Upgrading of High-Aluminum Hematite-Limonite Ore by High Temperature Reduction-Wet Magnetic Separation Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Experimental Procedures
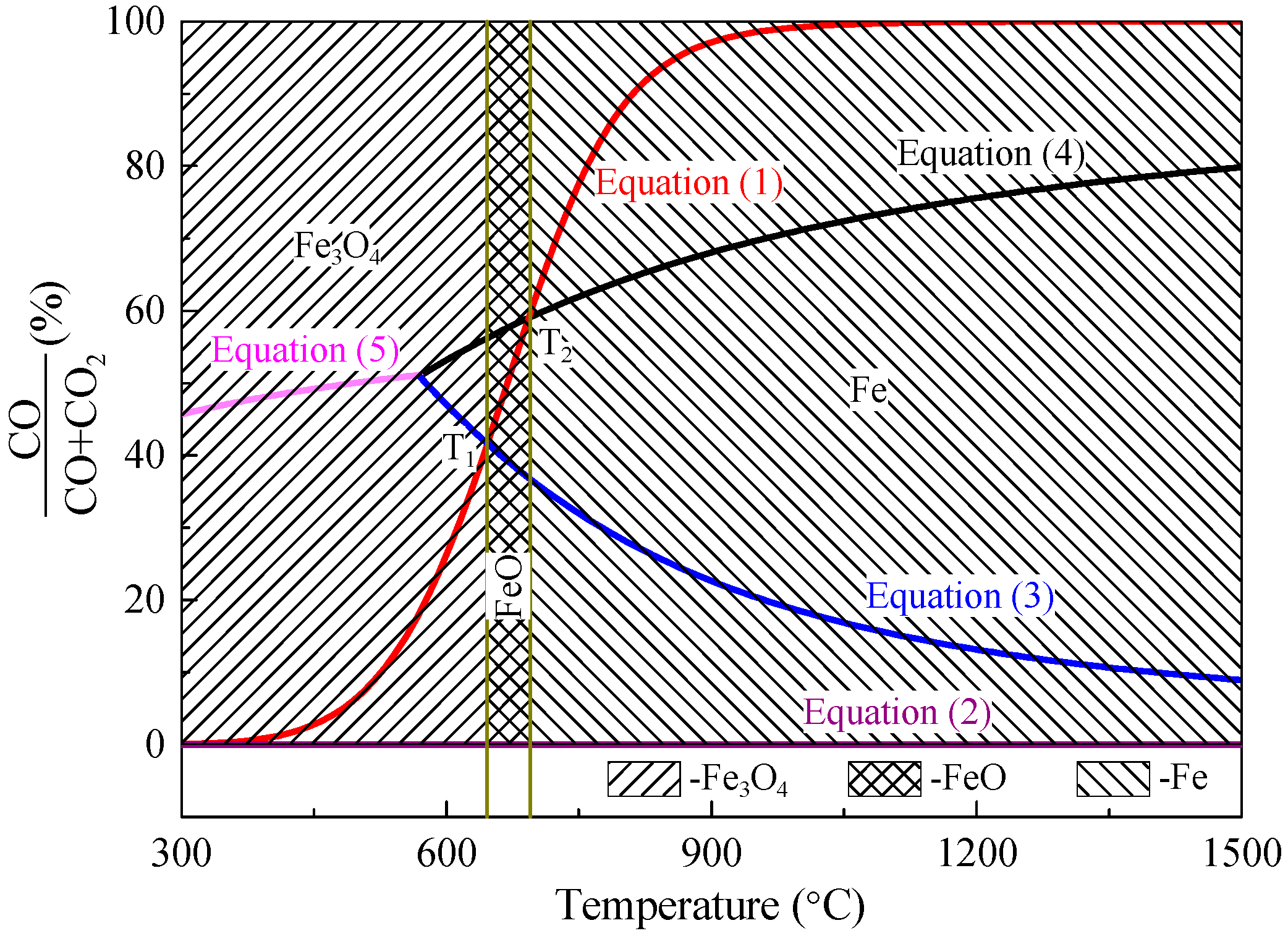
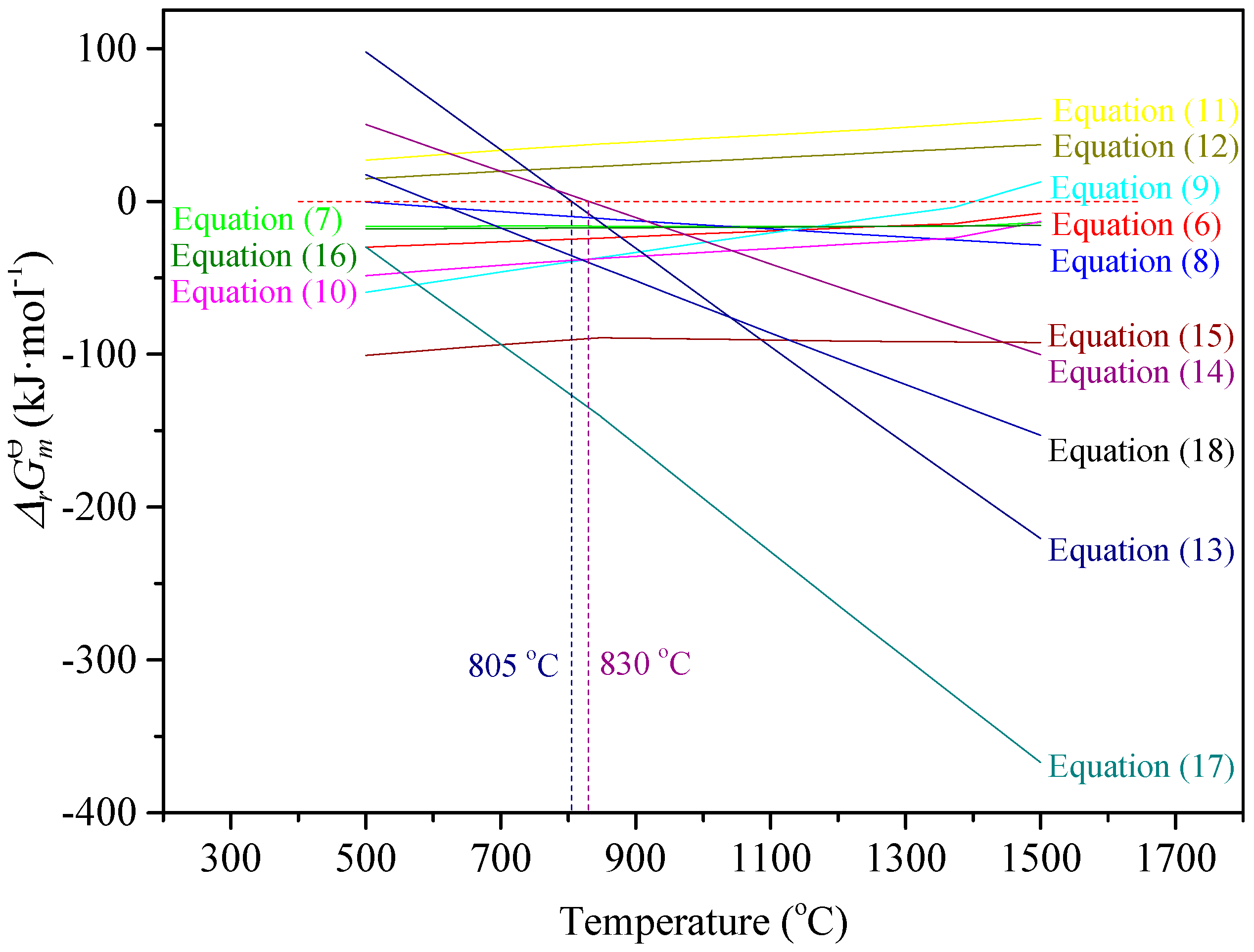
2.3. Thermodynamic Analysis of Reduction Process
3. Results and Discussion
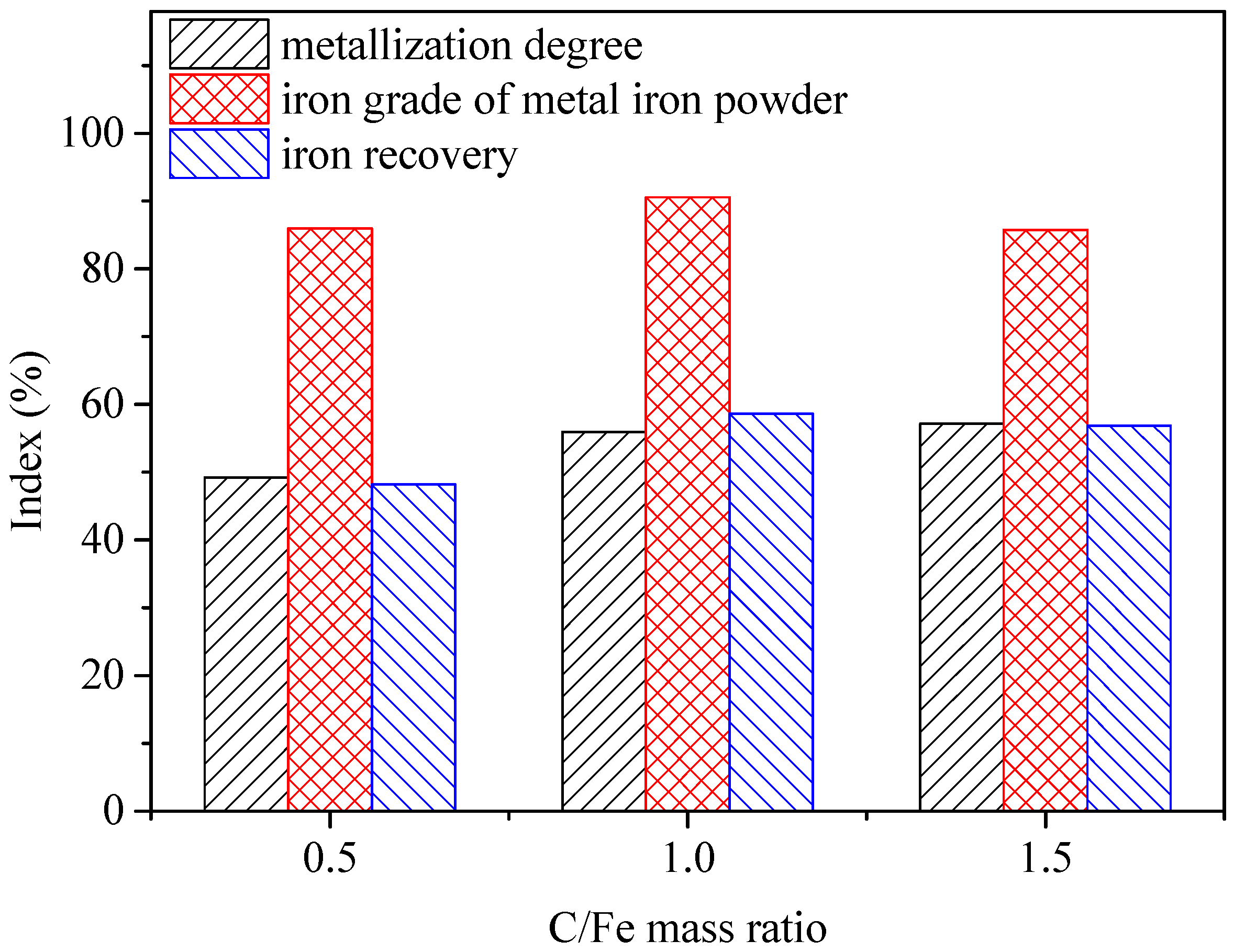
3.1. Dosage of Reductant
