Effects of Phyllanthus muellerianus Leaf-Extract on Steel-Reinforcement Corrosion in 3.5% NaCl-Immersed Concrete
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Setup
- Corrosion-rate (CR) measurements from linear polarization resistance using the three-electrode LPR Data Logger, Model MS1500L, obtained from Metal Samples® (Munford, AL, USA) [46,48], and that gave direct readout of CR in mpy unit. The three-electrode system by the instrument include a brass plate auxiliary electrode, a Ag/AgCl SCE reference electrode (EDT direct-ION®, Dover, UK) and the steel-reinforcement working electrode [40,49,50]. The LPR Data Logger instrument was connected to the steel-reinforced concrete specimen using typical electrochemical cell setup detailed in [26,51].
2.3. Initiation of Corrosion Test-Data Analyses—Statistical Distribution Fitting
2.4. Corrosion Noise-Resistance (Rn) Analyses
2.5. Surface Coverage and Inhibition Efficiency Analyses
2.6. Adsorption Isotherm Modeling
3. Results and Discussion
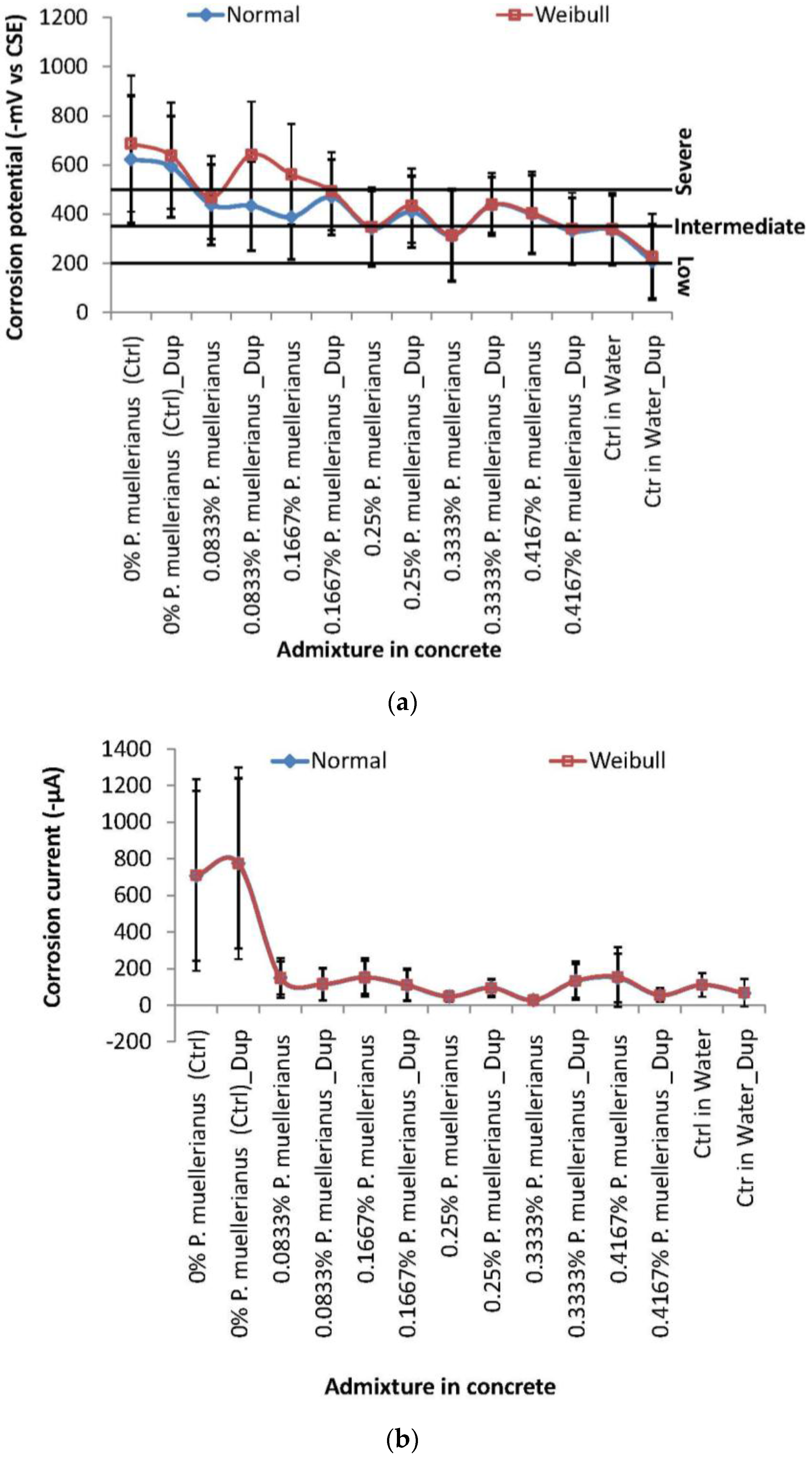
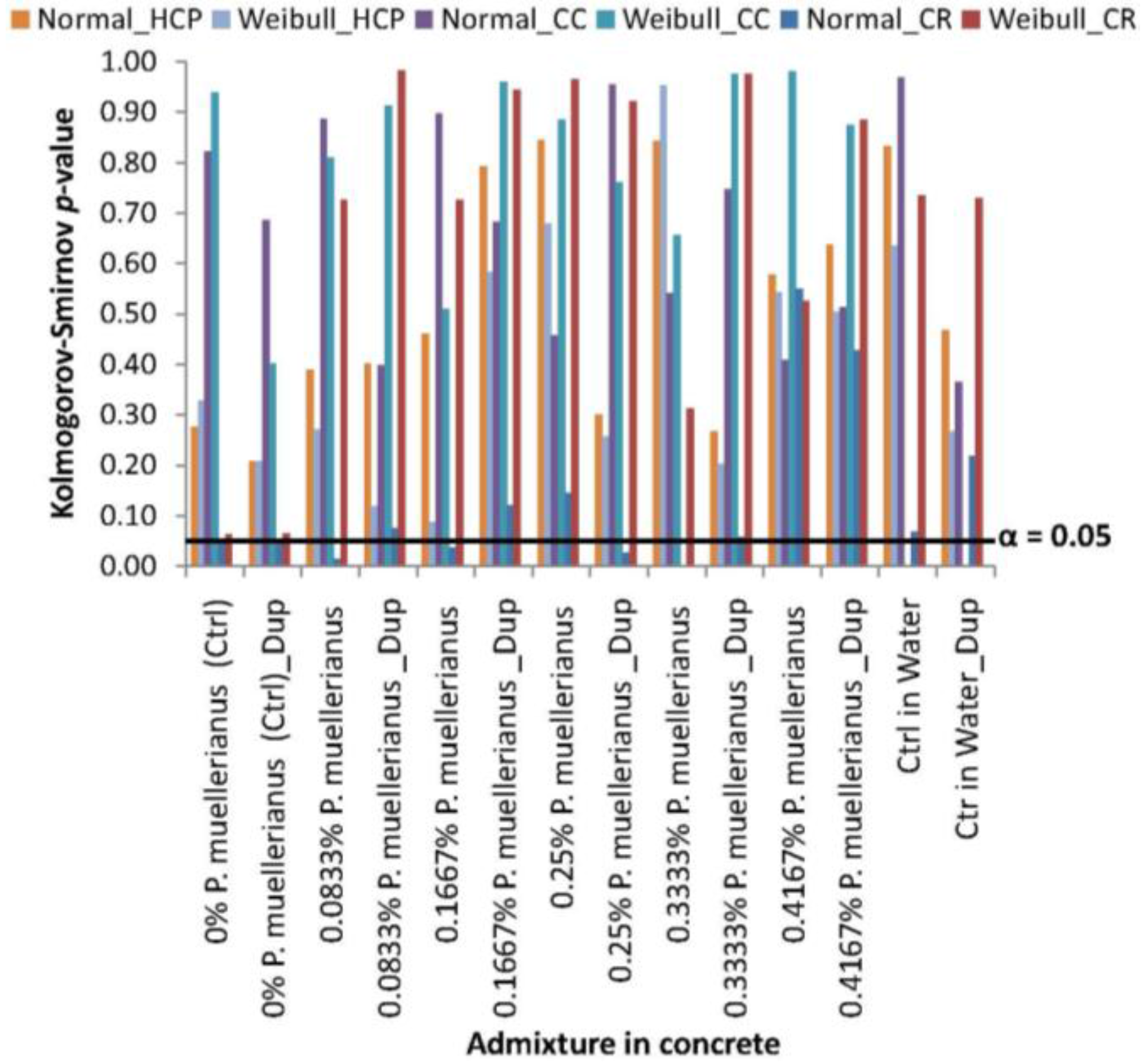
3.1. Statistical Distribution Fitting and Analyses of Corrosion Test-Variables
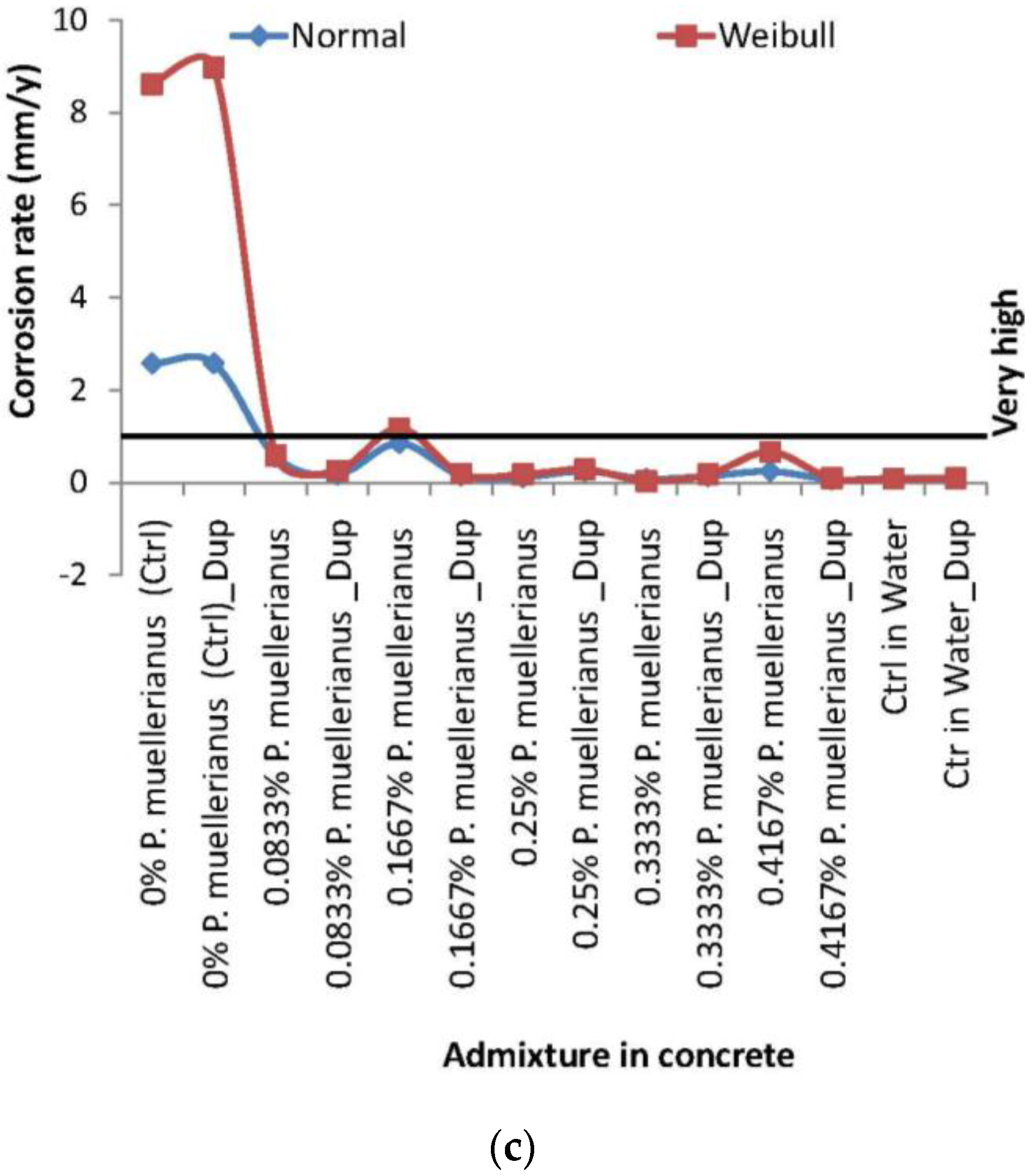
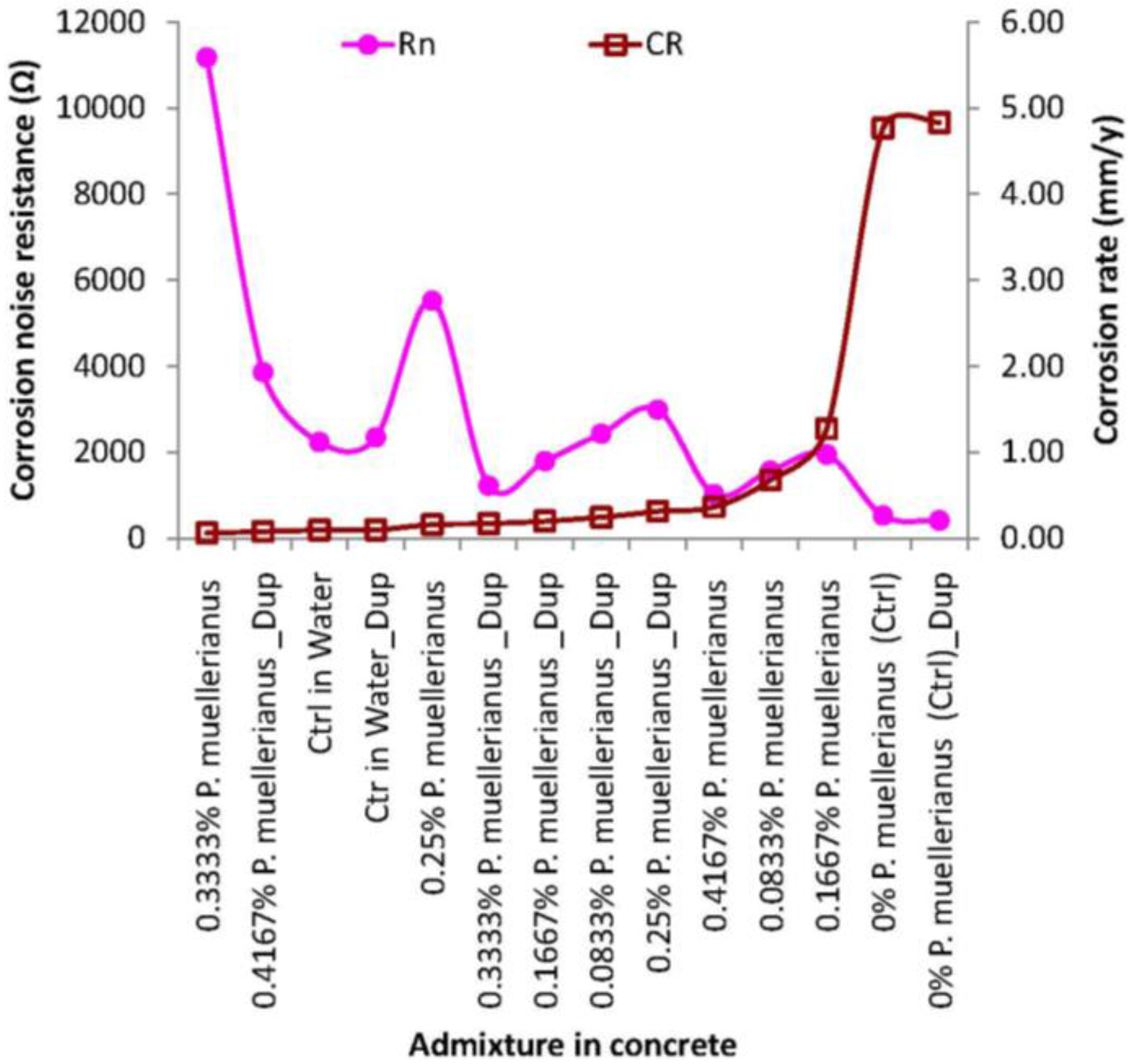
3.2. Corrosion Rate and Corrosion Noise Resistance for Correlation Modeling
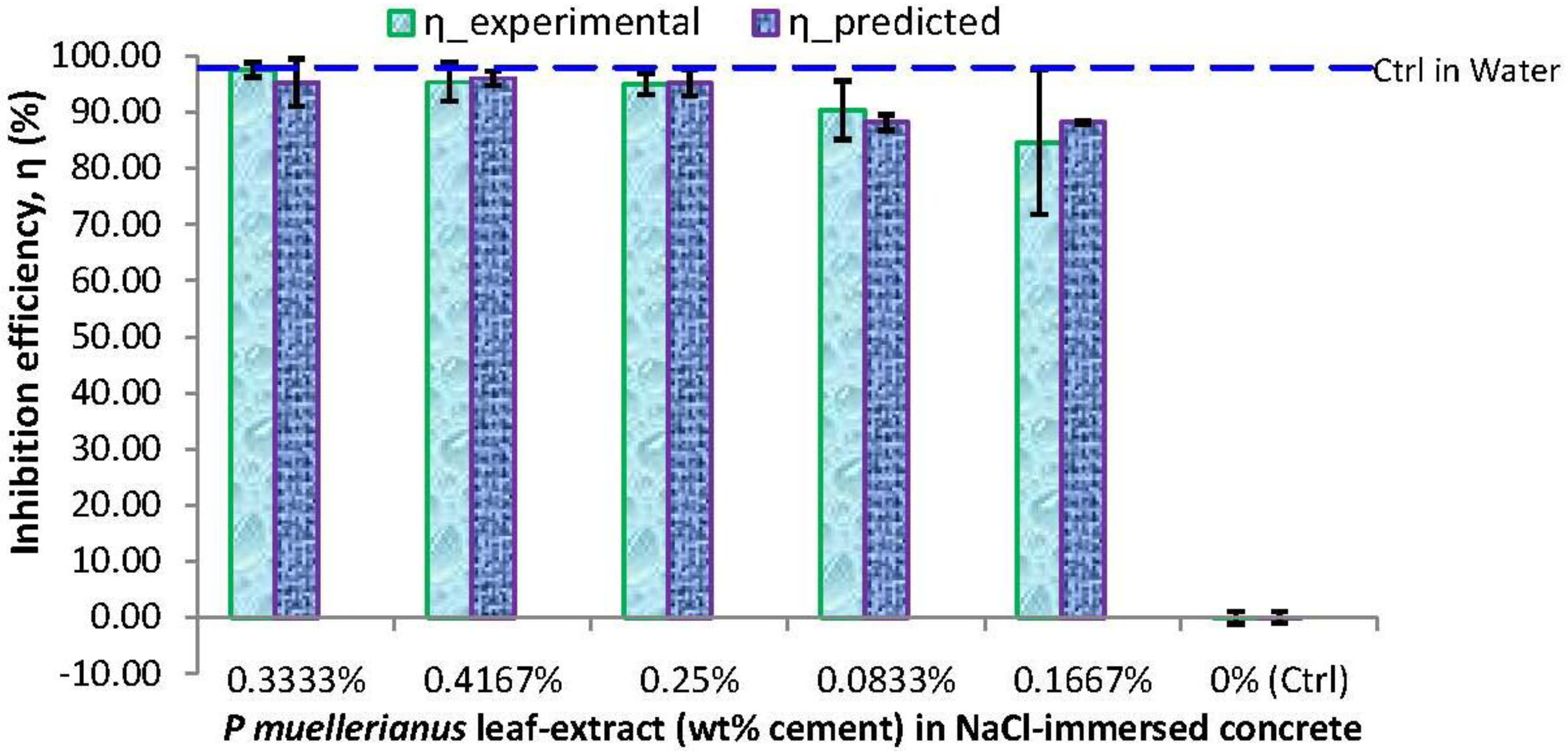
3.3. Corrosion Inhibition Effects by Experimental and Correlated Model
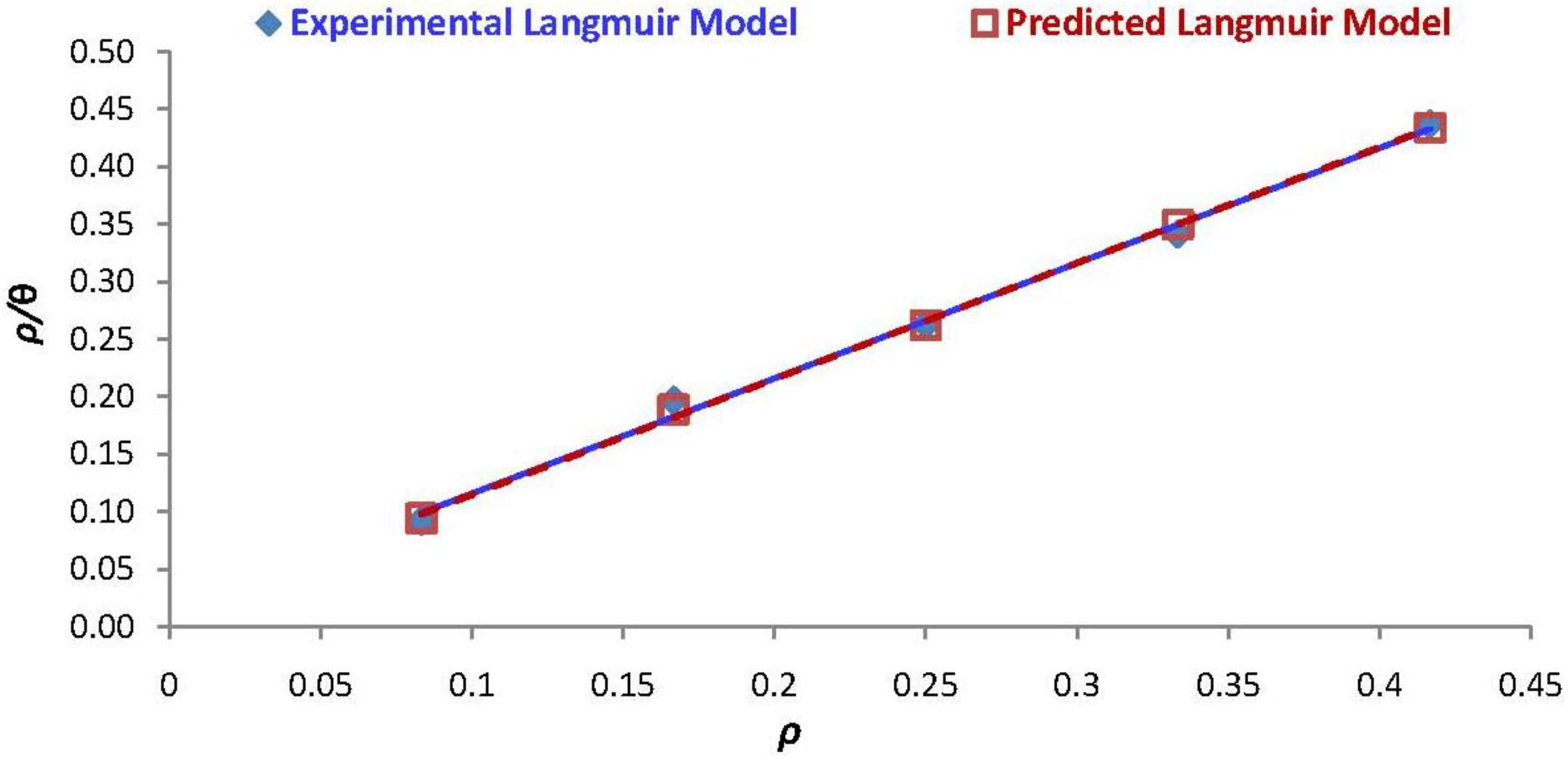
3.4. Adsorption Isotherm Model of Experimental and Correlated Data
3.5. Organic Bio-Constituent Model from P. muellerianus Leaf-Extract
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yang, Z.; Fischer, H.; Polder, R. Laboratory investigation of the influence of two types of modified hydrotalcites on chloride ingress into cement mortar. Cem. Concr. Compos. 2015, 58, 105–113. [Google Scholar] [CrossRef]
- Rosas, O.; Maya-Visuet, E.; Castaneda, H. Effect of chloride ions on the electrochemical performance of LDX 2003 alloy in concrete and simulated concrete-pore solutions. J. Appl. Electrochem. 2014, 44, 631–646. [Google Scholar] [CrossRef]
- Okeniyi, J.O. C10H18N2Na2O10 inhibition and adsorption mechanism on concrete steel-reinforcement corrosion in corrosive environments. J. Assoc. Arab Univ. Basic Appl. Sci. 2016, 20, 39–48. [Google Scholar] [CrossRef]
- Hu, J.; Koleva, D.A.; van Breugel, K. Corrosion performance of reinforced mortar in the presence of polymeric nano-aggregates: Electrochemical behavior, surface analysis, and properties of the steel/cement paste interface. J. Mater. Sci. 2012, 47, 4981–4995. [Google Scholar] [CrossRef]
