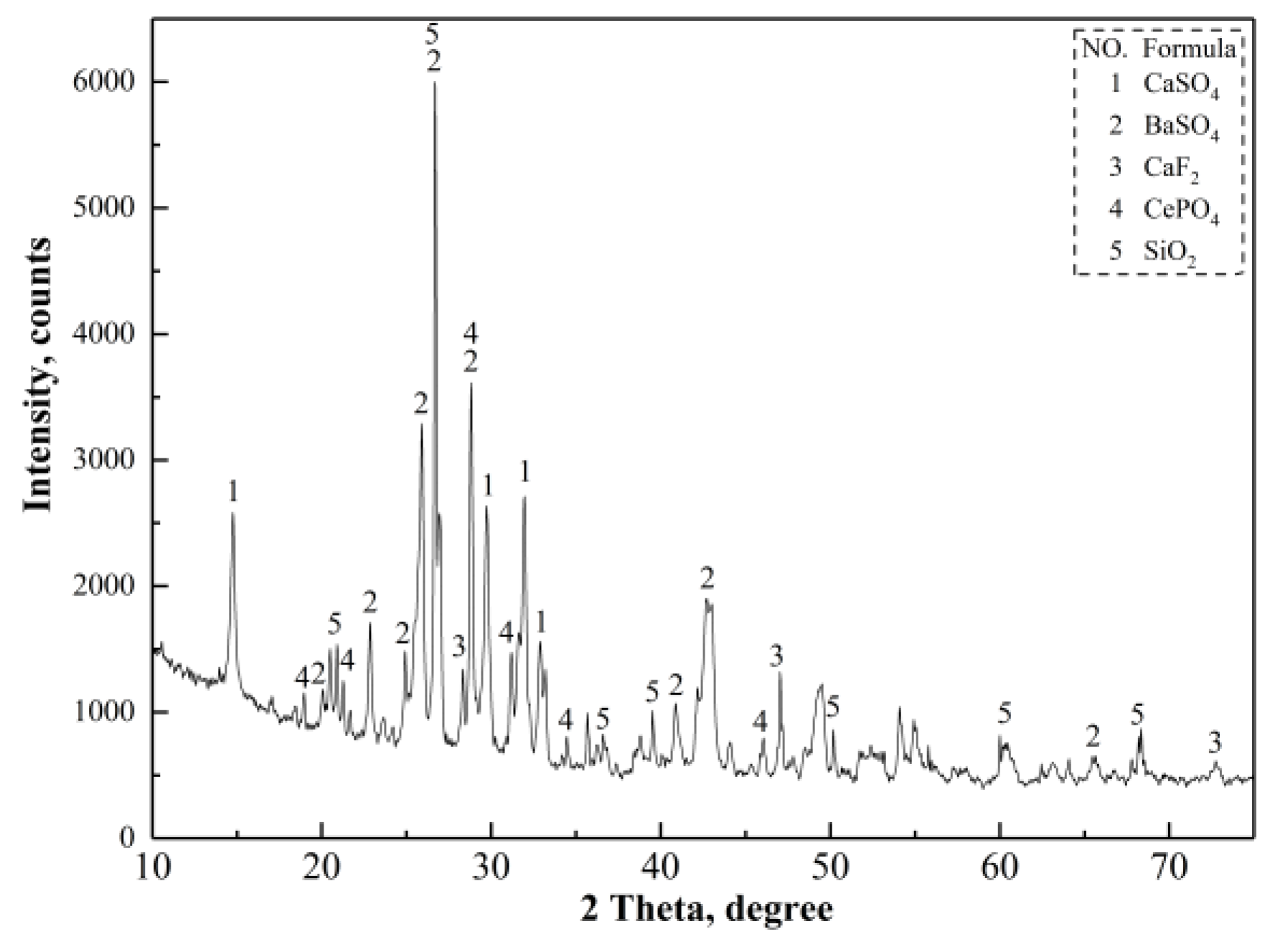
Figure 12 shows the results of thermogravimetry (TG) and differential scanning calorimetry (DSC) analysis results for (NH
4)
2SO
4. The analytical equipment used was the SDT 2960 Simultaneous DSC-TGA manufactured by TA Instruments (New Castle,Delaware, DE, USA). The TG–DSC curve of (NH
4)
2SO
4 indicated thermal decomposition between 200–500 °C, with the process expressed using Equations (14)–(19) [
16,
17,
18]. Obviously, the thermal decomposition of (NH
4)
2SO
4 is a complex process. Thermal decomposition reactions can be divided into three steps [
20,
21], as shown in
Table 6. The intermediate products during thermal decomposition, NH
4HSO
4, (NH
4)
3H(SO
4)
2, NH
2SO
3H, and (NH
4)
2S
2O
7, are known as acidifying agents. These acidifying agents perform better under "acidification" than the aqueous solution of (NH
4)
2SO
4. Thus, rare earth oxides can be decomposed into rare earth ions by acidifying agents within the temperature range of 200–500 °C.
