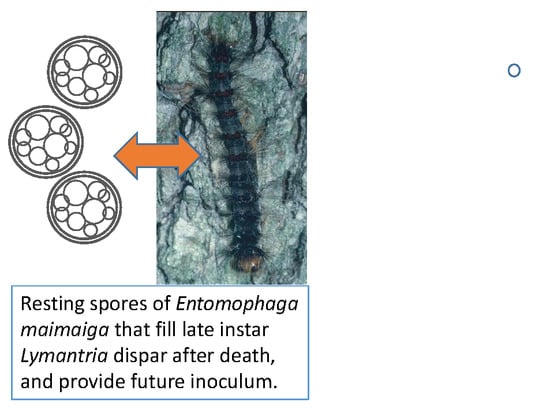
Sleeping Beauties: Horizontal Transmission via Resting Spores of Species in the Entomophthoromycotina
Abstract
1. Introduction
2. Systematics and Habits
3. General Biology
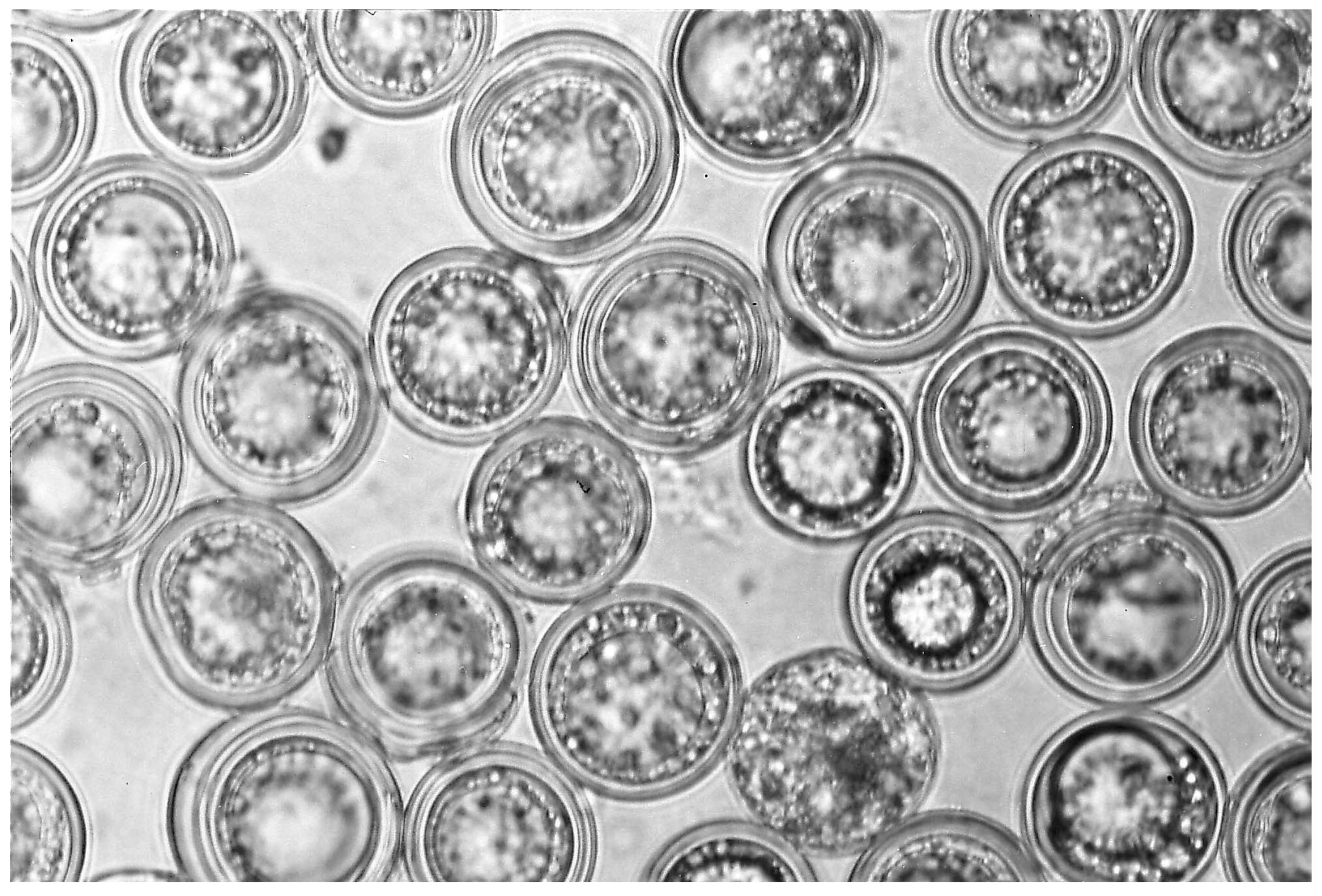