- Garcés, P.; Saura, P.; Zornoza, E.; Andrade, C. Influence of pH on the nitrite corrosion inhibition of reinforcing steel in simulated concrete pore solution. Corros. Sci. 2011, 53, 3991–4000. [Google Scholar] [CrossRef]
- Tam, C.T.; Lim, H.B.; Sisomphon, K. Carbonation of concrete in the tropical environment of Singapore. IES J. Part A Civ. Struct. Eng. 2008, 1, 146–153. [Google Scholar] [CrossRef]
- Zhu, X.; Zi, G.; Cao, Z.; Cheng, X. Combined effect of carbonation and chloride ingress in concrete. Constr. Build. Mater. 2016, 110, 369–380. [Google Scholar] [CrossRef]
- Faustino, P.; Brás, A.; Ripper, T. The effect of corrosion inhibitors on the modelling of design lifetime of RC structures. Mater. Struct. 2015, 48, 1303–1319. [Google Scholar] [CrossRef]
- Okeniyi, J.O.; Loto, C.A.; Popoola, A.P.I. Corrosion inhibition of concrete steel-reinforcement in saline/marine simulating-environment by Rhizophora mangle L. Solid State Phenom. 2015, 227, 185–189. [Google Scholar] [CrossRef]
- Muthulingam, S.; Rao, B.N. Non-uniform time-to-corrosion initiation in steel reinforced concrete under chloride environment. Corros. Sci. 2014, 82, 304–315. [Google Scholar] [CrossRef]
- Rakanta, E.; Daflou, E.; Batis, G. Evaluation of organic corrosion inhibitor effectiveness into the concrete. In Measuring, Monitoring and Modeling Concrete Properties; Konsta-Gdoutos, M.S., Ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 605–611. [Google Scholar]
- Pang, L.; Li, Q. Service life prediction of RC structures in marine environment using long term chloride ingress data: Comparison between exposure trials and real structure surveys. Constr. Build. Mater. 2016, 113, 979–987. [Google Scholar] [CrossRef]
- Okeniyi, J.O.; Loto, C.A.; Popoola, A.P.I. Morinda lucida effects on steel-reinforced concrete in 3.5% NaCl: Implications for corrosion-protection of wind-energy structures in saline/marine environments. Energy Proced. 2014, 50, 421–428. [Google Scholar] [CrossRef]
- Biondini, F.; Camnasio, E.; Palermo, A. Lifetime seismic performance of concrete bridges exposed to corrosion. Struct. Infrastruct. Eng. 2014, 10, 880–900. [Google Scholar] [CrossRef]
- Boero, J.; Schoefs, F.; Yáñez-Godoy, H.; Capra, B. Time-function reliability of harbour infrastructures from stochastic modelling of corrosion. Eur. J. Environ. Civ. Eng. 2012, 16, 1187–1201. [Google Scholar] [CrossRef] [Green Version]
- Pakrashi, V.; Schoefs, F.; Memet, J.B.; O’Connor, A. ROC dependent event isolation method for image processing based assessment of corroded harbour structures. Struct. Infrastruct. Eng. 2010, 6, 365–378. [Google Scholar] [CrossRef]
