Corrosion and Discharge Behaviors of Mg-Al-Zn and Mg-Al-Zn-In Alloys as Anode Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microstructure Characterization
2.3. Hydrogen Collection
2.4. Electrochemical Measurements
3. Results and Discussion
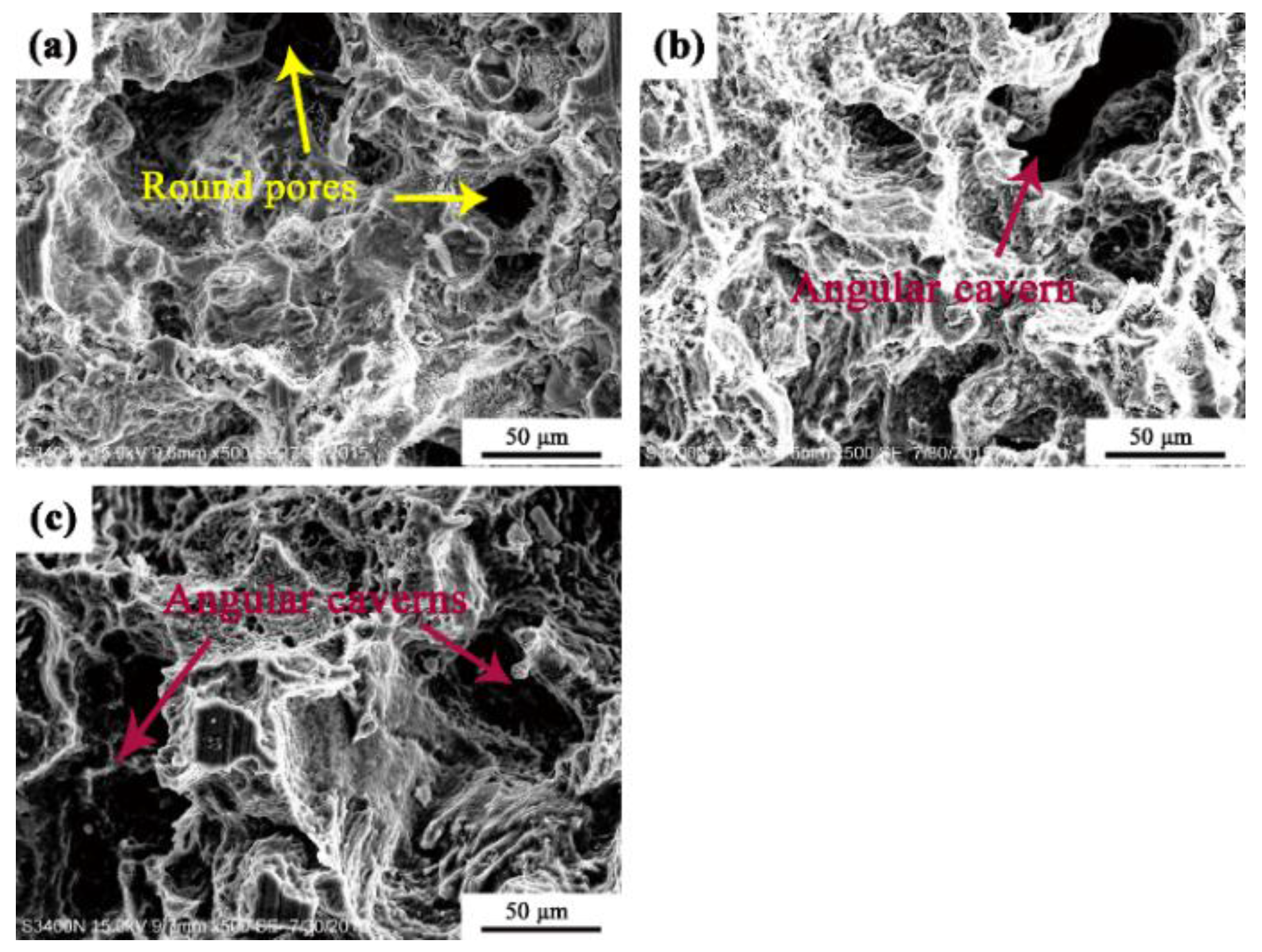
3.1. Microstructure
3.2. Hydrogen Collection
3.3. Open Circuit Potential
3.4. Potentiodynamic Polarization
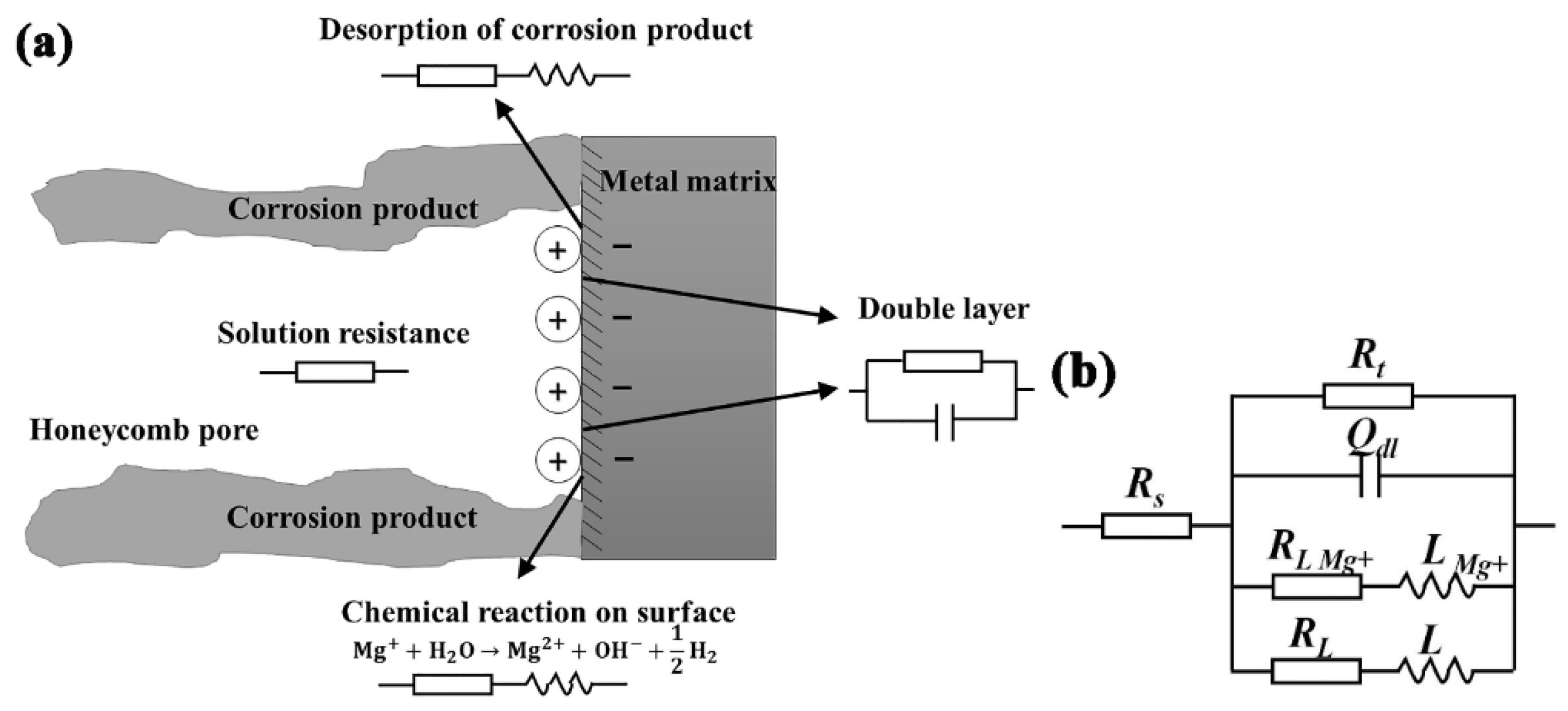
3.5. Electrochemical Impedance Spectroscopy (EIS)
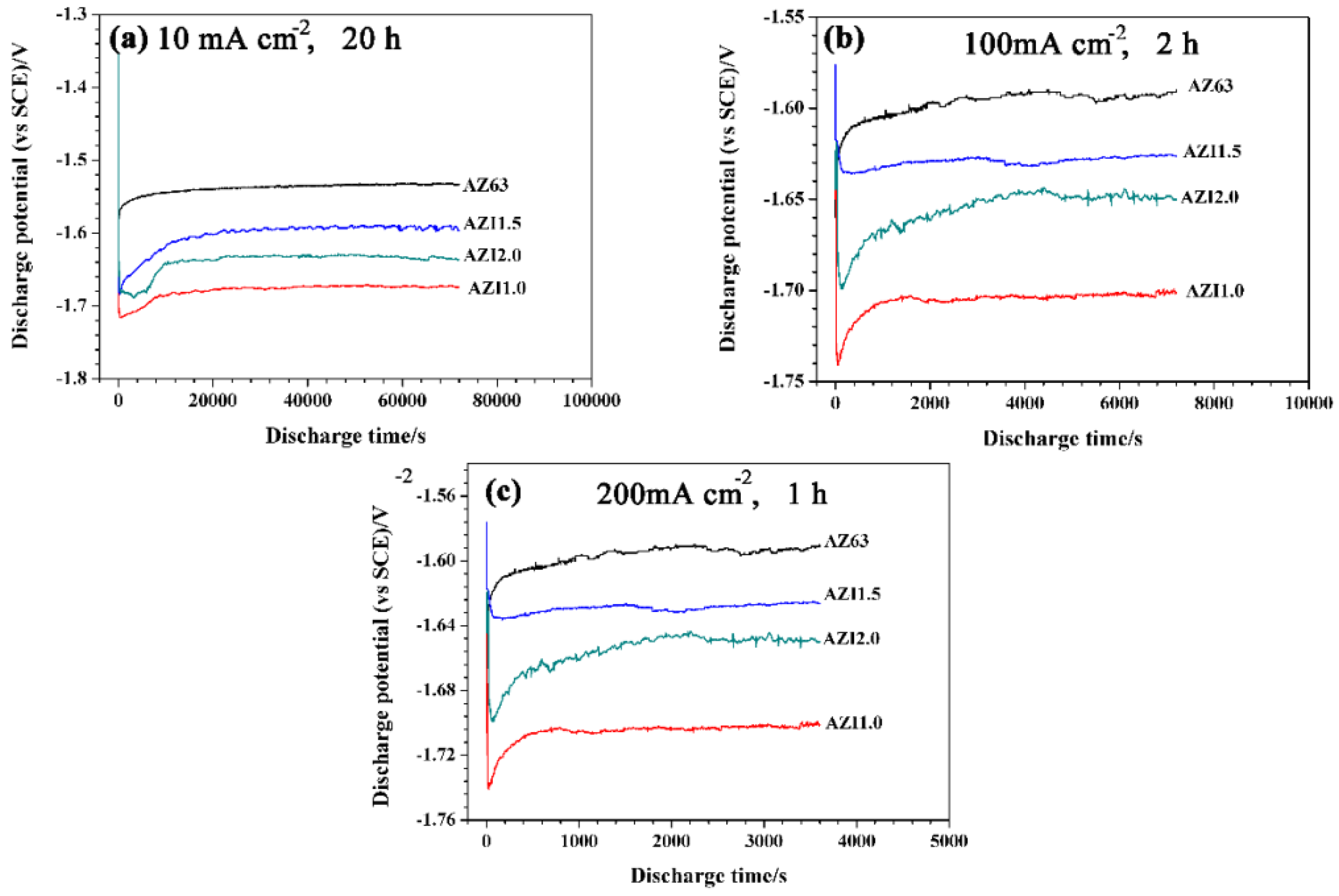
3.6. Galvanostatic Discharge
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Song, G.L.; Shi, Z. Corrosion mechanism and evaluation of anodized magnesium alloys. Corros. Sci. 2014, 85, 126–140. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.X.; Wang, R.C.; Peng, C.Q.; Shi, K.; Feng, Y. Effect of indium addition on corrosion of AP65 magnesium alloy. J. Cent. South. Univ. 2012, 19, 2086–2093. [Google Scholar] [CrossRef]
- Mostafa, A.; Medraj, M. Experimental investigation of the Mg-Nd-Zn isothermal section at 300 °C. Metals 2015, 5, 84–101. [Google Scholar] [CrossRef]
- Pardo, A.; Merino, M.C.; Arrabal, R.; Casajus, P.; Mohedano, M.; Feliu, S., Jr.; Merino, S. Corrosion behavior of AZ magnesium alloys with Al and Al-11Si thermal spray coatings. Corrosion 2011, 67. [Google Scholar] [CrossRef]
- Grubač, Z.; Rončević, I.Š.; Metikoš-Huković, M. Corrosion properties of the Mg alloy coated with polypyrrole films. Corros. Sci. 2016, 102, 310–316. [Google Scholar] [CrossRef]
- Li, X.; Weng, Z.; Yuan, W.; Luo, X.; Wong, H.M.; Liu, X.; Wu, S.; Yeung, K.W.K.; Zheng, Y.; Chu, P.K. Corrosion resistance of dicalcium phosphate dihydrate/poly(lactic-co-glycolic acid) hybrid coating on AZ31 magnesium alloy. Corros. Sci. 2016, 102, 209–221. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Gnedenkov, S.V. Protective properties of inhibitor-containing composite coatings on a Mg alloy. Corros. Sci. 2016, 102, 348–354. [Google Scholar] [CrossRef]