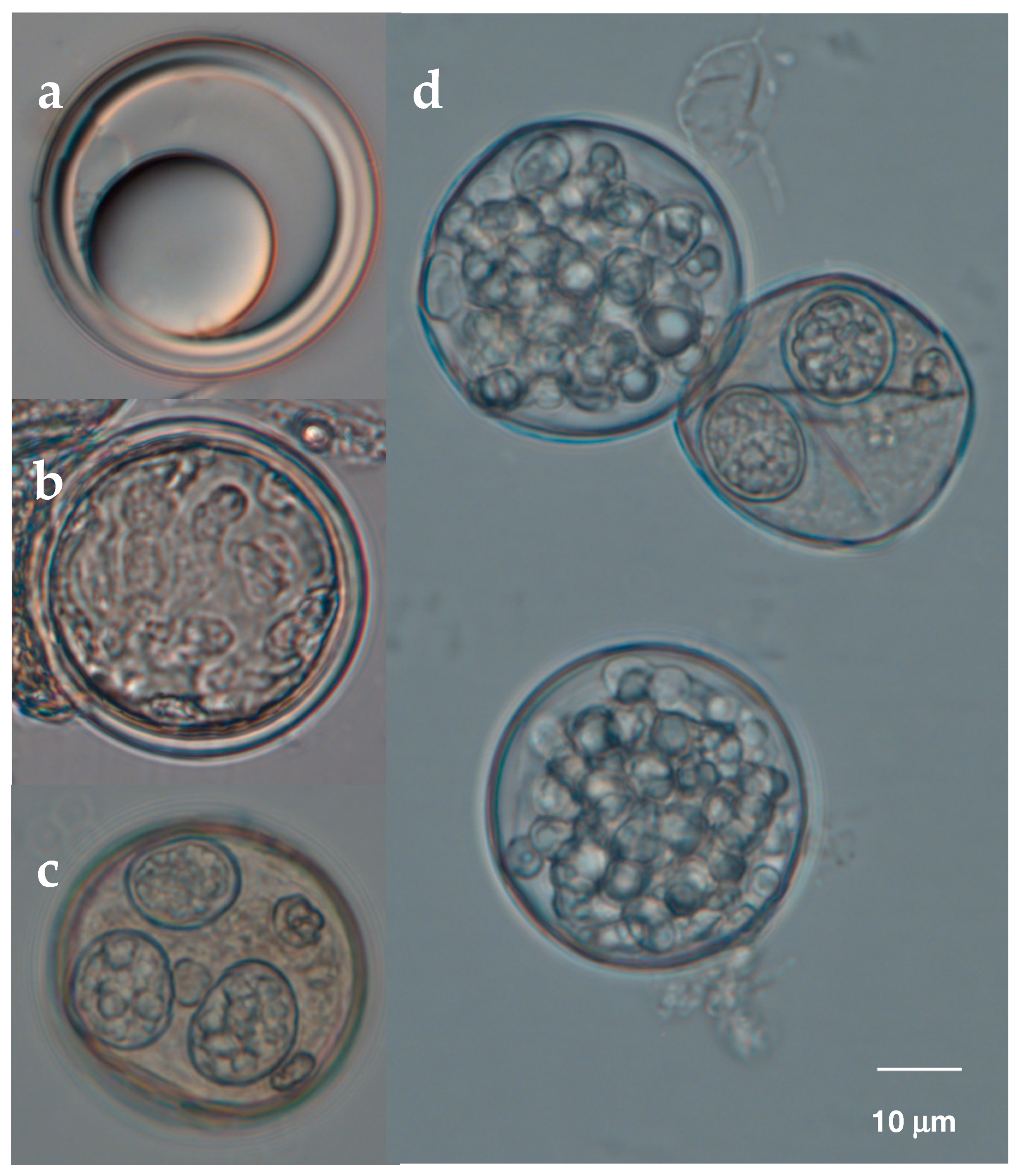
3.1. Resting Spores Versus Conidia and Species Identity
3.2. Resting Spore Production
3.3. Differential Locations of Cadavers Producing Resting Spores versus Conidia: Six Case Histories
- Massospora cicadina and Periodical Cicadas (Magicicada spp.)