- Cusson, D.; Qian, S.; Chagnon, N.; Baldock, B. Corrosion-inhibiting systems for durable concrete bridges. I: Five-year field performance evaluation. J. Mater. Civ. Eng. 2008, 20, 20–28. [Google Scholar] [CrossRef]
- Söylev, T.A.; McNally, C.; Richardson, M. Effectiveness of amino alcohol-based surface-applied corrosion inhibitors in chloride-contaminated concrete. Cem. Concr. Res. 2007, 37, 972–977. [Google Scholar] [CrossRef]
- Mehta, P.K. Concrete in the Marine Environment; CRC Press: New York, NY, USA, 2002. [Google Scholar]
- Yousif, H.A.; Al-Hadeethi, F.F.; Al-Nabilsy, B.; Abdelhadi, A.N. Corrosion of steel in high-strength self-compacting concrete exposed to saline environment. Int. J. Corros. 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Tarighat, A.; Zehtab, B. Structural reliability of reinforced concrete beams/columns under simultaneous static loads and steel reinforcement corrosion. Arab. J. Sci. Eng. 2016. [Google Scholar] [CrossRef]
- Budelmann, H.; Holst, A.; Wichmann, H.J. Non-destructive measurement toolkit for corrosion monitoring and fracture detection of bridge tendons. Struct. Infrastruct. Eng. 2014, 10, 492–507. [Google Scholar] [CrossRef]
- Omotosho, O.A.; Okeniyi, J.O.; Ajayi, O.O.; Loto, C.A. Effect of synergies of K2Cr2O7, K2CrO4, NaNO2 and aniline inhibitors on the corrosion potential response of steel reinforced concrete in saline medium. Int. J. Environ. Sci. 2012, 2, 2346–2259. [Google Scholar]
- Asipita, S.A.; Ismail, M.; Majid, M.Z.; Majid, Z.A.; Abdullah, C.; Mirza, J. Green Bambusa arundinacea leaves extract as a sustainable corrosion inhibitor in steel reinforced concrete. J. Clean Prod. 2014, 67, 139–146. [Google Scholar] [CrossRef]
- Patel, N.S.; Jauhariand, S.; Mehta, G.N.; Al-Deyab, S.S.; Warad, I.; Hammouti, B. Mild steel corrosion inhibition by various plant extracts in 0.5 M sulphuric acid. Int. J. Electrochem. Sci. 2013, 8, 2635–2655. [Google Scholar]
- Ismail, M.; Raja, P.B.; Salawu, A.A. Developing deeper understanding of green inhibitors for corrosion of reinforcing steel in concrete. In Handbook of Research on Recent Developments in Materials Science and Corrosion Engineering Education; Lim, H.L., Ed.; IGI Global: Hershey, PA, USA, 2015; pp. 118–146. [Google Scholar] [CrossRef]
- Khan, G.; Newaz, K.M.; Basirun, W.J.; Ali, H.B.; Faraj, F.L.; Khan, G.M. Application of natural product extracts as green corrosion inhibitors for metals and alloys in acid pickling processes—A review. Int. J. Electrochem. Sci. 2015, 10, 6120–6134. [Google Scholar]