- Ha, H.Y.; Kang, J.Y.; Yang, J.; Yim, C.D.; You, B.S. Role of Sn in corrosion and passive behavior of extruded Mg-5 wt%Sn alloy. Corros. Sci. 2016, 102, 355–362. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Gnedenkov, S.V. Localized corrosion of the Mg alloys with inhibitor-containing coatings: SVET and SIET studies. Corros. Sci. 2016, 102, 269–278. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, C.; Song, L.; Zeng, R.; Li, S.; Cui, H. Fabrication of the superhydrophobic surface on magnesium alloy and its corrosion resistance. J. Mater. Sci. Technol. 2015, 31, 1139–1143. [Google Scholar] [CrossRef]
- Kotoka, R.; Yarmolenko, S.; Pai, D.; Sankar, J. Corrosion behavior of reactive sputtered Al2O3 and ZrO2 thin films on Mg disk immersed in saline solution. J. Mater. Sci. Technol. 2015, 31, 873–880. [Google Scholar] [CrossRef]
- Tekumalla, S.; Seetharaman, S.; Almajid, A.; Gupta, M. Mechanical properties of magnesium-rare earth alloy systems: A review. Metals 2015, 5, 1–39. [Google Scholar] [CrossRef]
- Besenhard, J.O.; Winter, M. Advances in battery technology: Rechargeable magnesium batteries and novel negative-electrode materials for lithium ion batteries. Chemphyschem 2002, 3, 155–159. [Google Scholar] [CrossRef]
- Shinohara, M.; Araki, E.; Mochizuki, M.; Kanazawa, T.; Suyehiro, K. Practical application of a sea-water battery in deep-sea basin and its performance. J. Power Sources 2009, 187, 253–260. [Google Scholar] [CrossRef]
- Hasvold, O.; Lian, T.; Haakaas, E.; Storkersen, N.; Perelman, O.; Cordier, S. Clipper: A long-range, autonomous underwater vehicle using magnesium fuel and oxygen from the sea. J. Power Sources 2004, 136, 232–239. [Google Scholar] [CrossRef]