- Neozygites fresenii and Cotton Aphids (Aphis gossypii)
- Furia virescens and the True Armyworm (Mythimna unipuncta)
- Erynia aquatica and Aedes spp. Mosquitoes
- Eryniopsis lampyridarum and Soldier Beetles (Chauliognathus spp.)
- Entomophaga maimaiga and Gypsy Moth (Lymantria dispar)
3.3.1. Massospora cicadina Infections in Periodical Cicadas
3.3.2. Neozygites fresenii Infections in Aphis spp.
3.3.3. Furia virescens in the True Armyworm
3.3.4. Erynia aquatica Infections in Aedes spp. Mosquitoes
3.3.5. Eryniopsis lampyridarum Infections in Soldier Beetles
3.3.6. Entomophaga maimaiga and Gypsy Moth, Lymantria dispar
4. Resting Spores in the Environment
4.1. Quantification of Resting Spores in Soil
4.2. Spatial Distribution and Densities in the Environment
4.3. Resting Spore Activity
4.3.1. Dormancy and Germination
4.3.2. Horizontal Transmission Due to Resting Spores
4.3.3. Persistence
4.4. Ecosystem Level Interactions
5. Use of Resting Spores for Control
6. Conclusions: Importance of Resting Spores to Epizootiology
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sinclair, R.; Boone, S.A.; Greenberg, D.; Keim, P.; Gerba, C.P. Persistence of Category A select agents in the environment. Appl. Environ. Microbiol. 2007, 74, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Ewald, P.W. Evolution of Infectious Diseases; Oxford Univsity Press: Oxford, UK, 1994. [Google Scholar]
- Clayton, D.H.; Tompkins, D.M. Ectoparasite virulence is linked to mode of transmission. Proc. R. Soc. Lond. B 1994, 256, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.D.; Logsdon, J.M., Jr.; Kelley, S.E. An empirical study of the evolution of virulence under both horizontal and vertical transmission. Evolution 2005, 59, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Bałazy, S. Flora of Poland. Fungi (Mycota), Vol XXIV, Entomophthorales; Polish Academy of Sciences: Krakow, Poland, 1993. [Google Scholar]
- Pell, J.; Eilenberg, J.; Hajek, A.E.; Steinkraus, D.C. Biology, ecology and pest management potential of Entomophthorales. In Fungi as Biocontrol Agents: Progress, Problems and Potential; Butt, T.M., Jackson, C.W., Magan, N., Eds.; CABI Publishing: Wallingford, UK, 2001; pp. 71–153. [Google Scholar]
- Nielsen, C.; Jensen, A.B.; Eilenberg, J. Survival of entomophthoralean fungi infecting aphids and higher flies during unfavorable conditions and implications for conservation biological control. In Use of Entomopathogenic Fungi in Biological Pest Management; Ekesi, S., Maniana, N.K., Eds.; Research Signpost: Kerala, India, 2007; pp. 13–38. [Google Scholar]
- Humber, R.A. Entomophthoromycota: A new phylum and reclassification for entomophthoroid fungi. Mycotaxon 2012, 120, 477–492. [Google Scholar] [CrossRef]
- James, T.Y.; Kauff, F.; Schoch, C.; Matheny, P.B.; Hofstetter, V.; Cox, C.J.; Celio, G.; Gueidan, C.; Fraker, E.; Miadlikowska, J.; et al. Reconstructing the early evolution of fungi using a six-gene phylogeny. Nature 2006, 443, 818–822. [Google Scholar] [CrossRef] [PubMed]