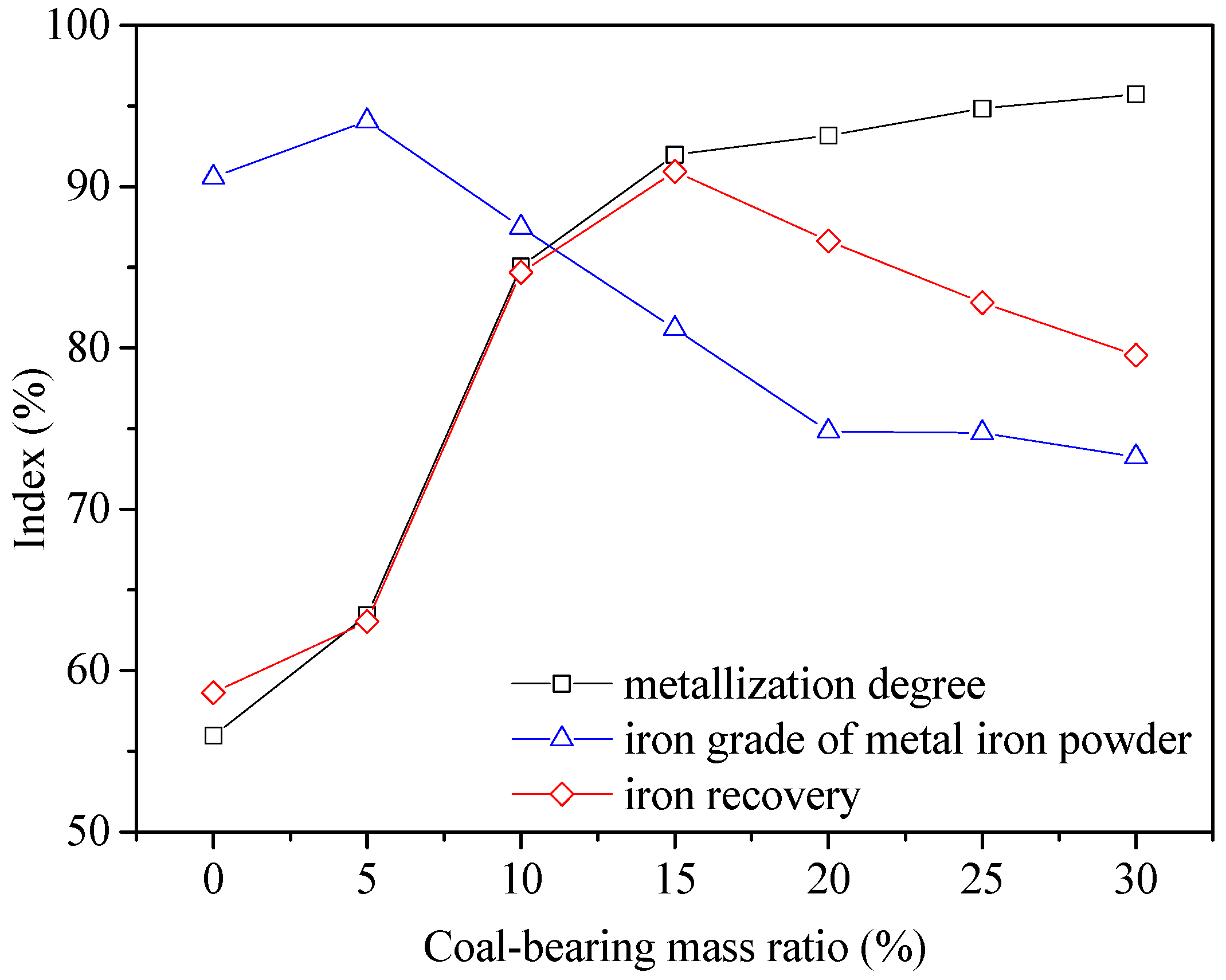
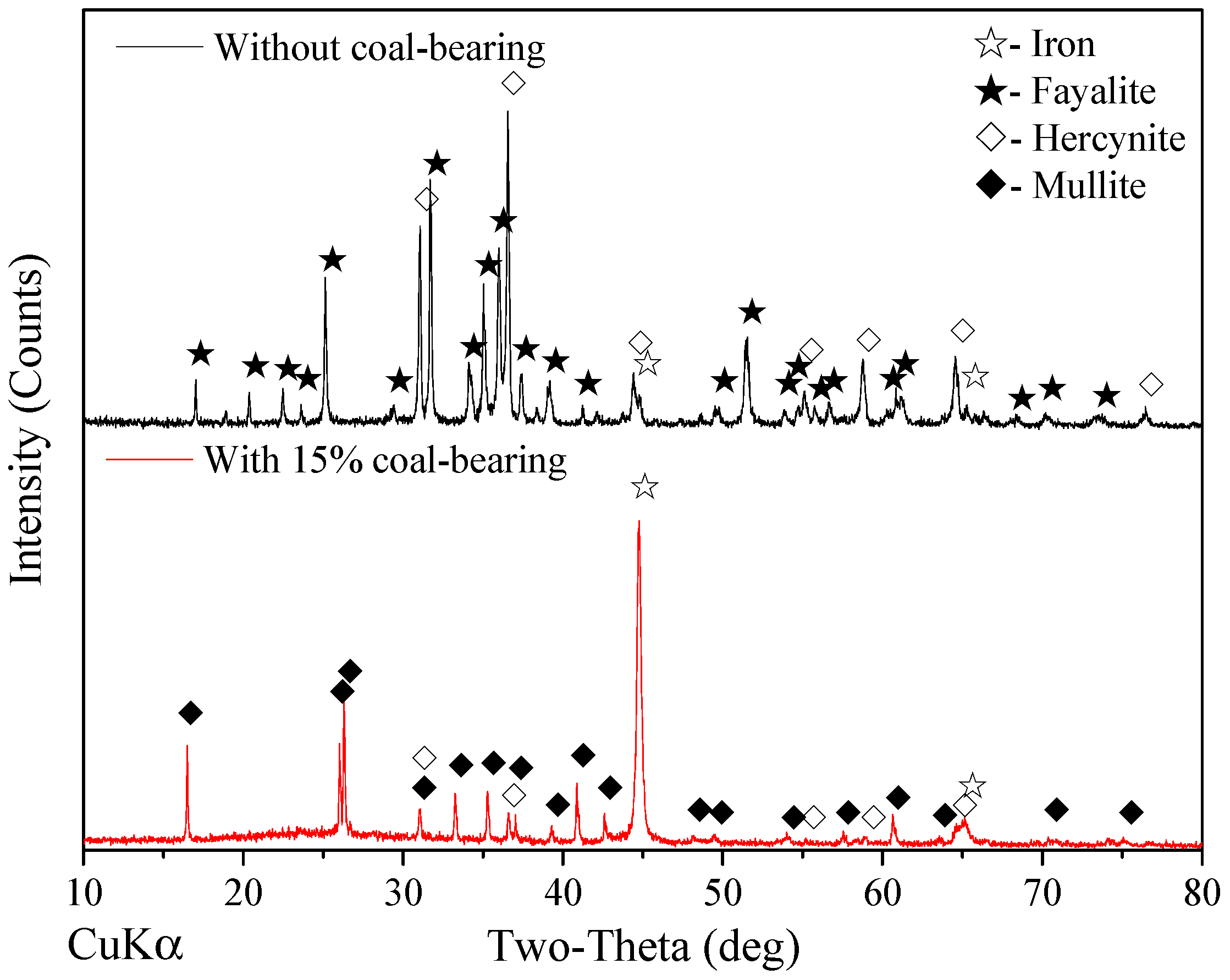
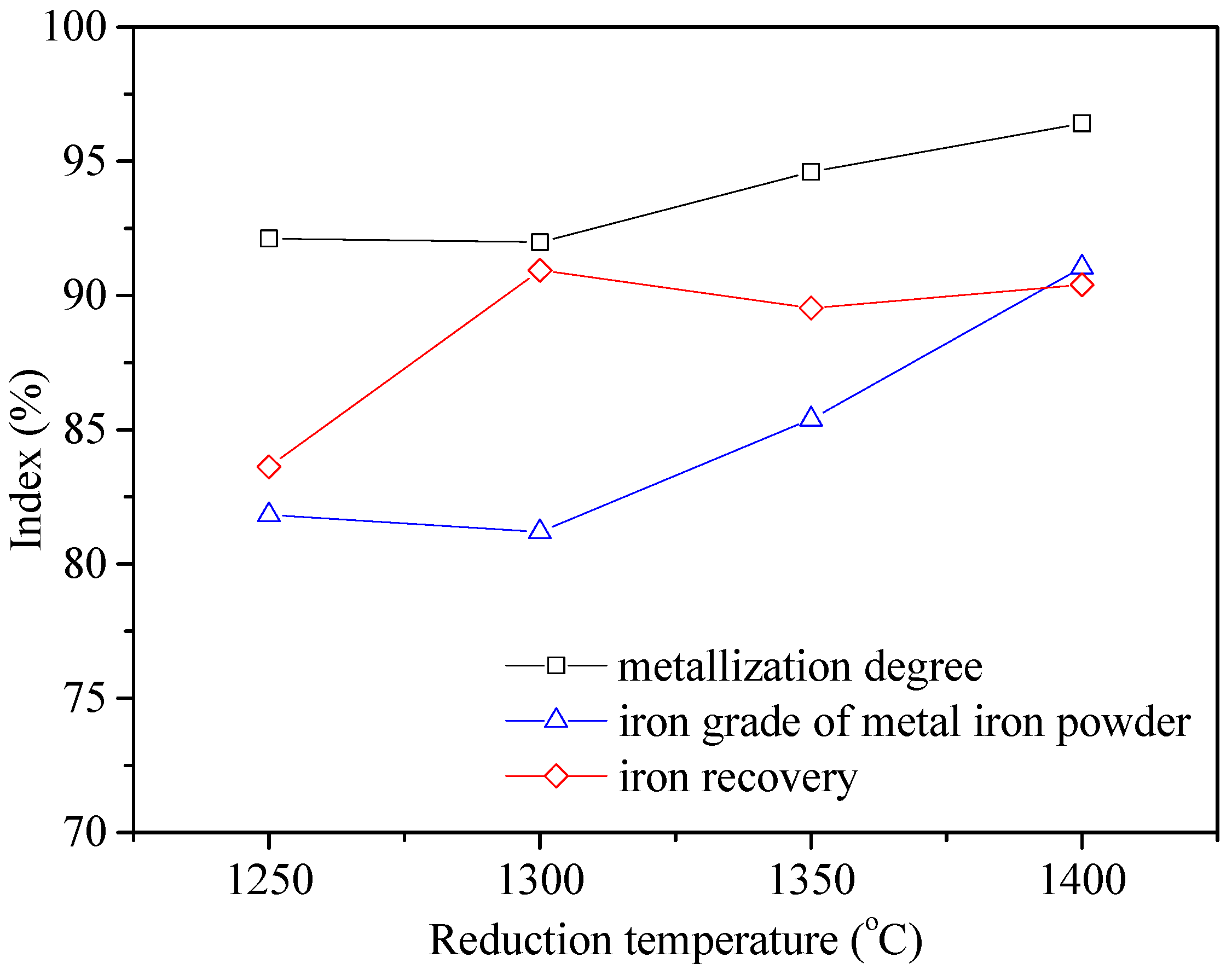
3.2. Optimization of Reduction Parameters
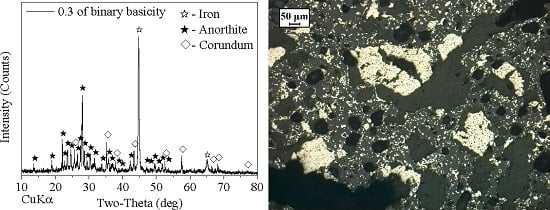