- Abdulrahman, A.S.; Ismail, M.; Hussain, M.S. Corrosion inhibitors for steel reinforcement in concrete: A review. Sci. Res. Essays 2011, 6, 4152–4162. [Google Scholar] [CrossRef]
- Okafor, P.C.; Ikpi, M.E.; Uwah, I.E.; Ebenso, E.E.; Ekpe, U.J.; Umoren, S.A. Inhibitory action of Phyllanthus amarus extracts on the corrosion of mild steel in acidic media. Corros. Sci. 2008, 50, 2310–2317. [Google Scholar] [CrossRef]
- Etteyeb, N.; Dhouibi, L.; Takenouti, H.; Triki, E. Protection of reinforcement steel corrosion by phenyl phosphonic acid pre-treatment PART I: Tests in solutions simulating the electrolyte in the pores of fresh concrete. Cem. Concr. Compos. 2015, 55, 241–249. [Google Scholar] [CrossRef]
- Ormellese, M.; Lazzari, L.; Goidanich, S.; Fumagalli, G.; Brenna, A. A study of organic substances as inhibitors for chloride-induced corrosion in concrete. Corros. Sci. 2009, 51, 2959–2968. [Google Scholar] [CrossRef]
- Etteyeb, N.; Dhouibi, L.; Sanchez, M.; Alonso, C.; Andrade, C.; Triki, E. Electrochemical study of corrosion inhibition of steel reinforcement in alkaline solutions containing phosphates based components. J. Mater. Sci. 2007, 42, 4721–4730. [Google Scholar] [CrossRef]
- Okeniyi, J.O.; Omotosho, O.A.; Ogunlana, O.O.; Okeniyi, E.T.; Owoeye, T.F.; Ogbiye, A.S.; Ogunlana, E.O. Investigating prospects of Phyllanthus muellerianus as eco-friendly/sustainable material for reducing concrete steel-reinforcement corrosion in industrial/microbial environment. Energy Proced. 2015, 74, 1274–1281. [Google Scholar] [CrossRef]
- Agyare, C.; Lechtenberg, M.; Deters, A.; Petereit, F.; Hensel, A. Ellagitannins from Phyllanthus muellerianus (Kuntze) Exell.: Geraniin and furosin stimulate cellular activity, differentiation and collagen synthesis of human skin keratinocytes and dermal fibroblasts. Phytomedicine 2011, 18, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Zirihi, G.N.; Mambu, L.; Guédé-Guina, F.; Bodo, B.; Grellier, P. In vitro antiplasmodial activity and cytotoxicity of 33 West African plants used for treatment of malaria. J. Ethnopharmacol. 2005, 98, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Okeniyi, J.O.; Loto, C.A.; Popoola, A.P.I. Electrochemical performance of Phyllanthus muellerianus on the corrosion of concrete steel-reinforcement in industrial/microbial simulating-environment. Port. Electrochim. Acta 2014, 32, 199–211. [Google Scholar] [CrossRef]