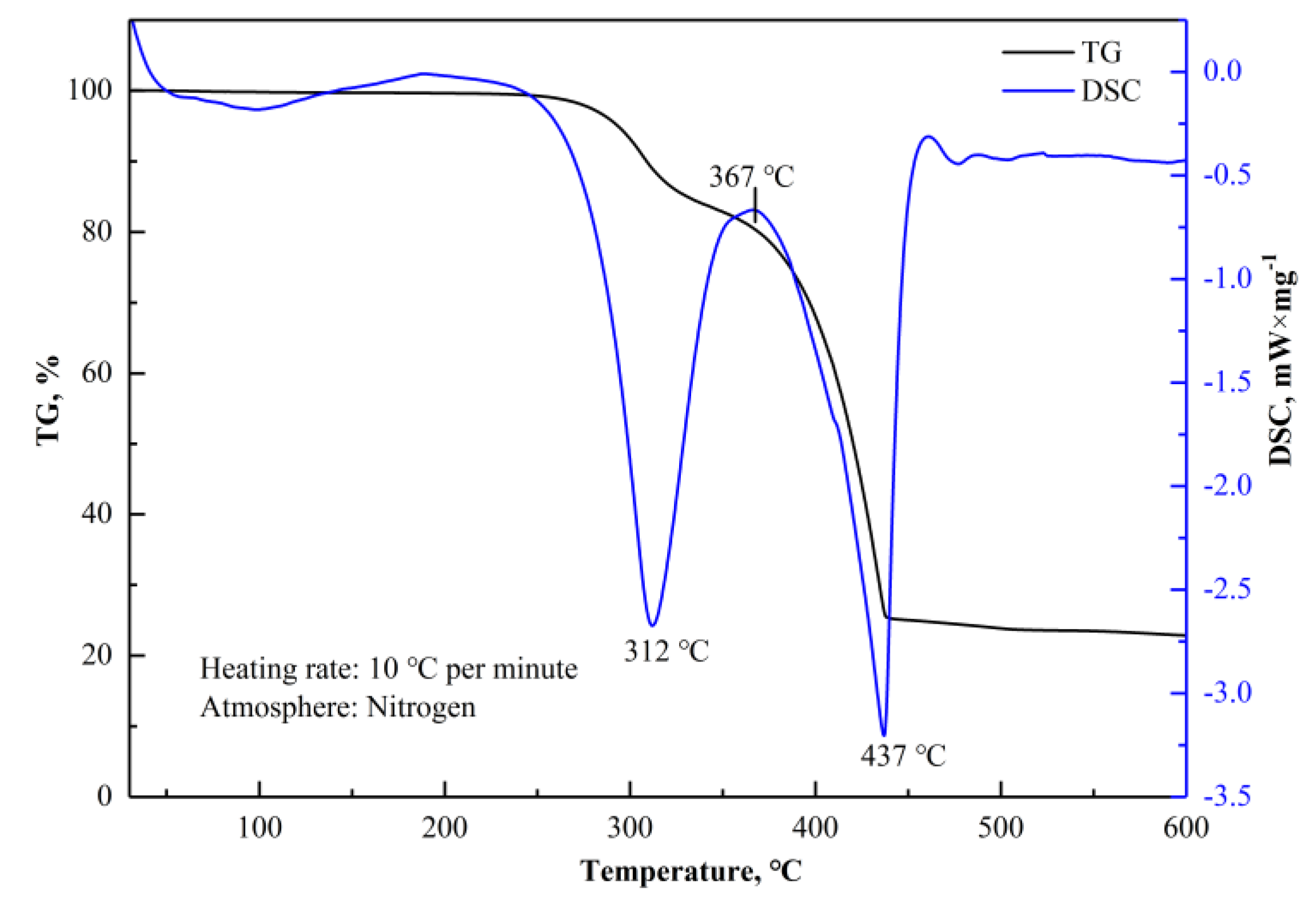
Figure 13 shows the TG–DSC curve of the mixture of the magnetic separation tailings and (NH
4)
2SO
4 in a mass ratio of 4:1. In contrast to the TG–DSC curve of (NH
4)
2SO
4 shown in
Figure 12, the number of the endothermic peaks and the peak temperature of the endothermic peaks changed. The peak at 312 °C indicated the occurrence of thermal decomposition of (NH
4)
2SO
4 and the formation of NH
4RE(SO
4)
2·4H
2O. The peak at 437 °C indicated the thermal decomposition of NH
4RE(SO
4)
2·4H
2O and residual acidifying agents.