- White, M.M.; James, T.Y.; O’Donnell, K.; Cafaro, M.J.; Tanabe, Y.; Sugiyama, J. Phylogeny of the Zygomycota based on nuclear ribosomal requence data. Mycologia 2006, 98, 872–884. [Google Scholar] [CrossRef] [PubMed]
- Hibbett, D.S.; Binder, M.; Bischoff, J.F.; Blackwell, M.; Cannon, P.F.; Eriksson, O.E.; Huhndorf, S.; James, T.; Kirk, P.M.; Lücking, R.; et al. A higher-level phylogenetic classification of the Fungi. Mycol. Res. 2007, 111, 509–547. [Google Scholar] [CrossRef] [PubMed]
- Gryganski, A.P.; Humber, R.A.; Smith, M.E.; Miadlikowska, J.; Wu, S.; Voigt, K.; Walther, G.; Anishchenko, I.M.; Vilgalys, R. Molecular phylogeny of the Entomophthoromycota. Mol. Phylogen. Evol. 2012, 65, 682–694. [Google Scholar] [CrossRef] [PubMed]
- Gryganski, A.P.; Humber, R.A.; Smith, M.E.; Hodge, K.; Huang, B.; Voigt, K.; Vilgalys, R. Phylogenetic lineages in Entomophthoromycota. Persoonia 2013, 30, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Spatafora, J.W.; Chang, Y.; Benny, G.L.; Lazarus, K.; Smith, M.E.; Berbee, M.L.; Bonito, G.; Corradi, N.; Grigoriev, I.; Gryganskyi, A.; et al. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 2016, 108, 1028–1046. [Google Scholar] [CrossRef] [PubMed]
- Hajek, A.E.; Gryganskyi, A.; Bittner, T.; Liebherr, J.K.; Liebherr, J.H.; Moulton, J.K.; Jensen, A.B.; Humber, R.A. Phylogenetic placement of two species known only from resting spores: Zoophthora independentia sp. nov. and Z. porteri comb nov. (Entomophthorales: Entomophthoraceae). J. Invertebr. Pathol. 2016, 140, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Humber, R.A. Synopsis of a revised classification for the Entomophthorales (Zygomycotina). Mycotaxon 1989, 34, 441–460. [Google Scholar]
- Keller, S. Fungal structures and biology. In Arthropod-Pathogenic Entomophthorales: Biology, Ecology and Identification; Keller, S., Ed.; COST Office: Luxembourg, 2007; pp. 27–54. [Google Scholar]
- Keller, S. Systematics, taxonomy and identification. In Arthropod-Pathogenic Entomophthorales: Biology, Ecology and Identification; Keller, S., Ed.; COST Office: Luxembourg, 2007; pp. 111–126. [Google Scholar]
- Keller, S. The arthropod-pathogenic Entomophthorales from Switzerland—is central Europe the centre of their global species-richness? Mitt. Schweiz Entomol. Gesellsch. 2008, 81, 39–51. [Google Scholar]
- Keller, S.; Petrini, O. Keys to the identification of arthropod pathogenic genera of the families Entomophthoraceae and Neozygitaceae (Zygomycetes), with description of three new subfamilies and a new genus. Sydowia 2005, 57, 23–53. [Google Scholar]
- Humber, R.A. The systematics of the genus Strongwellsea (Zygomycetes: Entomophthorales). Mycologia 1976, 68, 1042–1060. [Google Scholar] [CrossRef] [PubMed]
- Eidam, E. Basidiobolus, eine neue Gattung der Entomophthoraceen. Beitrage Biol. 1886, 4, 181–251. [Google Scholar]
- Keller, S. Validation of the combination Apterivorax acaricida (Petch) S. Keller. Sydowia 2006, 58, 75. [Google Scholar]