- Patel, J.R.; Tripathi, P.; Sharma, V.; Chauhan, N.S.; Dixit, V.K. Phyllanthus amarus: Ethnomedicinal uses, phytochemistry and pharmacology: A review. J. Ethnopharmacol. 2011, 138, 286–313. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, M.; Rajendran, S.; Sathiyabama, J.; Krishnaveni, A.; Shanthy, P.; Manimaran, N.; Shyamaladevi, B. Corrosion inhibition by an aqueous extract of Phyllanthus amarus. Port. Electrochim. Acta 2011, 29, 429–444. [Google Scholar] [CrossRef]
- ASTM G109-99a. Standard Test Method for Determining the Effects of Chemical Admixtures on the Corrosion of Embedded Steel Reinforcement in Concrete Exposed to Chloride Environments; ASTM International: West Conshohocken, PA, USA, 2005. [Google Scholar]
- Yang, Y.; Scantlebury, J.D.; Koroleva, E.V. A study of calcareous deposits on cathodically protected mild steel in artificial seawater. Metals 2015, 5, 439–456. [Google Scholar] [CrossRef]
- Zafeiropoulou, T.; Rakanta, E.; Batis, G. Performance evaluation of organic coatings against corrosion in reinforced cement mortars. Prog. Org. Coat. 2011, 72, 175–180. [Google Scholar] [CrossRef]
- Mennucci, M.M.; Banczek, E.P.; Rodrigues, P.R.; Costa, I. Evaluation of benzotriazole as corrosion inhibitor for carbon steel in simulated pore solution. Cem. Concr. Compos. 2009, 31, 418–424. [Google Scholar] [CrossRef]
- Okeniyi, J.O.; Loto, C.A.; Popoola, A.P.I. Anticorrosion performance of Anthocleista djalonensis on steel-reinforced concrete in a sulphuric-acid medium. HKIE Trans. 2016. [Google Scholar] [CrossRef]
- Okeniyi, J.O.; Omotosho, O.A.; Ajayi, O.O.; Loto, C.A. Effect of potassium-chromate and sodium-nitrite on concrete steel-rebar degradation in sulphate and saline media. Constr. Build. Mater. 2014, 50, 448–456. [Google Scholar] [CrossRef]
- ASTM C876-91 R99. Standard Test Method for Half-Cell Potentials of Uncoated Reinforcing Steel in Concrete; ASTM International: West Conshohocken, PA, USA, 2005. [Google Scholar]
- Okeniyi, J.O.; Omoniyi, O.M.; Okpala, S.O.; Loto, C.A.; Popoola, A.P.I. Effect of ethylenediaminetetraacetic disodium dihydrate and sodium nitrite admixtures on steel-rebar corrosion in concrete. Eur. J. Environ. Civ. Eng. 2013, 17, 398–416. [Google Scholar] [CrossRef]
- Abdelaziz, G.E.; Abdelalim, A.M.; Fawzy, Y.A. Evaluation of the short and long-term efficiencies of electro-chemical chloride extraction. Cem. Concr. Res. 2009, 39, 727–732. [Google Scholar] [CrossRef]
- Sastri, V.S. Green Corrosion Inhibitors, Theory and Practice; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Okeniyi, J.O.; Oladele, I.O.; Omoniyi, O.M.; Loto, C.A.; Popoola, A.P.I. Inhibition and compressive-strength performance of Na2Cr2O7 and C10H14N2Na2O8·2H2O in steel-reinforced concrete in corrosive environments. Can. J. Civ. Eng. 2015, 42, 408–416. [Google Scholar] [CrossRef]