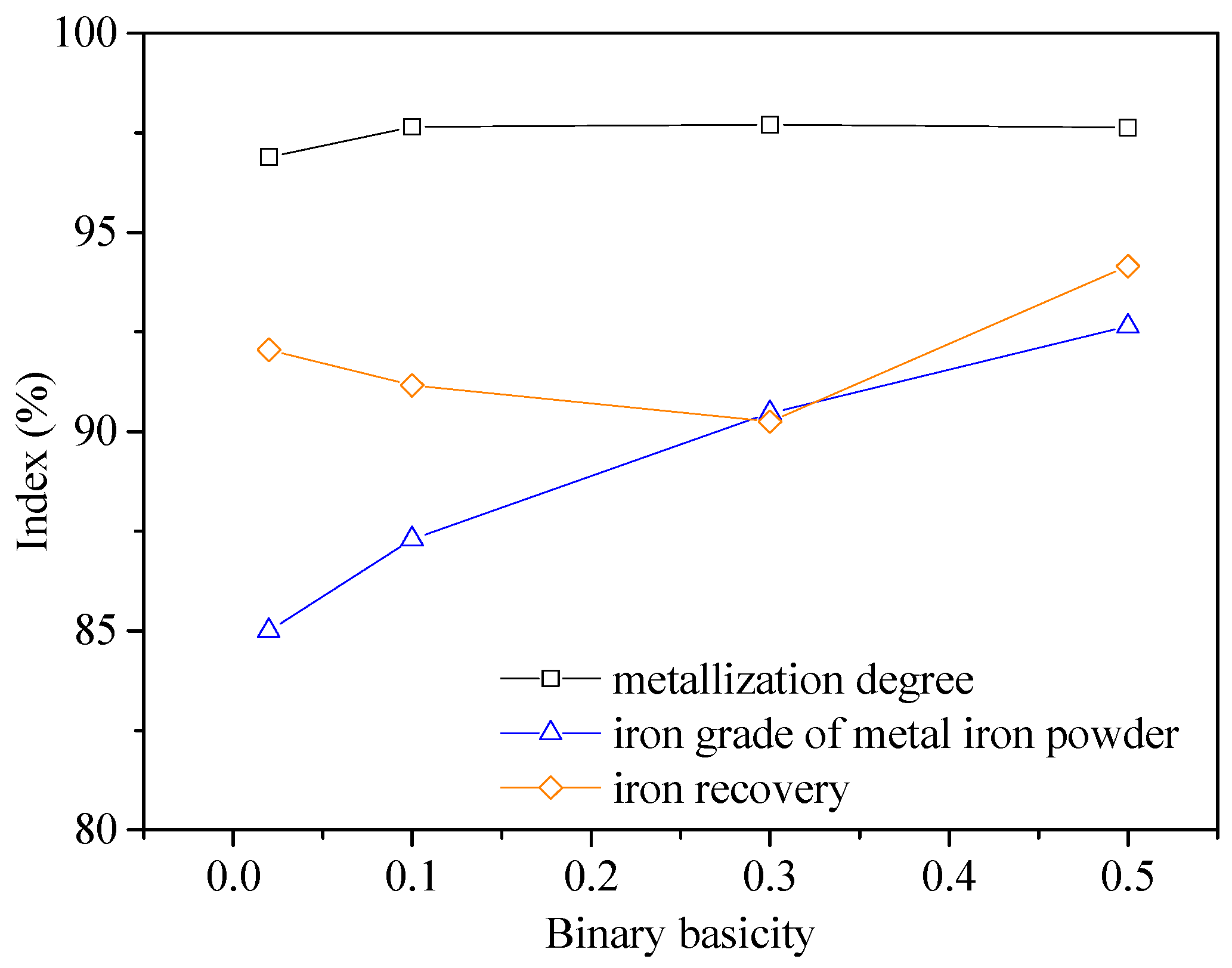
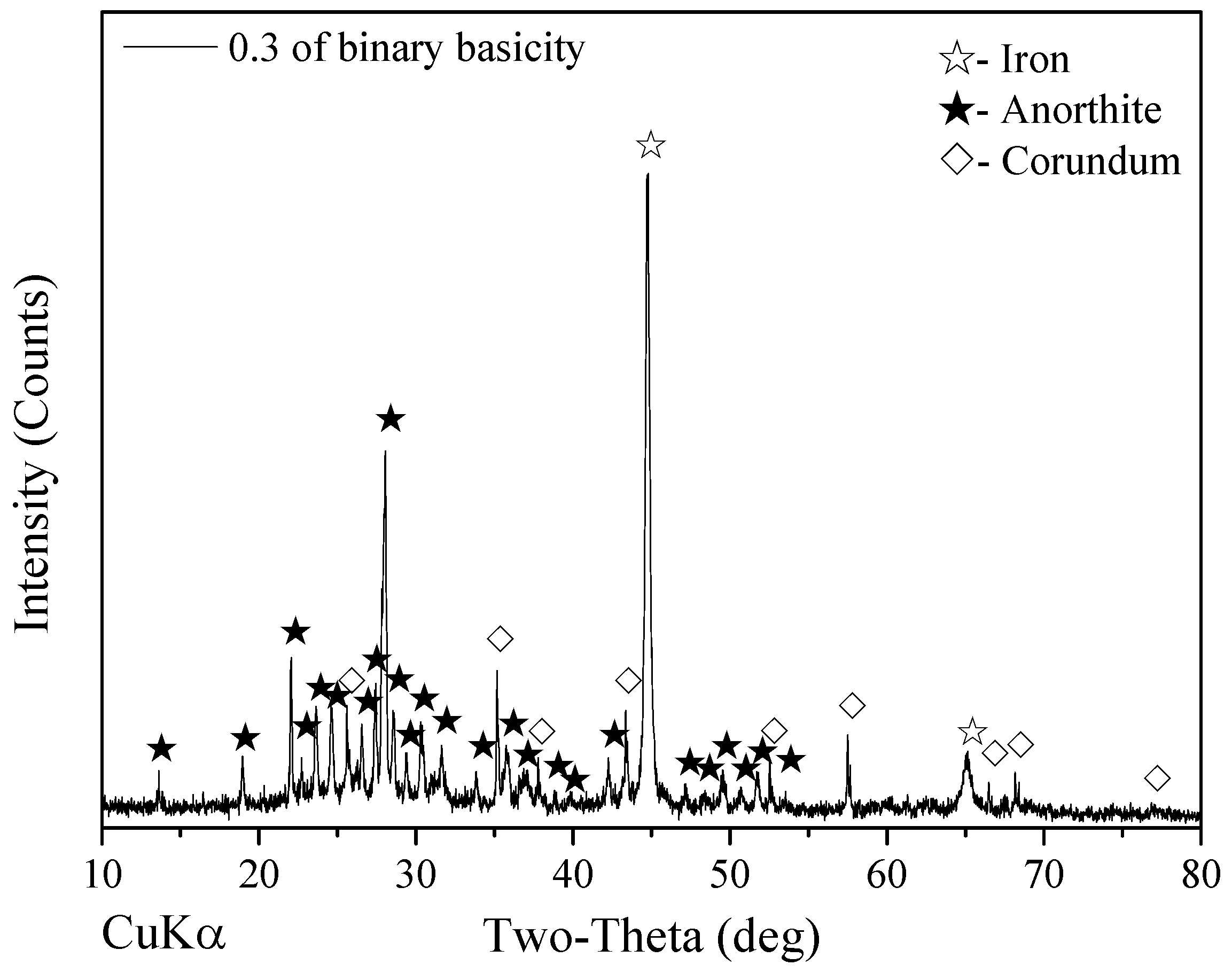
3.3. Effect of Binary Basicity
4. Conclusions
- (1)
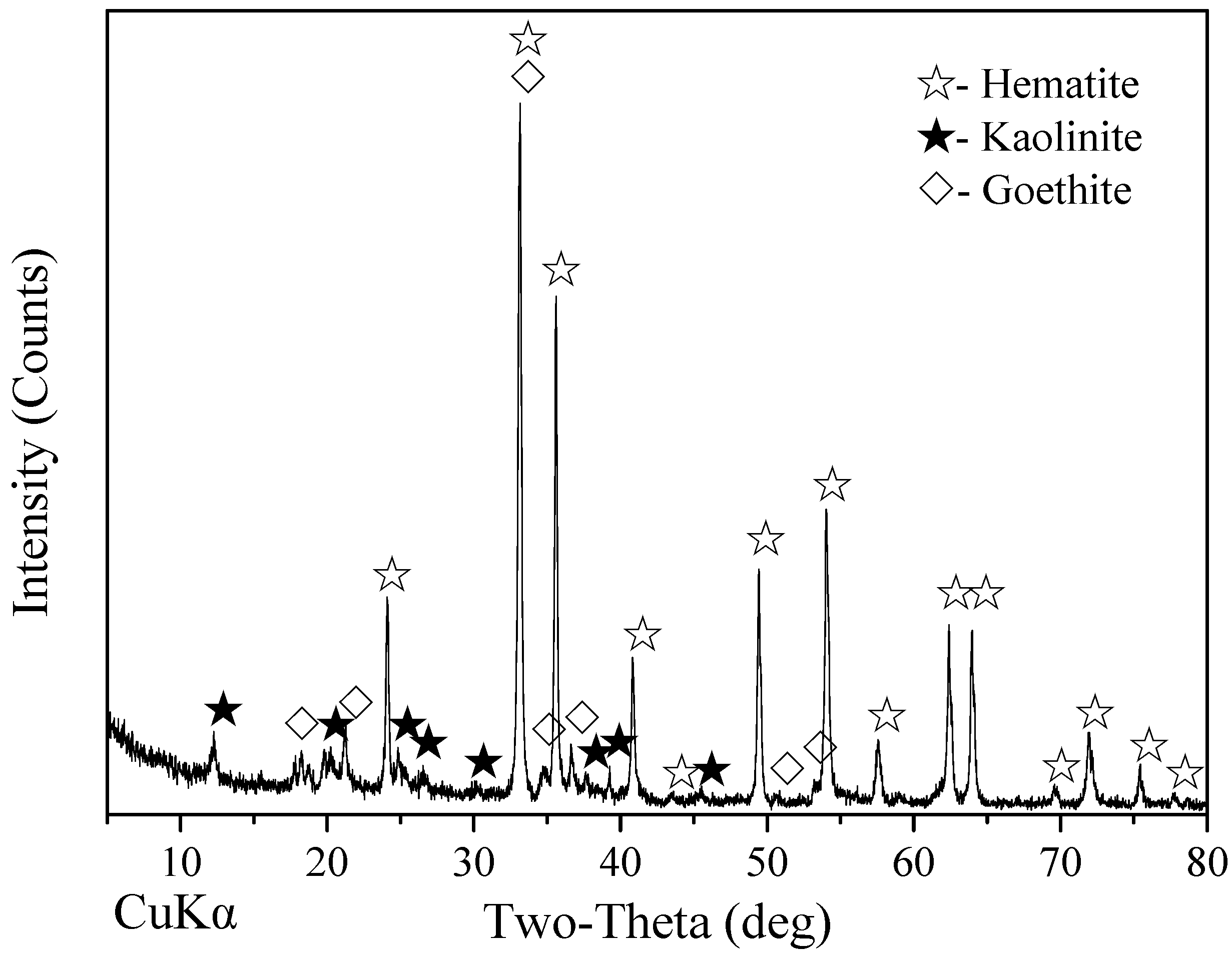