- Johnston, S.; Shi, Z.; Atrens, A. The influence of pH on the corrosion rate of high-purity Mg, AZ91 and ZE41 in bicarbonate buffered hanks’ solution. Corros. Sci. 2015, 101, 182–192. [Google Scholar] [CrossRef]
- Pathak, S.S.; Mendon, S.K.; Blanton, M.D.; Rawlins, J.W. Magnesium-based sacrificial anode cathodic protection coatings (Mg-rich primers) for aluminum alloys. Metals 2012, 2, 353–376. [Google Scholar] [CrossRef]
- Khoo, T.; Somers, A.; Torriero, A.A.J.; MacFarlane, D.R.; Howlett, P.C.; Forsyth, M. Discharge behaviour and interfacial properties of a magnesium battery incorporating trihexyl(tetradecyl)phosphonium based ionic liquid electrolytes. Electrochim. Acta 2013, 87, 701–708. [Google Scholar] [CrossRef]
- Cao, D.; Wu, L.; Wang, G.; Lv, Y. Electrochemical oxidation behavior of Mg-Li-Al-Ce-Zn and Mg-Li-Al-Ce-Zn-Mn in sodium chloride solution. J. Power Sources 2008, 183, 799–804. [Google Scholar] [CrossRef]
- Huang, D.B.; Hu, J.Y.; Song, G.L.; Guo, X.P. Self-corrosion, galvanic corrosion and inhibition of GW103 and AZ91D Mg alloys in ethylene glycol solution. Corros. Eng. Sci. Techn. 2013, 48, 155–160. [Google Scholar] [CrossRef]
- Andrei, M.; Di Gabriele, F.; Bonora, P.L.; Scantlebury, D. Corrosion behaviour of magnesium sacrificial anodes in tap water. Mater. Corros. 2003, 54, 5–11. [Google Scholar] [CrossRef]
- Wang, N.G.; Wang, R.C.; Peng, C.Q.; Hu, C.W.; Yan, F.; Bing, P. Research progress of magnesium anodes and their applications in chemical power sources. Trans. Nonferr. Met. Soc. China 2014, 24, 2427–2439. [Google Scholar] [CrossRef]
- Tahreen, N.; Zhang, D.F.; Pan, F.S.; Jiang, X.Q.; Li, D.Y.; Chen, D.L. Hot deformation and work hardening behavior of an extruded Mg-Zn-Mn-Y alloy. J. Mater. Sci. Technol. 2015, 31, 1161–1170. [Google Scholar] [CrossRef]