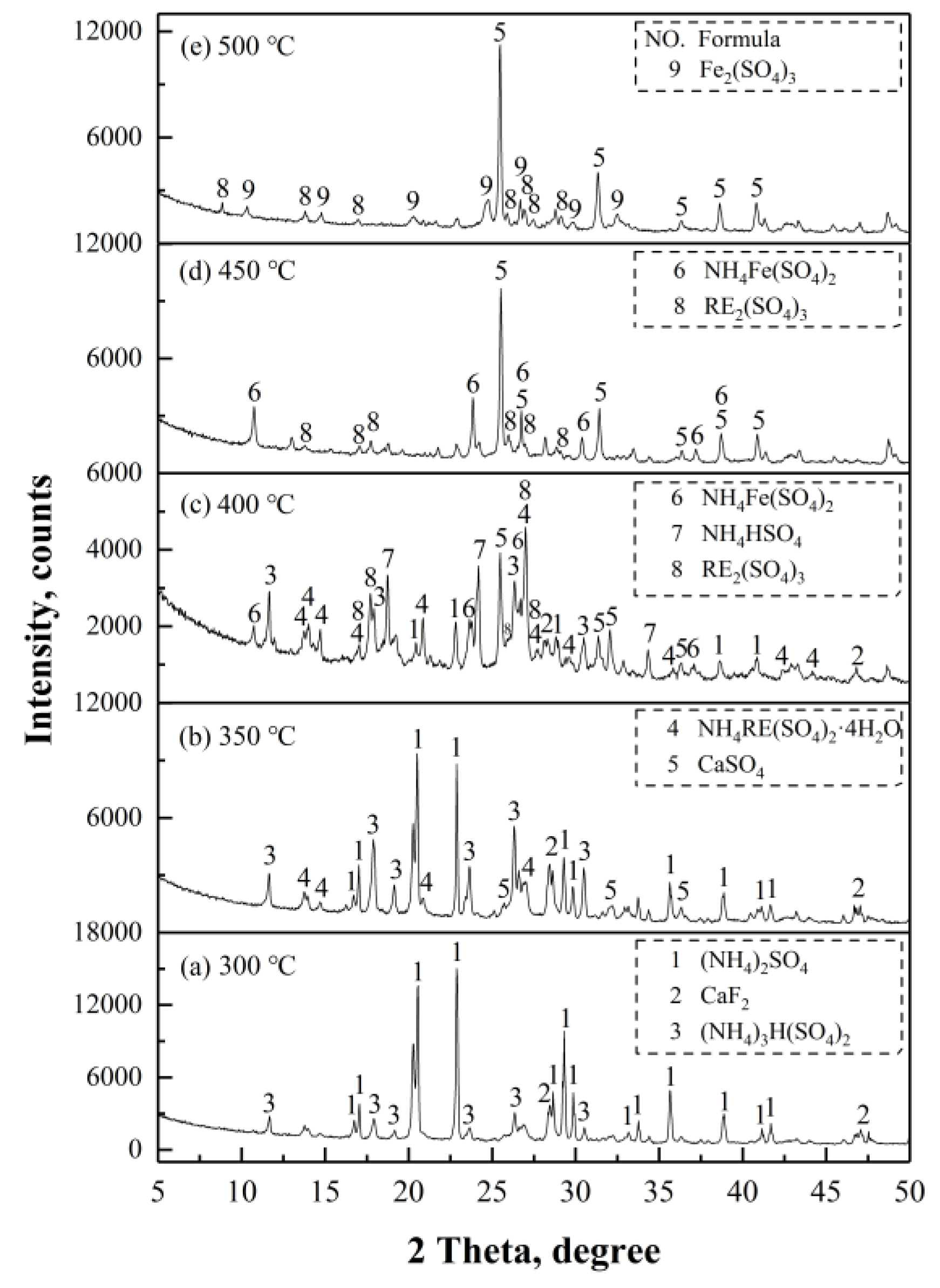
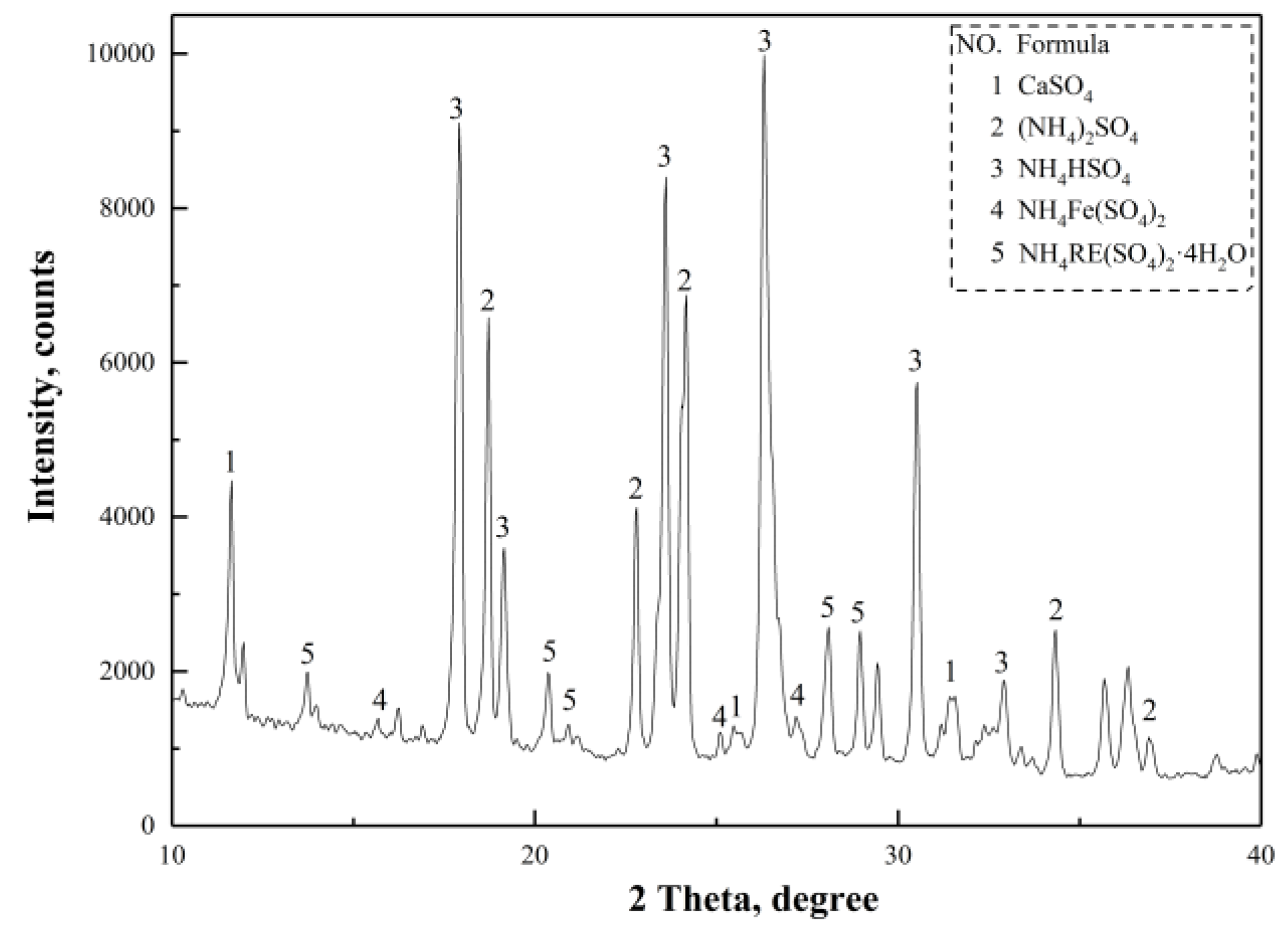
(NH
4)
3H(SO
4)
2 was the only product observed in
Figure 14a, indicating that the dominant reaction was Equation (15), when the roasting temperature reached 300 °C. As for the increase of temperature, the reactions of Equations (20)–(25), (31), and (32) occurred at 350 °C, corresponding to the first endothermic peak in
Figure 13, with the products of CaSO
4 and NH
4RE(SO
4)
2·4H
2O as shown in
Figure 14b. The formation of NH
4Fe(SO
4)
2, NH
4HSO
4 and RE
2(SO
4)
3 at 400 °C, shown in
Figure 14c, implied that NH
4RE(SO
4)
2·4H
2O and (NH
4)
2SO
4 continued to decompose, and ferruginous minerals (residual Fe
2O
3 and Fe
3O
4, NaFeSi
2O
6, FeS) reacted with the acidifying agents. The characteristic peaks of (NH
4)
2SO
4, NH
4HSO
4 and NH
4RE(SO
4)
2∙4H
2O disappeared at 450 °C, as shown in
Figure 14d, which were attributed to the complete decomposition of NH
4RE(SO
4)
2·4H
2O, (NH
4)
2SO
4, and the residual acidifying agents, corresponding to the second endothermic peak in
Figure 13. When the temperature reached 500 °C, NH
4Fe(SO
4)
2 completely decomposed into Fe
2(SO
4)
3, as shown in
Figure 14e. The reactions between the magnetic separation tailings and (NH
4)
2SO
4 which may occur at roasting temperatures from 200 to 500 °C, are expressed with Equations (20)–(32). In addition, the reaction (Equation (33)) between CeO
2 and (NH
4)
2SO
4 at 250 °C was also included, which is confirmed by Mudher K.D.S et al. [
13].