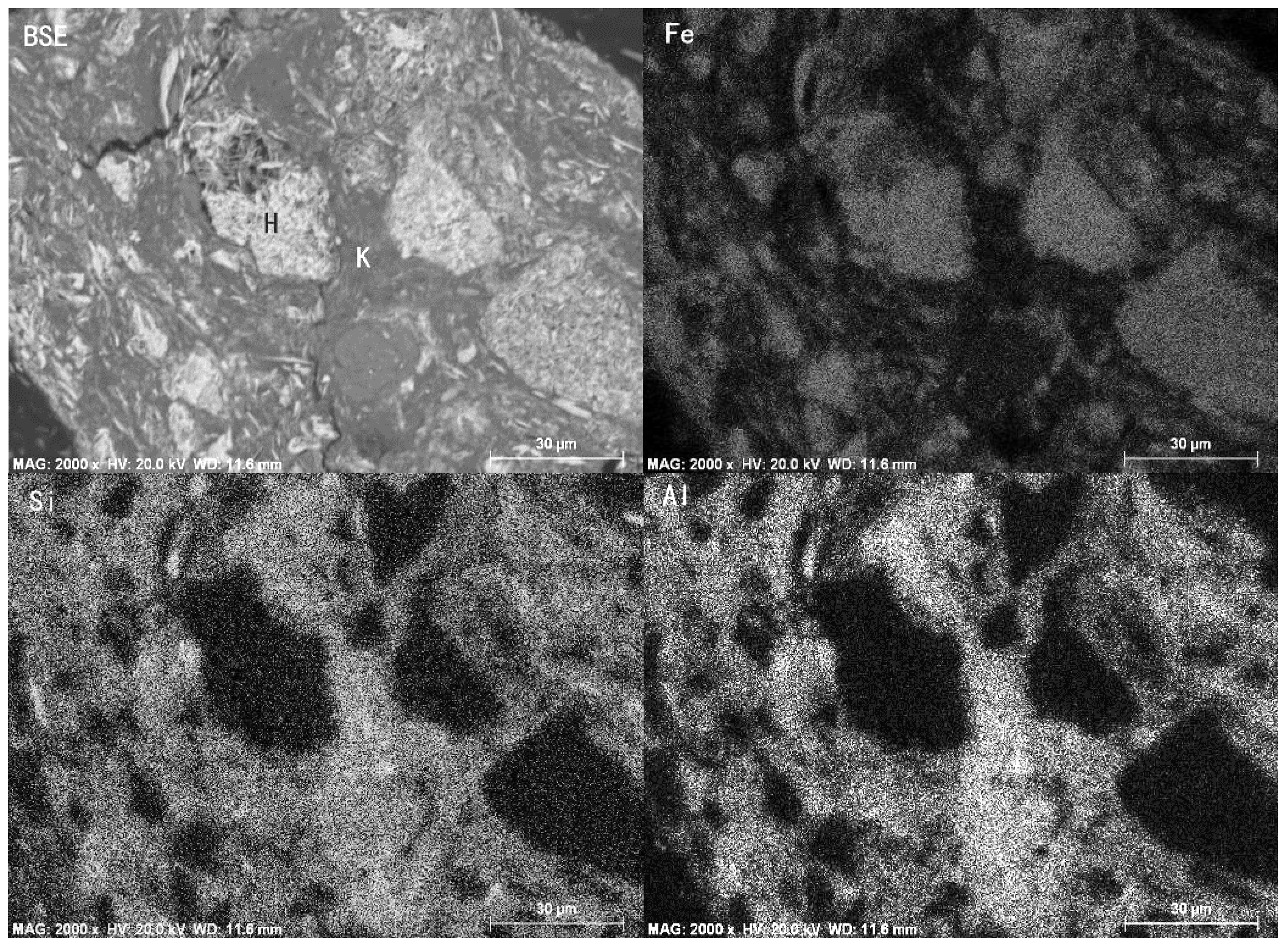
- A high-aluminum hematite-limonite ore, assaying 41.92% Fetotal, 13.74% Al2O3 and 13.96% SiO2, was used as the raw material to manufacture metal iron powder, where iron minerals mainly occur in hematite and goethite and aluminum minerals exist in kaolinite. Hematite is closely disseminated with kaolinite, leading to harder separation between iron and alumina.