- Gusieva, K.; Davies, C.H.J.; Scully, J.R.; Birbilis, N. Corrosion of magnesium alloys: The role of alloying. Int. Mater. Rev. 2015, 60, 169–194. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, K.; Hu, Y.; Li, S.; Tan, X.; Chen, F.; Yu, Z. Discharge behavior of Mg-4 wt%Ga-2 wt%Hg alloy as anode for seawater activated battery. Electrochim. Acta 2011, 56, 8224–8231. [Google Scholar] [CrossRef]
- Wang, P.; Li, J.; Guo, Y.; Yang, Z.; Xia, F.; Wang, J. Effect of Sn on microstructure and electrochemical properties of Mg alloy anode materials. Rare Met. Mater. Eng. 2012, 41, 2095–2099. [Google Scholar]
- Song, G.L.; Atrens, A. Corrosion mechanisms of magnesium alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Huang, S.; Wei, Y.; Xi, X.; Cai, K.; Pan, F. Effects of Y on the microstructure, mechanical and bio-corrosion properties of Mg-Zn-Ca bulk metallic glass. J. Mater. Sci. Technol. 2014, 30, 1255–1261. [Google Scholar] [CrossRef]
- Anik, M.; Guneşdoğdu, I.M. Corrosion characteristics of alloy AZ63 in buffered neutral solutions. Mater. Des. 2010, 31, 3100–3105. [Google Scholar] [CrossRef]
- Altun, H.; Sen, S. Studies on the influence of chloride ion concentration and pH on the corrosion and electrochemical behaviour of AZ63 magnesium alloy. Mater. Des. 2004, 25, 637–643. [Google Scholar] [CrossRef]
- Zazoua, A.; Azzouz, N. An investigation on the use of indium to increase dissolution of AlZn anodes in sea water. Mater. Des. 2008, 29, 806–810. [Google Scholar] [CrossRef]
- Wang, N.; Wang, R.; Peng, C.; Peng, B.; Feng, Y.; Hu, C. Discharge behaviour of Mg-Al-Pb and Mg-Al-Pb-In alloys as anodes for Mg-air battery. Electrochim. Acta 2014, 149, 193–205. [Google Scholar] [CrossRef]