- Silaimani, S.M.; Vivekanandan, G.; Veeramani, P. Nano-nickel-copper alloy deposit for improved corrosion resistance in marine environment. Int. J. Environ. Sci. Technol. 2015, 12, 2299–2306. [Google Scholar] [CrossRef]
- Okeniyi, J.O.; Ambrose, I.J.; Okpala, S.O.; Omoniyi, O.M.; Oladele, I.O.; Loto, C.A.; Popoola, P.A.I. Probability density fittings of corrosion test-data: Implications on C6H15NO3 effectiveness on concrete steel-rebar corrosion. Sadhana 2014, 39, 731–764. [Google Scholar] [CrossRef]
- ASTM G16-95 R04. Standard Guide for Applying Statistics to Analysis of Corrosion Data; ASTM International: West Conshohocken, PA, USA, 2005. [Google Scholar]
- Okeniyi, J.O.; Okeniyi, E.T.; Atayero, A.A. Programming development of Kolmogorov-Smirnov goodness-of-fit testing of data normality as a Microsoft Excel® library function. J. Softw. Syst. Dev. 2015. [Google Scholar] [CrossRef]
- Okeniyi, J.O.; Okeniyi, E.T. Implementation of Kolmogorov-Smirnov p-value computation in Visual Basic®: Implication for Microsoft Excel® library function. J. Stat. Comput. Simul. 2012, 82, 1727–1741. [Google Scholar] [CrossRef]
- Pham, H. Basic statistical concepts. In Springer Handbook of Engineering Statistics; Pham, H., Ed.; Springer-Verlag: London, UK, 2006; pp. 3–48. [Google Scholar]
- Okeniyi, J.O.; Loto, C.A.; Popoola, A.P.I. Electrochemical performance of Anthocleista djalonensis on steel-reinforcement corrosion in concrete immersed in saline/marine simulating-environment. Trans. Indian Inst. Met. 2014, 67, 959–969. [Google Scholar] [CrossRef]
- Lai, C.D.; Pra Murthy, D.N.; Xie, M. Weibull distributions and their applications. In Springer Handbook of Engineering Statistics; Pham, H., Ed.; Springer-Verlag: London, UK, 2006; pp. 63–78. [Google Scholar]
- Eden, D.A. Electrochemical noise. In Uhlig’s Corrosion Handbook, 2nd ed.; Revie, R.W., Ed.; Wiley: New York, NY, USA, 2000; pp. 1227–1238. [Google Scholar]
- Kelly, R.G.; Inman, M.E.; Hudson, J.L. Analysis of electrochemical noise for type 410 stainless steel in chloride solutions. In Electrochemical Noise Measurement for Corrosion Applications: ASTM STP 1277; Kearns, J.R., Scully, J.R., Roberge, P.R., Eds.; American Society for Testing and Materials: West Conshohocken, PA, USA, 1996; pp. 101–103. [Google Scholar]
- Okeniyi, J.O.; Popoola, A.P.I.; Loto, C.A. Corrosion test-data modeling for C10H18N2Na2O10 performance on steel-rebar in NaCl-immersed concrete. In CORROSION 2015; NACE International: Houston, TX, USA, 2015. [Google Scholar]