- (2)
- Good quality metal iron powder assaying 90.46% Fetotal was produced at an overall iron recovery of 90.25% under the following conditions: balling the mixture of iron ore with 15% coal and CaCO3 fines at 0.3 basicity, reducing the dried pellets at 1350 °C for 25 min and with a total C/Fe mass ratio of 1.0, grinding the reduced pellets up to 95% passing at 0.074 mm and magnetic separation in a Davis Tube at 0.10 T of magnetic field intensity.
- (3)
- The process provides a potential way to utilize high-aluminum iron ore, where the good quality metal iron powder can be used as the burden for EAF and nonmagnetic tailings can be applied in ceramic and glass industries.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Steel Statistical Yearbook 2015; World Steel Association: Brussels, Belgium, 2015; p. 105.
- Zhang, K.; Kleit, A.N. Mining rate optimization considering the stockpiling: A theoretical economics and real option model. Resour. Policy 2016, 47, 87–94. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, M.; Li, G.; Sun, N.; Zeng, J.; Qiu, G. Novel process for treatment of high-aluminum limonite ore by reduction roasting with addition of sodium salts. Chin. J. Nonferr. Met. 2010, 20, 565–571. [Google Scholar]
- Shu, C.; Tang, Y.; Wang, Z.; Zhang, Q. Research on sodium salt roasting-acid leaching technology for high-alumina and high-silicon refractory limonite. Min. Metall. Eng. 2012, 32, 62–65. [Google Scholar]
- Lu, L.; Holmes, R.J.; Manuel, J.R. Effects of alumina on sintering performance of hematite iron ores. ISIJ Int. 2007, 47, 349–358. [Google Scholar] [CrossRef]
- Tian, Q.; Shi, Y. Research on desulfurizing capacity of high Al2O3 content slag. Ironmaking 1998, 17, 32–33. [Google Scholar]
- Raghukumar, C.; Tripathy, S.K.; Mohanan, S. Beneficiation of Indian high alumina iron ore fines—A case study. Int. J. Min. Eng. Miner. Process. 2012, 1, 94–100. [Google Scholar] [CrossRef]
- Thella, J.S.; Mukherjee, A.K.; Srikakulapu, N.G. Processing of high alumina iron ore slimes using classification and flotation. Powder Technol. 2012, 217, 418–426. [Google Scholar] [CrossRef]
- Jain, V.; Rai, B.; Waghmare, U.V.; Tammishetti, V. Pradip Processing of Alumina-Rich Iron Ore Slimes: Is the Selective Dispersion-Flocculation-Flotation the Solution We Are Looking for the Challenging Problem Facing the Indian Iron and Steel Industry? Trans. Indian Inst. Met. 2013, 66, 447–456. [Google Scholar] [CrossRef]
- Chun, T.J.; Zhu, D.Q.; Pan, J.; He, Z. Preparation of metallic iron powder from red mud by sodium salt roasting and magnetic separation. Can. Metall. Quart. 2014, 53, 183–189. [Google Scholar] [CrossRef]
- Li, G.; Zhou, T.; Liu, M.; Jiang, T.; Fan, X. Novel process and mechanisms of aluminum-iron separation of high-aluminum limonite ore. Chin. J. Nonferr. Met. 2008, 18, 2087–2093. [Google Scholar]
- Li, G.; Liu, M.; Jiang, T.; Zhou, T.; Fan, X. Mineralogy charateristics and separation of aluminum and iron of high-aluminum iron ores. J. Cent. South Univ. 2009, 18, 1165–1171. [Google Scholar]
- Jiang, T.; Liu, M.; Li, G.; Sun, N.; Zeng, J.; Qiu, G. Effects of sodium-salt on Al-Fe separation by reduction roasting for high-aluminum content limonite. Chin. J. Nonferr. Met. 2010, 20, 1226–1233. [Google Scholar]
- Li, G.; Jiang, T.; Liu, M.; Zhou, T.; Fan, X.; Qiu, G. Beneficiation of high-aluminium-content hematite ore by soda ash roasting. Min. Proc. Ext. Met. Rev. 2010, 31, 150–164. [Google Scholar] [CrossRef]
- Chun, T.; Long, H.; Li, J. Alumina-iron separation of high alumina iron ore by carbothermic reduction and magnetic separation. Sep. Sci. Technol. 2015, 50, 760–766. [Google Scholar] [CrossRef]