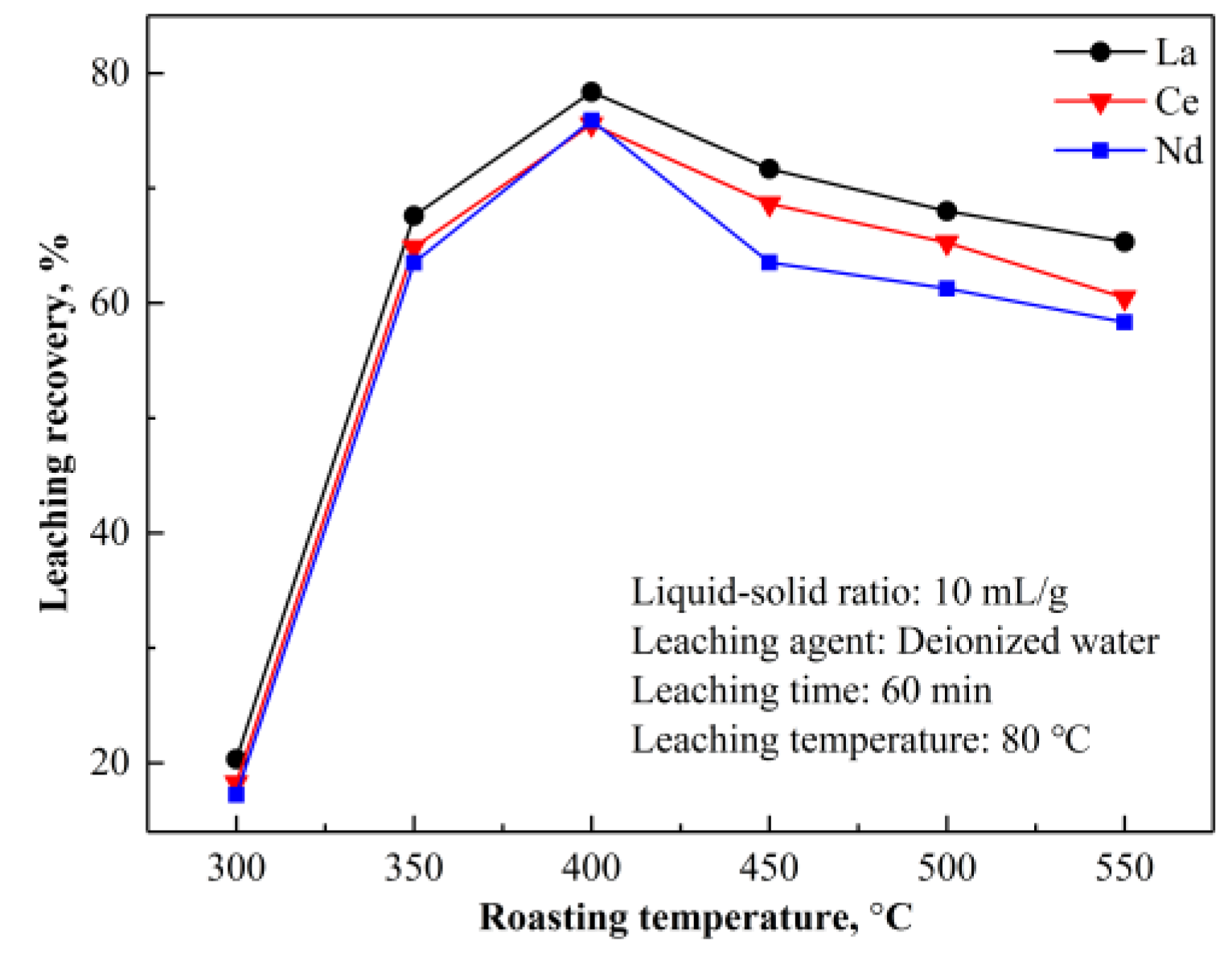
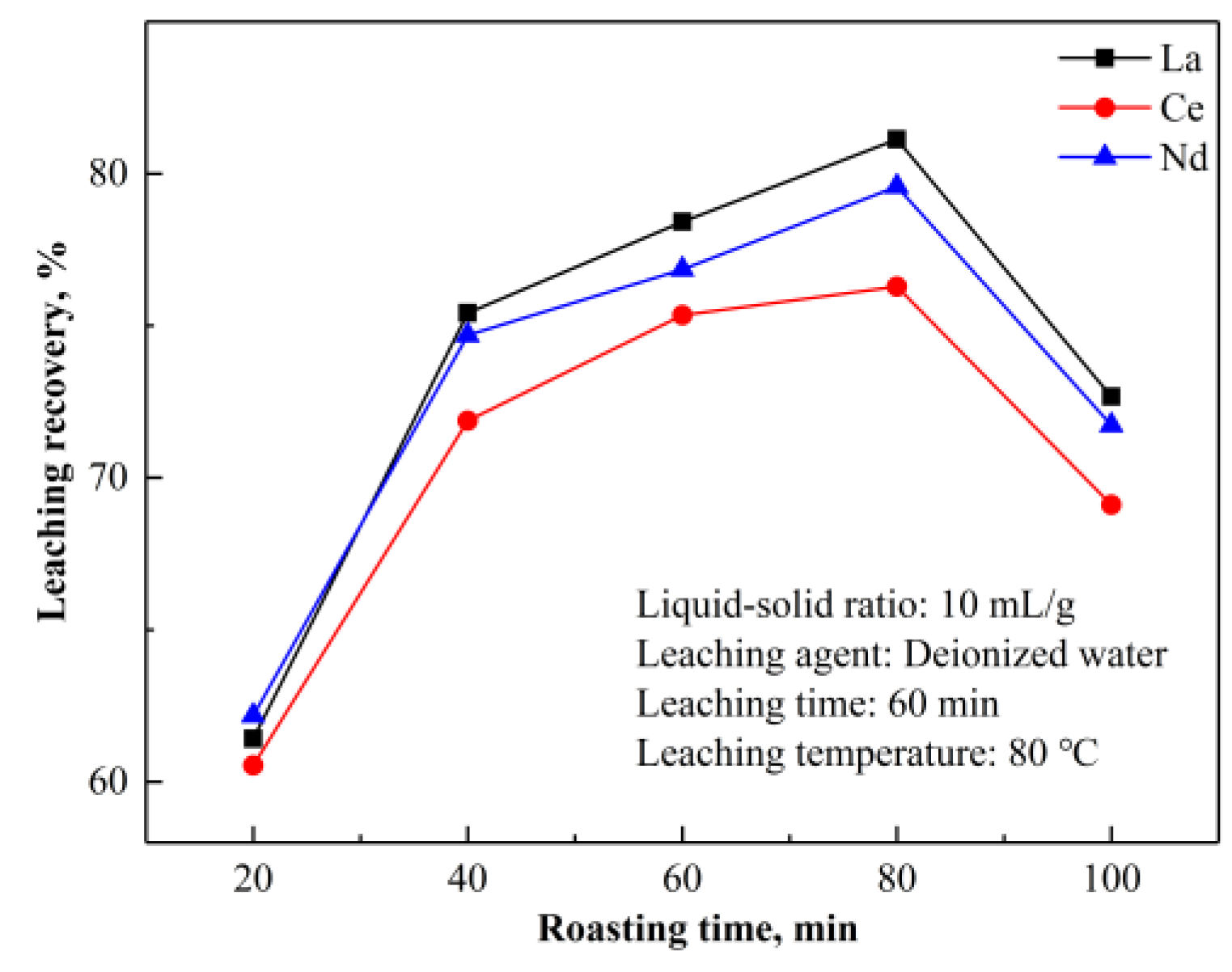
NH
4RE(SO
4)
2·4H
2O and RE
2(SO
4)
3 are soluble in water, providing a feasible way to extract REEs by water leaching. According to the