- Steinkraus, D.C.; Hollingsworth, R.G.; Slaymaker, P.H. Prevalence of Neozygites fresenii (Entomophthorales: Neozygotaceae) on cotton aphids (Homoptera: Aphididae) in Arkansas cotton. Environ. Entomol. 1995, 24, 465–474. [Google Scholar] [CrossRef]
- Van der Geest, L.P.S.; Elliot, S.L.; Breeuwer, J.A.J.; Beerling, E.A.M. Diseases of mites. Exp. Appl. Acarol. 2000, 24, 497–560. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ze’ev, I.S.; Kenneth, R.G.; Uziel, A. A reclassification of Entomophthora turbinata in Thaxterosporium gen. nov., Neozygitaceae fam. nov. (Zygomycetes: Entomophthorales). Mycotaxon 1987, 28, 313–326. [Google Scholar]
- Berda, H. Revision of the genus Ancylistes. Mycologia 1938, 30, 396–415. [Google Scholar] [CrossRef]
- Montalva, C.; Rocha, L.F.N.; Fernandes, E.K.K.; Luz, C.; Humber, R.A. Conidiobolus macrosporus (Entomophthorales), a mosquito pathogen in Central Brazil. J. Invertebr. Pathol. 2016, 139, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Bernard, E.C.; Arroyo, T.L. Development, distribution and host studies of the fungus Macrobiotophthora vermicola (Entomophthorales). J. Nematol. 1990, 22, 39–44. [Google Scholar] [PubMed]
- Tucker, B.E. A review of the nonentomogenous Entomophthorales. Mycotaxon 1981, 13, 481–505. [Google Scholar]
- Hajek, A.E. Pathology and epizootiology of Entomophaga maimaiga infections in forest Lepidoptera. Microbiol. Mol. Biol. Rev. 1990, 63, 814–835. [Google Scholar]
- Ramoska, W.A.; Hajek, A.E.; Ramos, M.E.; Soper, R.S. Infection of grasshoppers by members of the Entomophaga grylli species complex. J. Invertebr. Pathol. 1988, 52, 309–313. [Google Scholar] [CrossRef]
- Brobyn, P.J.; Wilding, N. Invasive and developmental processes of Entomophthora muscae infecting houseflies (Musca domestica). Trans. Br. Mycol. Soc. 1983, 80, 1–8. [Google Scholar] [CrossRef]
- Samson, R.A.; Ramakers, P.M.J.; Oswald, T. Entomophthora thripidum, a new fungal pathogen of Thrips tabaci. Can. J. Bot. 1979, 57, 1317–1323. [Google Scholar] [CrossRef]
- Anderson, J.F.; Ringo, S.L. Entomophthora aquatica sp. n. infecting larvae and pupae of floodwater mosquitoes. J. Invertebr. Pathol. 1969, 13, 386–393. [Google Scholar] [CrossRef]
- Hywel-Jones, N.L.; Webster, J. Mode of infection of Simulium by Erynia conica. Trans. Br. Mycol. Soc. 1986, 87, 381–387. [Google Scholar] [CrossRef]
- Filotas, M.J.; Hajek, A.E.; Humber, R.A. Prevalence and biology of Furia gastropachae (Zygomycetes: Entomophthorales) in populations of forest tent caterpillar (Lepidoptera: Lasiocampidae). Can. Entomol. 2003, 135, 359–378. [Google Scholar] [CrossRef]
- Duke, L.; Steinkraus, D.C.; English, J.E.; Smith, K.G. Infectivity of resting spores of Massospora cicadina (Entomophthorales: Entomophthoraceae), an entomopathogenic fungus of periodical cicadas (Magicicada spp.) (Homoptera: Cicadidae). J. Invertebr. Pathol. 2002, 80, 1–6. [Google Scholar] [CrossRef]
- Steinkraus, D.C.; Oliver, J.B.; Humber, R.A.; Gaylor, M.J. Mycosis of bandedwinged whitefly (Trialeurodes abutilonea) (Homoptera: Aleyrodidae) caused by Orthomyces aleyrodis gen. & sp. nov. (Entomophthorales: Entomophthoraceae). J. Invertebr. Pathol. 1998, 72, 1–8. [Google Scholar] [PubMed]