- Abidin, N.I.Z.; Atrens, A.D.; Martin, D.; Atrens, A. Corrosion of high purity Mg, Mg2Zn0.2Mn, ZE41 and AZ91 in Hank's solution at 37 °C. Corros. Sci. 2011, 53, 3542–3556. [Google Scholar] [CrossRef]
- Atrens, A.; Liu, M.; Abidin, N.I.Z. Corrosion mechanism applicable to biodegradable magnesium implants. Mater. Sci. Eng. B 2011, 176, 1609–1636. [Google Scholar] [CrossRef]
- Becerra, A.; Pekguleryuz, M. Effects of zinc, lithium, and indium on the grain size of magnesium. J. Mater. Res. 2009, 24, 1722–1729. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A. Understanding magnesium corrosion—a framework for improved alloy performance. Adv. Eng. Mater. 2003, 5, 837–858. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, T.; Wang, F. Effect of microcrystallization on corrosion resistance of AZ91D alloy. Electrochim. Acta 2006, 51, 2845–2850. [Google Scholar] [CrossRef]
- Song, G.L.; Bowles, A.L.; StJohn, D.H. Corrosion resistance of aged die cast magnesium alloy AZ91D. Mater. Sci. Eng. A 2004, 366, 74–86. [Google Scholar] [CrossRef]
- Liu, W.; Cao, F.; Chang, L.; Zhang, Z.; Zhang, J. Effect of rare earth element Ce and La on corrosion behavior of AM60 magnesium alloy. Corros. Sci. 2009, 51, 1334–1343. [Google Scholar] [CrossRef]
- Zhao, M.C.; Liu, M.; Song, G.L.; Atrens, A. Influence of pH and chloride ion concentration on the corrosion of Mg alloy ZE41. Corros. Sci. 2008, 50, 3168–3178. [Google Scholar] [CrossRef]
- Reboul, M.C.; Gimenez, P.; Rameau, J.J. A proposed activation mechanism for Al anodes. Corrosion 1984, 40, 366–371. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A.; Dargusch, M. Influence of microstructure on the corrosion of diecast AZ91D. Corros. Sci. 1999, 41, 249–273. [Google Scholar] [CrossRef]
- Udhayan, R.; Bhatt, D.P. On the corrosion behaviour of magnesium and its alloys using electrochemical techniques. J. Power Sources 1996, 63, 103–107. [Google Scholar] [CrossRef]