above analysis and TG-DSC curves in
Figure 12 and
Figure 13, the roasting temperature should be controlled below 400 °C to avoid rapid decomposition and mass loss of (NH
4)
2SO
4, which is detrimental to the formation of NH
4RE(SO
4)
2·4H
2O.
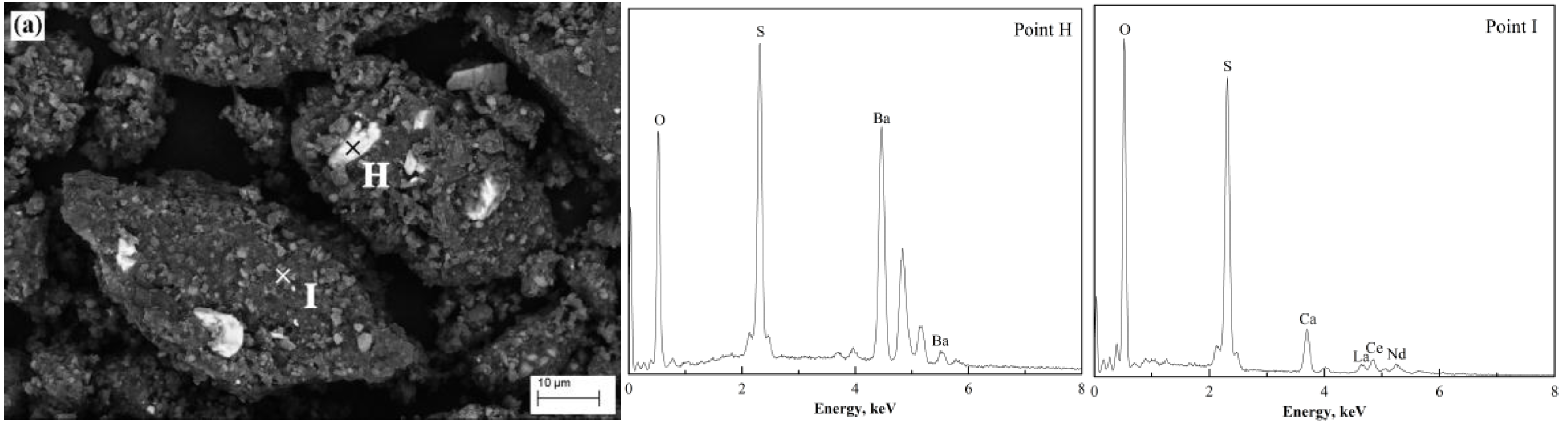
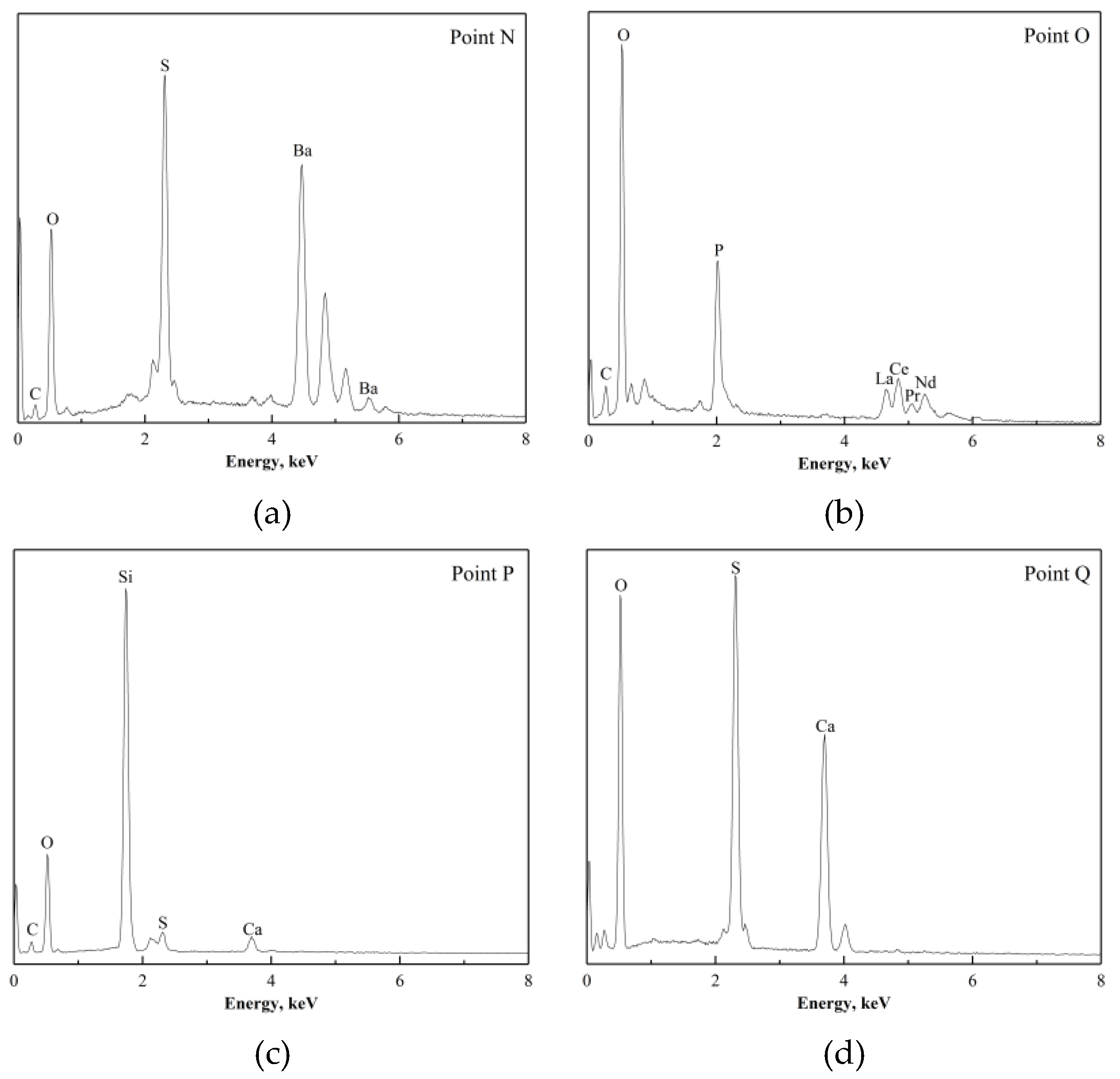
The morphology of the products after (NH
4)
2SO
4 activation roasting at various temperatures was examined by SEM-EDS.