- Stjernberg, J.; Olivas-Ogaz, M.A.; Antti, M.L.; Ion, J.C.; Lindblom, B. Laboratory scale study of the degradation of mullite/corundum refractories by reaction with alkali-doped deposit materials. Ceram. Int. 2013, 39, 791–800. [Google Scholar] [CrossRef]
- Stjernberg, J.; Antti, M.; Nordin, L.; Odén, M. Degradation of refractory bricks used as thermal insulation in rotary kilns for iron ore pellet production. Int. J. Appl. Ceram. Technol. 2009, 6, 717–726. [Google Scholar] [CrossRef]
- Zhu, D.; Zhou, X.; Pan, J.; Luo, Y. Direct reduction and beneficiation of a refractory siderite lump. Miner. Process. Extr. Metall. 2014, 123, 246–250. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, D.; Pan, J.; Wu, T. Utilization of waste copper slag to produce directly reduced iron for weathering resistant steel. ISIJ Int. 2015, 55, 1347–1352. [Google Scholar]
- Huang, X. Ferrous Metallurgy Theory, 4th ed.; Metallurgical Industry Press: Beijing, China, 2005; pp. 413–437. [Google Scholar]
- Zhang, B.; Wang, Z.; Gong, X.; Guo, Z. Study on reduction of high-aluminum iron ores with gas in a fluidized bed (in Chinese). J. Chin. Soc. Rare Earth. 2013, 30, 774–780. [Google Scholar]
- Wei, Y.; Sun, T.; Kou, J.; Yu, W.; Cao, Y. Effect of coal dosage on direct reduction roasting of refractory iron ore briquettes. J. Cent. South Univ. 2013, 44, 1305–1311. [Google Scholar]
- Lu, W.; Huang, D.F. The evolution of ironmaking process based on coal-containing iron ore agglomerates. ISIJ Int. 2001, 41, 807–812. [Google Scholar] [CrossRef]
- Gu, X.; Dong, W.; Chen, Y.; Li, Y. Investigation on the glass-anorthite insulating composites with low dielectric constant and low sintering temperature. Rare Metal Mater. Eng. 2007, 36, 464–467. [Google Scholar]
- Liu, Q.; Pan, Z.; Li, Q.; Ruan, Y. Preparation of anorthite lightweight thermal insulating brick and the formation process of anorthite. Bull. Chin. Ceram. Soc. 2010, 29, 1269–1274. [Google Scholar]

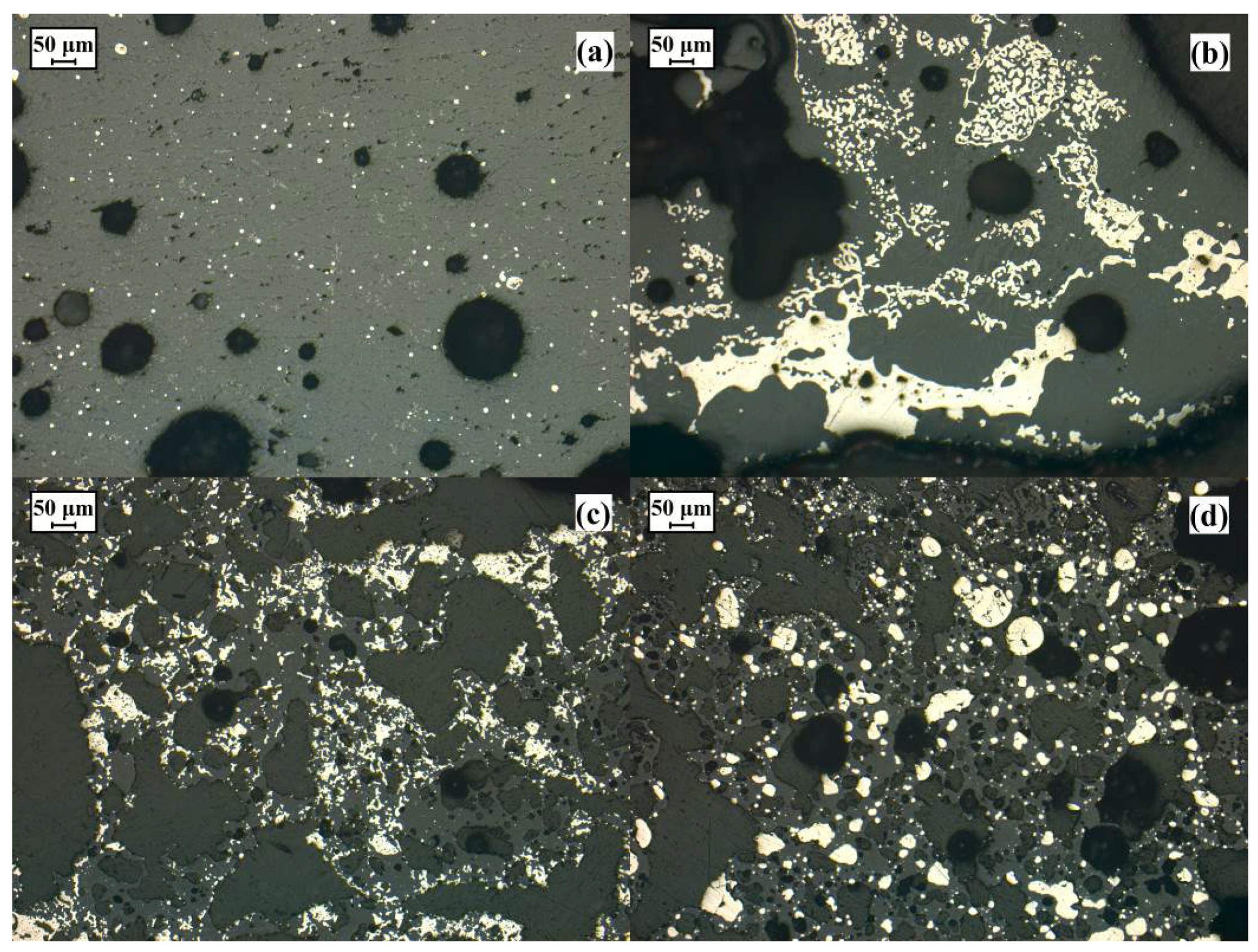


| Chemical Compositions (wt. %) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fetotal | FeO | Al2O3 | SiO2 | CaO | MgO | Mn | K2O | Na2O | P | S | Pb | Zn | LOI |
| 41.92 | 0.17 | 13.74 | 13.96 | 0.13 | 0.88 | 1.24 | 0.42 | 0.03 | 0.13 | 0.01 | 0.64 | 0.21 | 7.20 |
| Chemical Compositions (wt. %) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fetotal | Fe2O3 | SiO2 | Al2O3 | CaO | MgO | K2O | Na2O | P | S |
| 11.80 | 16.70 | 27.62 | 8.02 | 24.94 | 1.34 | 0.16 | 1.34 | 0.01 | 12.92 |
| Sample | Fetotal | Al2O3 | SiO2 | CaO | MgO | MnO | K2O | Na2O | P | S |
|---|---|---|---|---|---|---|---|---|---|---|
| Metal iron powder | 90.46 | 4.57 | 3.57 | 0.48 | 0.23 | 0.42 | 0.09 | 0.04 | 0.025 | 0.007 |
| Nonmagnetic tailings | 9.97 | 34.64 | 37.99 | 8.63 | 1.71 | 2.81 | 1.64 | 0.27 | 0.040 | 0.101 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Zhu, D.; Pan, J.; Luo, Y.; Liu, X. Upgrading of High-Aluminum Hematite-Limonite Ore by High Temperature Reduction-Wet Magnetic Separation Process. Metals 2016, 6, 57. https://doi.org/10.3390/met6030057
Zhou X, Zhu D, Pan J, Luo Y, Liu X. Upgrading of High-Aluminum Hematite-Limonite Ore by High Temperature Reduction-Wet Magnetic Separation Process. Metals. 2016; 6(3):57. https://doi.org/10.3390/met6030057
Chicago/Turabian StyleZhou, Xianlin, Deqing Zhu, Jian Pan, Yanhong Luo, and Xinqi Liu. 2016. "Upgrading of High-Aluminum Hematite-Limonite Ore by High Temperature Reduction-Wet Magnetic Separation Process" Metals 6, no. 3: 57. https://doi.org/10.3390/met6030057