- Zhang, T.; Meng, G.; Shao, Y.; Cui, Z.; Wang, F. Corrosion of hot extrusion AZ91 magnesium alloy. Part П: Effect of rare earth element neodymium (Nd) on the corrosion behavior of extruded alloy. Corros. Sci. 2011, 53, 2934–2942. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Han, E.; Dong, J.; Ke, W. AC impedance spectroscopy study of the corrosion behavior of an AZ91 magnesium alloy in 0.1 M sodium sulfate solution. Electrochim. Acta 2007, 52, 3299–3309. [Google Scholar] [CrossRef]
- Chavarin, J.U. Electrochemical investigations of the activation mechanism of aluminum. Corrosion 1991, 47, 472–479. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, T.; Shao, Y.; Meng, G.; Wang, F. Effect of alternating voltage treatment on the corrosion resistance of pure magnesium. Corros. Sci. 2009, 51, 1772–1779. [Google Scholar] [CrossRef]
- Song, G.L. Effect of tin modification on corrosion of AM70 magnesium alloy. Corros. Sci. 2009, 51, 2063–2070. [Google Scholar] [CrossRef]
- Zhang, T.; Shao, Y.; Meng, G.; Cui, Z.; Wang, F. Corrosion of hot extrusion AZ91 magnesium alloy: I-relation between the microstructure and corrosion behavior. Corros. Sci. 2011, 53, 1960–1968. [Google Scholar] [CrossRef]
- Suresh Kannan, A.R.; Muralidharan, S.; Sarangapani, K.B.; Balaramachandran, V.; Kapali, V. Corrosion and anodic behaviour of zinc and its ternary alloys in alkaline battery electrolytes. J. Power Sources 1995, 57, 93–98. [Google Scholar] [CrossRef]
- Nestoridi, M.; Pletcher, D.; Wood, R.J.K.; Wang, S.; Jones, R.L.; Stokes, K.R.; Wilcock, I. The study of aluminium anodes for high power density Al/air batteries with brine electrolytes. J. Power Sources 2008, 178, 445–455. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, R.C.; Peng, C.Q. Influence of Ga and In on microstructure and electrochemical properties of Mg anodes. Trans. Nonferr. Met. Soc. China 2013, 23, 2650–2656. [Google Scholar] [CrossRef]