Figure 15a shows the particles roasted at 400 °C. The particles were covered with sulfates (NH
4RE(SO
4)
2·4H
2O, CaSO
4, (NH
4)
2SO
4, etc.) as indicated by the letter I in
Figure 15a. The white phase (marked as “H” in
Figure 15a) on the surface of particles was identified as BaSO
4.
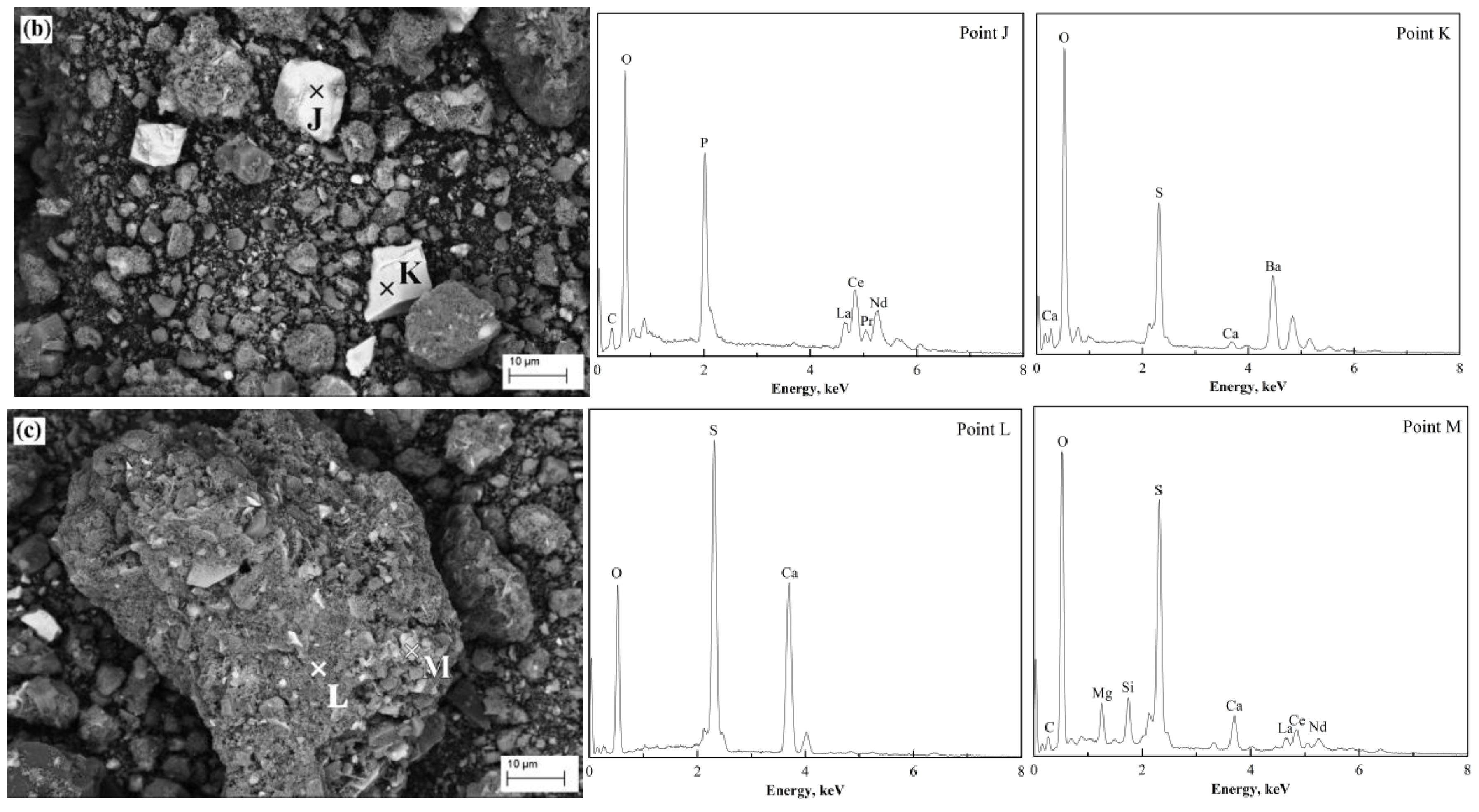
The micrograph of roasting products at 500 °C shows a non-uniform distribution in particle size, as shown in
Figure 15b,c. The bright phases (marked as “J” and “K”) were REPO
4 and BaSO
4 respectively, indicating that they hardly reacted with (NH
4)
2SO
4 or the acidifying agents. The larger particle in
Figure 15c consisted of CaSO
4 (marked as “L”) and other sulfates (marked as “M”) such as RE
2(SO
4)
3 and MgSO
4. The morphology shown in
Figure 15c was different from the one shown in
Figure 15a, because there was no residual (NH
4)
2SO
4 on the surface, after roasting at 500 °C.