- Bouhrira, K.; Chetouani, A.; Zerouali, D.; Hammouti, B.; Yahyi, A.; Et-Touhami, A.; Yahyaoui, R.; Touzani, R. Theoretical investigation of inhibition of the corrosion of A106 steel in NaCl solution by di-n-butyl bis (thiophene-2-carboxylato-O,O′) tin (IV). Res. Chem. Intermed. 2014, 40, 569–586. [Google Scholar] [CrossRef]
- Asegbeloyin, J.; Ejikeme, P.; Olasunkanmi, L.; Adekunle, A.; Ebenso, E. A novel Schiff base of 3-acetyl-4-hydroxy-6-methyl-(2H)pyran-2-one and 2,2’-(ethylenedioxy)diethylamine as potential corrosion inhibitor for mild steel in acidic medium. Materials 2015, 8, 2918–2934. [Google Scholar] [CrossRef]
- Peme, T.; Olasunkanmi, L.; Bahadur, I.; Adekunle, A.; Kabanda, M.; Ebenso, E. Adsorption and corrosion inhibition studies of some selected dyes as corrosion inhibitors for mild steel in acidic medium: Gravimetric, electrochemical, quantum chemical studies and synergistic effect with Iodide ions. Molecules 2015, 20, 16004–16029. [Google Scholar] [CrossRef] [PubMed]
- Afia, L.; Salghi, R.; Zarrouk, A.; Zarrok, H.; Bazzi, E.H.; Hammouti, B.; Zougagh, M. Comparative study of corrosion inhibition on mild steel in HCl medium by three green compounds: Arganiaspinosa press cake, kernels and hulls extracts. Trans. Indian Inst. Met. 2013, 66, 43–49. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Mehrizad, A.; Aghaie, M.; Gharbani, P.; Dastmalchi, S.; Monajjemi, M.; Zare, K. Comparison of 4-chloro-2-nitrophenol adsorption on single-walled and multi-walled carbon nanotubes. Iran. J. Environ. Health Sci. Eng. 2012, 9, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bungey, J.H.; Millard, S.G.; Grantham, M.G. Testing of Concrete in Structures, 4th ed.; Taylor & Francis: New York, NY, USA, 2006. [Google Scholar]
- Broomfield, J.P. Corrosion of Steel in Concrete: Understanding, Investigation and Repair; Taylor & Francis e-Library: New York, NY, USA, 2003. [Google Scholar]
- McCarter, W.J.; Vennesland, Ø. Sensor systems for use in reinforced concrete structures. Constr. Build. Mater. 2004, 18, 351–358. [Google Scholar] [CrossRef]
- Coffey, R.; Dorai-Raj, S.; O’Flaherty, V.; Cormican, M.; Cummins, E. Modeling of pathogen indicator organisms in a small-scale agricultural catchment using SWAT. Hum. Ecol. Risk Assess. Int. J. 2013, 19, 232–253. [Google Scholar] [CrossRef]
- Okeniyi, J.O.; Ambrose, I.J.; Oladele, I.O.; Loto, C.A.; Popoola, P.A.I. Electrochemical performance of sodium dichromate partial replacement models by triethanolamine admixtures on steel-rebar corrosion in concretes. Int. J. Electrochem. Sci. 2013, 8, 10758–10771. [Google Scholar]