- Zhao, H.; Bian, P.; Ju, D. Electrochemical performance of magnesium alloy and its application on the sea water battery. J. Environ. Sci. 2009, 21 (Suppl. 1), S88–S91. [Google Scholar] [CrossRef]
- Bessone, J.B.; Flamini, D.O.; Saidman, S.B. Comprehensive model for the activation mechanism of Al-Zn alloys produced by indium. Corros. Sci. 2005, 47, 95–105. [Google Scholar] [CrossRef]

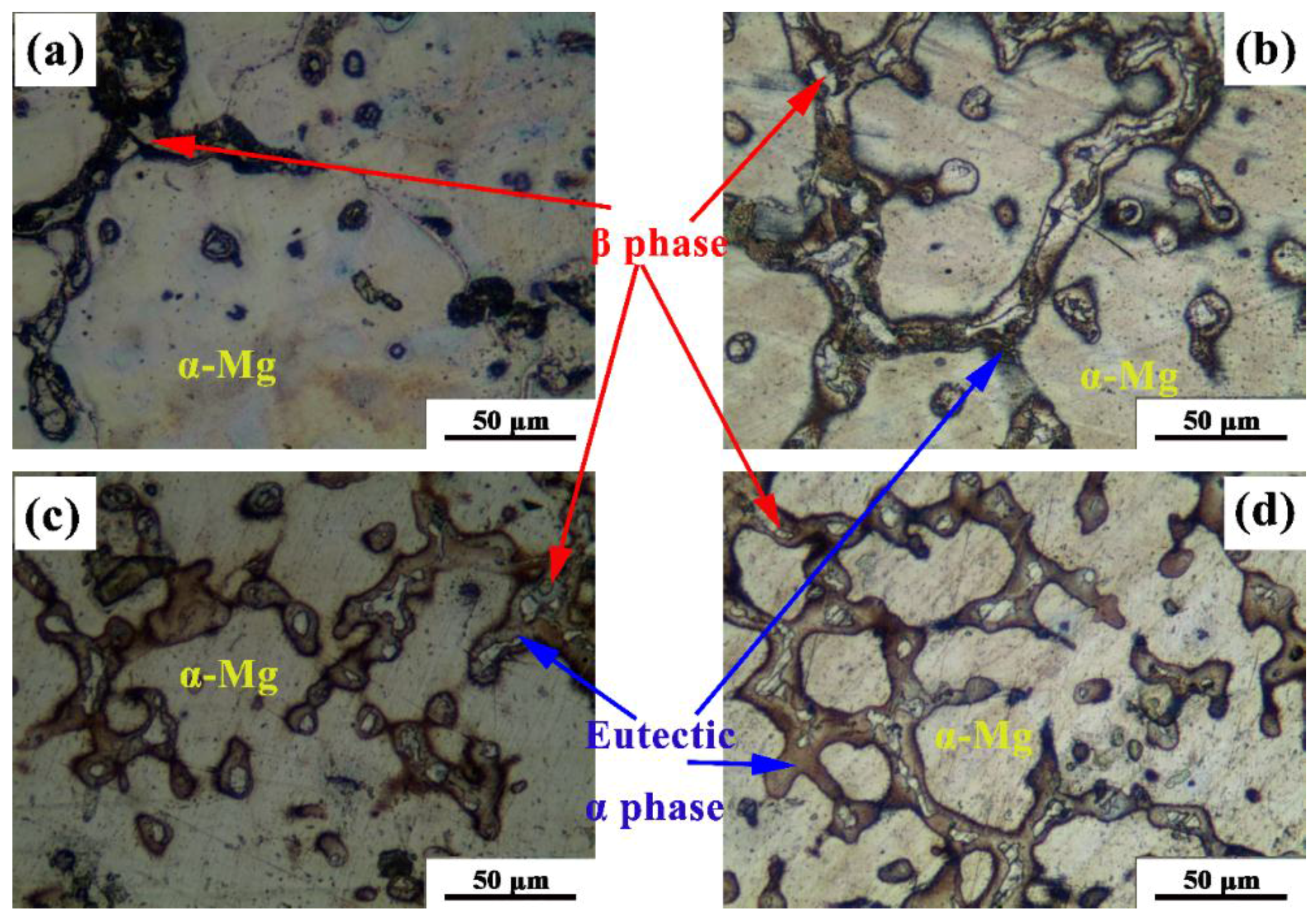

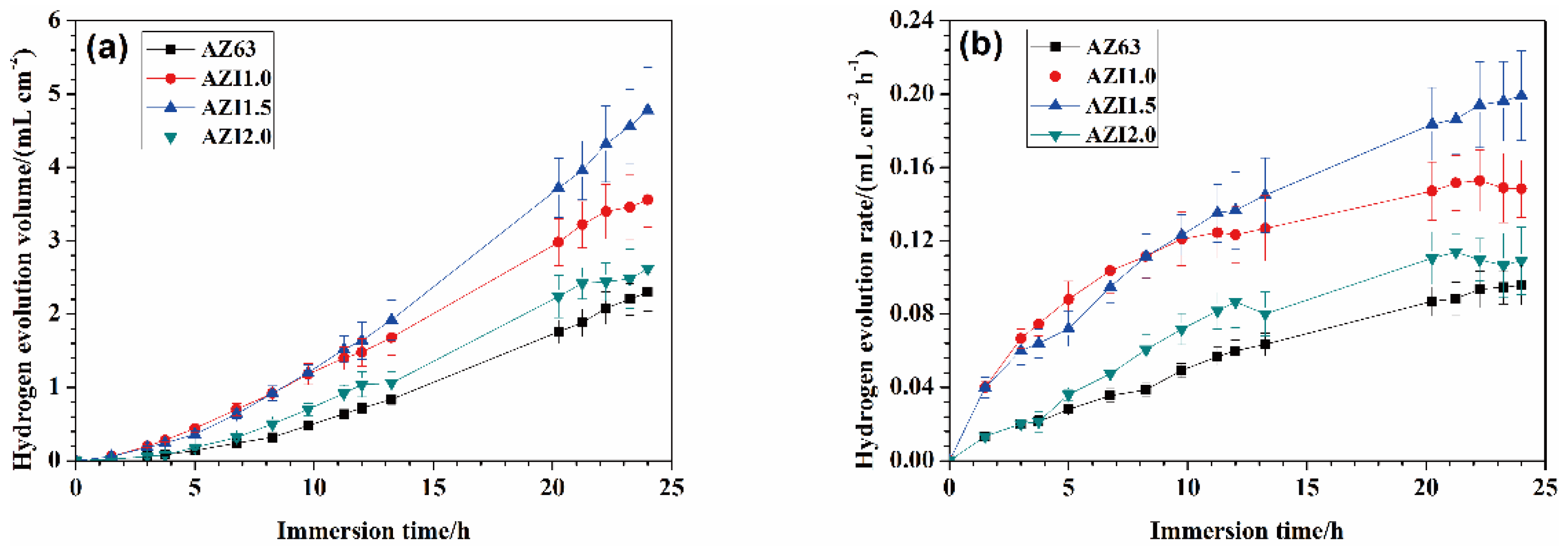

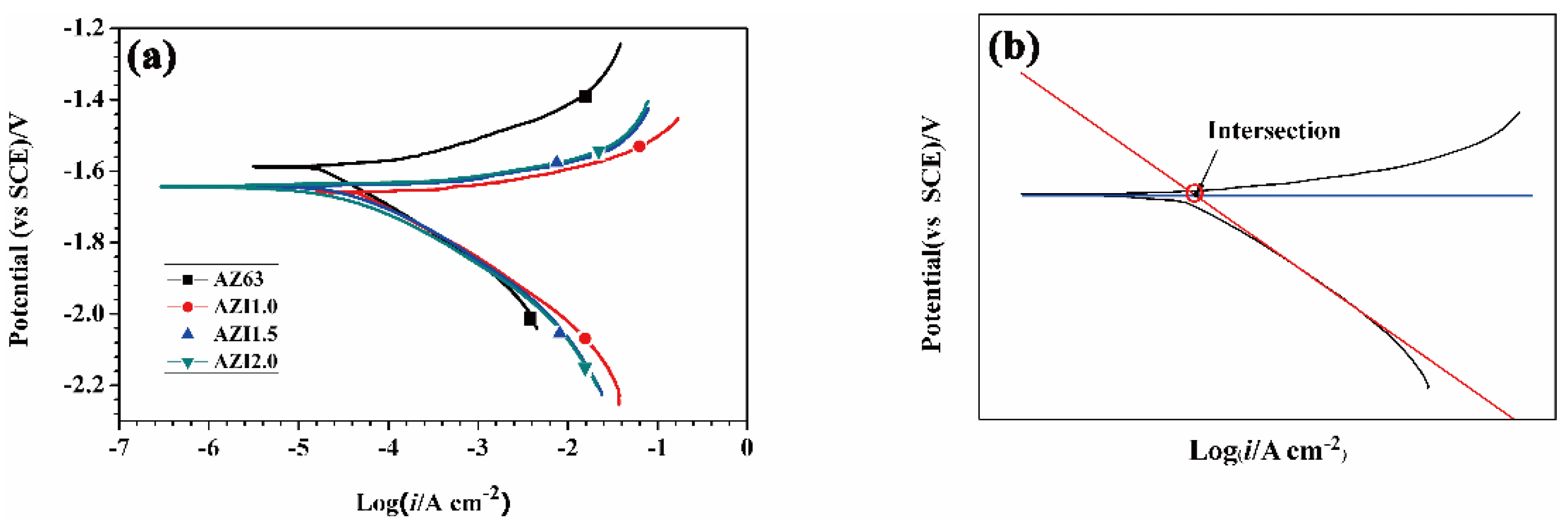
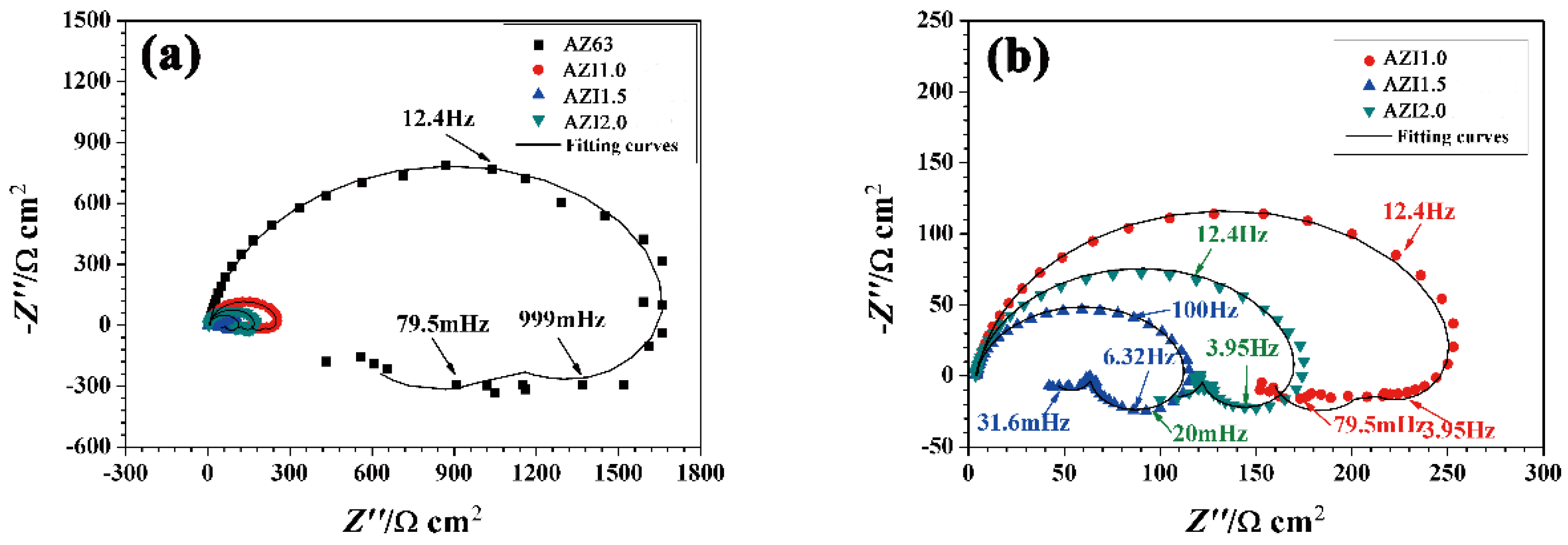

| Alloys | Al | Zn | Mn | In | Mg |
|---|---|---|---|---|---|
| AZ63 | 5.683 | 2.681 | 0.301 | – | balance |
| AZI1.0 (AZ63 + 1.0% In) | 5.492 | 2.784 | 0.313 | 0.926 | balance |
| AZI1.5 (AZ63 + 1.5% In) | 5.749 | 2.901 | 0.296 | 1.443 | balance |
| AZI2.0 (AZ63 + 2.0% In) | 5.699 | 2.824 | 0.322 | 1.896 | balance |
| Alloys | Ecorr (vs. SCE)/V | icorr/(µA·cm−2) | bc/(mV·dec−1) | ba/(mV·dec−1) |
|---|---|---|---|---|
| AZ63 | −1.587 | 18.7 | −134.3 | 57.8 |
| AZI1.0 | −1.658 | 45.0 | −148.1 | 23.2 |
| AZI1.5 | −1.642 | 52.7 | −163.4 | 16.1 |
| AZI2.0 | −1.621 | 47.2 | −153.8 | 22.0 |
| Alloy | Rs/(Ω·cm2) | Rt/(Ω·cm2) | Ydl/(Ω−1·cm−2·sn) | ndl | RL1/(Ω·cm2) | L/(Ω cm2·s) |
|---|---|---|---|---|---|---|
| AZ63 | 7.0 | 1811 | 9.1 × 10−6 | 9.1 × 10−1 | 2980 | 441.2 |
| AZ63 + 1% In | 4.5 | 261.1 | 1.0 × 10−5 | 9.3 × 10−1 | 902 | 26.3 |
| AZ63 + 1.5% In | 3.9 | 113.5 | 1.4 × 10−5 | 9.1 × 10−1 | 121 | 980.6 |
| AZ63 + 2% In | 3.8 | 173 | 1.3 × 10−5 | 9.2 × 10−1 | 360.8 | 4351 |
| Alloy | RL2/(Ω·cm2) | L/(Ω·cm2·s) | τc/s | τL1/s | τL2/s | Rp/(Ω·cm2) |
| AZ63 | 948 | 4912 | 1.3 × 10−2 | 1.5 × 10−1 | 5.2 | 514.8 |
