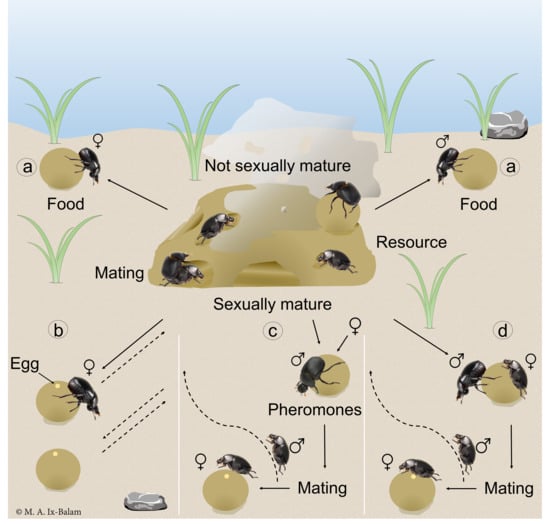
The Rolling of Food by Dung Beetles Affects the Oviposition of Competing Flies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Samples
2.2. Behavioural Analysis
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Halffter, G.; Edmonds, D.W. The Nesting Behavior of Dung Beetles (Scarabeinae): An Ecological and Evolutive Approach; Instituto de Ecología: México D.F., Mexico, 1982. [Google Scholar]
- Scholtz, C.H.; Davis, A.L.V.; Kryger, U. Evolutionary Biology and Conservation of Dung Beetles; Pensoft Publishers: Sofia, Bulgaria, 2009; ISBN 978-954-642-517-1. [Google Scholar]
- Hanski, I.; Cambefort, Y. Competition in dung beetles. In Dung Beetles Ecology; Hanski, I., Cambefort, Y., Eds.; Princeton University Press: Princeton, NJ, USA, 1991; pp. 305–329. ISBN 0-691-08739-3. [Google Scholar]
- Bornemissza, G.F. Insectary studies on the control of dung breeding flies by the activity of the dung beetle, Onthophagus gazella F. (Coleoptera: Scarabaeinae). Aust. Entomol. 1970, 9, 31–41. [Google Scholar] [CrossRef]
- Bellés, X.; Favila, M.E. Protection chimique du nid chez Canthon cyanellus cyanellus LeConte (Col. Scarabaeidae). Bull. Soc. Entomol. Fr. 1983, 88, 602–607. [Google Scholar]
- Hirschberger, P.; Degro, H.N. Oviposition of the dung beetle Aphodius ater in relation to the abundance of yellow dungfly larvae (Scatophaga stercoraria). Ecol. Entomol. 1996, 21, 352–357. [Google Scholar] [CrossRef]
- Favila, M.E. Chemical labelling of the food ball during rolling by males of the subsocial coleopteran Canthon cyanellus cyanellus Leconte (Scarabaeidae). Insectes Soc. 1988, 35, 125–129. [Google Scholar] [CrossRef]
- Cortez, V.; Favila, M.E.; Verdú, J.R.; Ortiz, A.J. Behavioral and antennal electrophysiological responses of a predator ant to the pygidial gland secretions of two species of Neotropical dung roller beetles. Chemoecology 2012, 22, 29–38. [Google Scholar] [CrossRef]
- Cortez, V.; Verdú, J.R.; Ortiz, A.J.; Trigos, Á.R.; Favila, M.E. Chemical diversity and potential biological functions of the pygidial gland secretions in two species of Neotropical dung roller beetles. Chemoecology 2015, 25, 201–213. [Google Scholar] [CrossRef]
- Burger, B.V.; Petersen, W.G.B. Semiochemicals of the Scarabaenae: VI. Identification of EAD-active constituents of abdominal secretion of male dung beetle, Kheper nigroaeneus. J. Chem. Ecol. 2002, 28, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Burger, B.V.; Petersen, W.G.B.; Weber, W.G.; Munro, Z.M. Semiochemicals of the Scarabaeinae. VII: Identification and synthesis of EAD-active constituents of abdominal sex attracting secretion of the male dung beetle, Kheper subaeneus. J. Chem. Ecol. 2002, 28, 2527–2539. [Google Scholar] [CrossRef] [PubMed]
- Burger, B.V.; Petersen, W.G.B.; Tribe, G.D. Semiochemicals of the Scarabaeinae, V: Characterization of the defensive secretion of the dung beetle Oniticellus egregius. Z. Naturforsch. C 1995, 50, 681–684. [Google Scholar] [CrossRef]
- Burger, B.V. First investigation of the semiochemistry of South African dung beetle species. In Neurobiology of Chemical Communication; Mucignat-Caretta, C., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 57–97. ISBN 978-1-4665-5341-5. [Google Scholar]
- Falqueto, S.A.; Vaz-De-Mello, F.; Schoereder, J.H. Are fungivorous Scarabaeidae less specialist? Ecol. Austral 2005, 15, 17–22. [Google Scholar]
- Capinera, J.L. Australian sheep blowfly, Lucilia cuprina Wiedemann (Diptera: Calliphoridae). In Encyclopedia of Entomology, 2nd ed.; Capinera, J.L., Ed.; Springer: Gainesville, FL, USA, 2008; Volume 4, pp. 335–338. ISBN 978-1-4020-6242-1. [Google Scholar]
- R Development Core Team. The R Foundation for Statistical Computing; Ver. 3.4.1; Vienna University of Technology: Vienna, Austria, 2014; Available online: http://www.r-project.org/ (accessed on 13 June 2018).