- Okeniyi, J.O.; Ogunlana, O.O.; Ogunlana, O.E.; Owoeye, T.F.; Okeniyi, E.T. Biochemical characterisation of the leaf of Morinda lucida: Prospects for environmentally-friendly steel-rebar corrosion-protection in aggressive medium. In TMS2015 Supplemental Proceedings; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 635–644. [Google Scholar] [CrossRef]
- Al-Amiery, A.; Al-Majedy, Y.; Kadhum, A.; Mohamad, A. New coumarin derivative as an eco-friendly inhibitor of corrosion of mild steel in acid medium. Molecules 2015, 20, 366–383. [Google Scholar] [CrossRef] [PubMed]
- Kadhum, A.; Mohamad, A.; Hammed, L.; Al-Amiery, A.; San, N.; Musa, A. Inhibition of mild steel corrosion in hydrochloric acid solution by new coumarin. Materials 2014, 7, 4335–4348. [Google Scholar] [CrossRef]
- Al-Amiery, A.; Kadhum, A.; Kadihum, A.; Mohamad, A.; How, C.; Junaedi, S. Inhibition of mild steel corrosion in sulfuric acid solution by new Schiff base. Materials 2014, 7, 787–804. [Google Scholar] [CrossRef]
- Morad, M.S. Corrosion inhibition of mild steel in sulfamic acid solution by S-containing amino acids. J. Appl. Electrochem. 2008, 38, 1509–1518. [Google Scholar] [CrossRef]



| λ | aλ |
|---|---|
| 0 | 0.6396 |
| 1 | 4.6268 |
| 2 | 29.3848 |
| 3 | 49.3821 |
| 4 | 31.1072 |
| 5 | 6.2572 |
| Source of Variations | df | SS | MS | F | p-Value |
|---|---|---|---|---|---|
| Treatment | 6 | 33.3438 | 5.5573 | 34.7807 | 0.0006 |
| Residual | 5 | 0.7989 | 0.1598 | ||
| Total | 11 | 34.1428 |
| Isotherm Parameter | Experimental Model | Predicted Model |
|---|---|---|
| Kads | 62.9422 | 71.1493 |
| R, correlation coefficient, (%) | 99.51 | 99.90 |
| RL, separation factor | 3.2995 × 10−3 | 2.9192 × 10–3 |
| ΔGads Gibbs free energy (kJ/mol) | –20.3492 | –20.6549 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okeniyi, J.O.; Loto, C.A.; Popoola, A.P.I. Effects of Phyllanthus muellerianus Leaf-Extract on Steel-Reinforcement Corrosion in 3.5% NaCl-Immersed Concrete. Metals 2016, 6, 255. https://doi.org/10.3390/met6110255
Okeniyi JO, Loto CA, Popoola API. Effects of Phyllanthus muellerianus Leaf-Extract on Steel-Reinforcement Corrosion in 3.5% NaCl-Immersed Concrete. Metals. 2016; 6(11):255. https://doi.org/10.3390/met6110255
Chicago/Turabian StyleOkeniyi, Joshua Olusegun, Cleophas Akintoye Loto, and Abimbola Patricia Idowu Popoola. 2016. "Effects of Phyllanthus muellerianus Leaf-Extract on Steel-Reinforcement Corrosion in 3.5% NaCl-Immersed Concrete" Metals 6, no. 11: 255. https://doi.org/10.3390/met6110255