- MacLeod, D.M.; Tyrrell, D.; Soper, R.S.; de Lyzer, A.J. Entomophthora bullata as a pathogen of Sarcophaga aldrichi. J. Invertebr. Pathol. 1973, 22, 75–79. [Google Scholar] [CrossRef]
- Shah, P.A.; Clark, S.J.; Pell, J.K. Assessment of aphid host range and isolate variability in Pandora neoaphidis (Zygomycetes: Entomophthorales). Biol. Contr. 2004, 29, 90–99. [Google Scholar] [CrossRef]
- Hajek, A.E. Entomophaga maimaiga reproductive output is determined by spore type initiating an infection. Mycol. Res. 1997, 101, 971–974. [Google Scholar] [CrossRef]
- Elliot, S.L.; Mumford, J.D.; de Moraes, G.J. The role of resting spores in the survival of the mite pathogenic fungus Neozygites floridana from Mononychellus tanajoa during dry periods in Brazil. J. Invertebr. Pathol. 2002, 81, 148–157. [Google Scholar] [CrossRef]
- Klingen, I.; Wærsted, G.; Westrum, K. Overwintering and prevalence of Neozygites floridana (Zygomycetes: Entomophthorales) in hibernating females of Tetranychus urticae (Acari: Tetranychidae) under cold climatic conditions in strawberries. Exp. Appl. Acarol. 2008, 46, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Hajek, A.E.; Humber, R.A. Formation and germination of Entomophaga maimaiga azygospores. Can. J. Bot. 1997, 75, 1739–1747. [Google Scholar] [CrossRef]
- Hajek, A.E.; Burke, A.; Nielsen, C.; Hannam, J.J.; Bauer, L.S. Nondormancy of Entomophaga maimaiga azygospores: Effects of isolate and cold storage. Mycologia 2008, 100, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Humber, R.A.; Ramoska, W.A. Variations in entomophthoralean life cycles: Practical implications. In Fundamental and Applied Aspects of Invertebrate Pathology; Samson, R.A., Vlak, J.M., Peters, D., Eds.; Foundation of the 4th International Colloquium of Invertebrate Pathology: Wageningen, The Netherlands, 1986; pp. 190–193. [Google Scholar]
- Steinkraus, D.C.; Kramer, J.P. Development of resting spores of Erynia aquatica (Zygomycetes: Entomophthoraceae) in Aedes aegypti (Diptera: Culicidae). Environ. Entomol. 1989, 18, 1147–1152. [Google Scholar] [CrossRef]
- Hajek, A.E.; Shimazu, M. Types of spores produced by Entomophaga maimaiga infecting the gypsy moth Lymantria dispar. Can. J. Bot. 1996, 74, 708–715. [Google Scholar] [CrossRef]
- Shimazu, M. Effect of rearing humidity of host insects on the spore types of Entomophaga maimaiga Humber, Shimazu et Soper (Entomophthorales: Entomophthoraceae). Appl. Entomol. Zool. 1987, 22, 394–397. [Google Scholar] [CrossRef]
- Huang, Z.-H.; Feng, M.-G. Resting spore formation of aphid-pathogenic fungus Pandora nouryi depends on the concentration of infective inoculum. Environ. Microbiol. 2008, 10, 1912–1916. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Feng, M.-G. Biotic and abiotic regulation of resting spore formation in vivo of obligate aphid pathogen Pandora nouryi: Modeling analysis and biological implication. J. Invertebr. Pathol. 2010, 103, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Feng, M.G.; Zhang, L.-Q. The role of temperature on in vivo resting spore formation of the aphid-specific pathogen Pandora nouryi (Zygomycota: Entomophthorales) under winter field conditions. Biocontr. Sci. Technol. 2012, 22, 93–100. [Google Scholar] [CrossRef]