| AZ63 + 1% In | 653.8 | 1570 | 2.2 × 10−3 | 2.9 × 10−2 | 2.4 | 154.6 |
| AZ63 + 1.5% In | 127.2 | 5.1 | 1.3 × 10−3 | 8.1 | 4 × 10−2 | 40.1 |
| AZ63 + 2% In | 376.7 | 18 | 1.5 × 10−3 | 12.1 | 4.8 × 10−2 | 89.2 |
| Alloys | Average Potentials (vs. SCE)/V | ||
|---|---|---|---|
| 10 mA·cm−2, 20 h | 100 mA·cm−2, 2 h | 200 mA·cm−2, 1 h | |
| AZ63 | −1.538 ± 0.007 | −1.553 ± 0.013 | −1.597 ± 0.007 |
| AZI1.0 | −1.679 ± 0.010 | −1.704 ± 0.007 | −1.705 ± 0.003 |
| AZI1.5 | −1.602 ± 0.019 | −1.634 ± 0.010 | −1.629 ± 0.003 |
| AZI2.0 | −1.639 ± 0.015 | −1.660 ± 0.012 | −1.655 ± 0.012 |
| Alloys | Current Efficiency (%) | ||
|---|---|---|---|
| 10 mA·cm−2, 20 h | 100 mA·cm−2, 2 h | 200 mA·cm−2, 1 h | |
| AZ63 | 59.1 | 62.9 | 59.4 |
| AZI1.0 | 66.1 | 71.8 | 71.2 |
| AZI1.5 | 71.5 | 70.0 | 68.6 |
| AZI2.0 | 65.4 | 73.2 | 74.4 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wan, K.; Jiang, Q.; Sun, H.; Li, Y.; Hou, B.; Zhu, L.; Liu, M. Corrosion and Discharge Behaviors of Mg-Al-Zn and Mg-Al-Zn-In Alloys as Anode Materials. Metals 2016, 6, 65. https://doi.org/10.3390/met6030065
Li J, Wan K, Jiang Q, Sun H, Li Y, Hou B, Zhu L, Liu M. Corrosion and Discharge Behaviors of Mg-Al-Zn and Mg-Al-Zn-In Alloys as Anode Materials. Metals. 2016; 6(3):65. https://doi.org/10.3390/met6030065
Chicago/Turabian StyleLi, Jiarun, Kai Wan, Quantong Jiang, Huyuan Sun, Yantao Li, Baorong Hou, Liwei Zhu, and Min Liu. 2016. "Corrosion and Discharge Behaviors of Mg-Al-Zn and Mg-Al-Zn-In Alloys as Anode Materials" Metals 6, no. 3: 65. https://doi.org/10.3390/met6030065
APA StyleLi, J., Wan, K., Jiang, Q., Sun, H., Li, Y., Hou, B., Zhu, L., & Liu, M. (2016). Corrosion and Discharge Behaviors of Mg-Al-Zn and Mg-Al-Zn-In Alloys as Anode Materials. Metals, 6(3), 65. https://doi.org/10.3390/met6030065