- Favila, M.E. Historia de vida y comportamiento de un escarabajo necrofago: Canthon cyanellus cyanellus LeConte (Coleoptera: Scarabaeinae). Folia Entomol. Mex. 2001, 40, 245–278. [Google Scholar]
- Pluot-Sigwalt, D. La diversité du système des glandes tégumentaires abdominales des scarabaeidae (S. Str.) (Coleoptera): Morphologie et répartition des structures cuticulaires. Ann. Soc. Entomol. Fr. 1995, 31, 295–348. [Google Scholar]
- Houston, W.W.K. Exocrine glands in the forelegs of dung beetles in the genus Onitis F. (Coleoptera: Scarabaeidae). Aust. Entomol. 1986, 25, 161–169. [Google Scholar] [CrossRef]
- Favila, M.E. Some ecological factors affecting the life-style of Canthon cyanellus cyanellus (Coleoptera Scarabaeidae): An experimental approach. Ethol. Ecol. Evol. 1993, 5, 319–328. [Google Scholar] [CrossRef]
- Cortes-Gallardo, V.; Favila, M.E. Actividad antifúngica del ácido 4-metoxi fenilácetico producido en las glándulas esternales de machos del escarabajo rodador Canthon cyanellus cyanellus (Coleoptera: Scarabaeinae). In Entomología Mexicana; Estrada, V.E., Equihua, M.A., Luna, L.C., Rosas, A.J., Eds.; Sociedad Mexicana de Entomología-Colegio de Postgraduados: Texcoco, Mexico, 2007; Volume 6, pp. 355–359. ISBN 968839517X. [Google Scholar]
- Emmens, R.L.; Murray, M.D. Bacterial odours as oviposition stimulants for Lucilia cuprina (Wiedemann) (Diptera: Calliphoridae), the Australian sheep blowfly. Bull. Entomol. Res. 1983, 73, 411–415. [Google Scholar] [CrossRef]
- Romero, A.; Broce, A.; Zurek, L. Role of bacteria in the oviposition behaviour and larval development of stable flies. Med. Vet. Entomol. 2006, 20, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.; Babor, D.; Duthie, B.; Babor, E.M.; Moore, M.; Gries, G. Proliferating bacterial symbionts on house fly eggs affect oviposition behaviour of adult flies. Anim. Behav. 2007, 74, 81–92. [Google Scholar] [CrossRef]
- Thompson, C.R.; Brogan, R.S.; Scheifele, L.Z.; Rivers, D.B. Bacterial interactions with necrophagous flies. Ann. Entomol. Soc. Am. 2013, 106, 799–809. [Google Scholar] [CrossRef]
- Zheng, L.; Crippen, T.; Holmes, L.; Singh, B.; Pimsler, M.; Benbow, M.; Tarone, A.; Dowd, S.; Yu, Z.; Vanlaerhoven, S.; et al. Bacteria mediate oviposition by the black soldier fly, Hermetia illucens (L.), (Diptera: Stratiomyidae). Sci. Rep. 2013, 3, 2563. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.; Geisreiter, C.; Gries, G. Ovipositing female house flies provision offspring larvae with bacterial food. Entomol. Exp. Appl. 2009, 133, 292–295. [Google Scholar] [CrossRef]
- Bing, X.; Attardo, G.M.; Vigneron, A.; Aksoy, E.; Scolari, F.; Malacrida, A.; Weiss, B.L.; Aksoy, S. Unravelling the relationship between the tsetse fly and its obligate symbiont Wigglesworthia: Transcriptomic and metabolomic landscapes reveal highly integrated physiological networks. Proc. R. Soc. B 2017, 284, 20170360. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ix-Balam, M.A.; A. Oliveira, M.G.; Louzada, J.; McNeil, J.N.; Lima, E. The Rolling of Food by Dung Beetles Affects the Oviposition of Competing Flies. Insects 2018, 9, 92. https://doi.org/10.3390/insects9030092
Ix-Balam MA, A. Oliveira MG, Louzada J, McNeil JN, Lima E. The Rolling of Food by Dung Beetles Affects the Oviposition of Competing Flies. Insects. 2018; 9(3):92. https://doi.org/10.3390/insects9030092
Chicago/Turabian StyleIx-Balam, Manuel A., Maria G. A. Oliveira, Júlio Louzada, Jeremy N. McNeil, and Eraldo Lima. 2018. "The Rolling of Food by Dung Beetles Affects the Oviposition of Competing Flies" Insects 9, no. 3: 92. https://doi.org/10.3390/insects9030092
APA StyleIx-Balam, M. A., A. Oliveira, M. G., Louzada, J., McNeil, J. N., & Lima, E. (2018). The Rolling of Food by Dung Beetles Affects the Oviposition of Competing Flies. Insects, 9(3), 92. https://doi.org/10.3390/insects9030092