- Thomsen, L.; Bresciani, J.; Eilenberg, J. Formation and germination of resting spores from different strains from the Entomophthora muscae complex produced by Musca domestica. Can. J. Bot. 2001, 79, 1076–1082. [Google Scholar]
- Thomsen, L.; Eilenberg, J. Entomophthora muscae resting spore formation in vivo in the host Delia radicum. J. Invertebr. Pathol. 2000, 76, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Glare, T.R.; Milner, R.J.; Chivers, G.A. Factors affecting the production of resting spores by Zoophthora radicans in the spotted alfalfa aphids, Therioaphis trifolii f. maculata. Can. J. Bot. 1989, 67, 848–855. [Google Scholar] [CrossRef]
- Duarte, V.S.; Westrum, K.; Ribeiro, A.E.L.; Gondim, M.G.C., Jr.; Klingen, I.; Delalibera, I., Jr. Abiotic and biotic factors affecting resting spore formation in the mite pathogen Neozygites floridana. Int. J. Microbiol. 2013, 2013. [Google Scholar] [CrossRef]
- Kramer, J.P. A mycosis of the blood-sucking snipe fly Symphoromyia hirta caused by Erynia ithacensis sp.n. (Entomophthoraceae). Mycopathologia 1981, 75, 159–164. [Google Scholar] [CrossRef]
- Steinkraus, D.C.; Kramer, J.P. Entomophthora scatophagae descr. Ampl., (Zygomycetes: Entomophthorales), a fungal pathogen of the yellow dung fly, Scatophaga stercoraria (Diptera: Anthomyiidae). Mycotaxon 1988, 32, 105–113. [Google Scholar]
- Steinkraus, D.C.; Kring, T.J.; Tugwell, N.P. Neozygites fresenii in Aphis gossypii on cotton. Southwest. Entomol. 1991, 16, 118–122. [Google Scholar]
- Roy, H.E.; Steinkraus, D.C.; Eilenberg, J.; Hajek, A.E.; Pell, J.K. Bizarre interactions and endgames: Entomopathogenic fungi and their arthropod hosts. Ann. Rev. Entomol. 2006, 51, 331–357. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, M.; Dybas, H.S. The periodical cicada problem. II. Evolution. Evolution 1966, 20, 466–505. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, M.; White, J.; Stanton, N. Dispersal of fungus-infected periodical cicadas to new habitat. Environ. Entomol. 1982, 11, 852–858. [Google Scholar] [CrossRef]
- Cooley, J.R.; Marshall, D.C.; Hill, K.B.R. A specialized fungal parasite (Massospora cicadina) hijacks the sexual signals of periodical cicadas (Hemiptera: Cicadidae: Magicicada). Sci. Rep. 2018, 8, 1432. [Google Scholar] [CrossRef] [PubMed]
- White, J.; Lloyd, M. A pathogenic fungus, Massospora cicadina Peck (Entomophthorales), in emerging nymphs of periodical cicadas. (Homoptera: Cicadidae). Environ. Entomol. 1983, 12, 1245–1252. [Google Scholar] [CrossRef]
- Steinkraus, D.C.; Hollingsworth, R.G.; Boys, G.O. Aerial spores of Neozygites fresenii (Entomophthorales: Neozygitaceae): Density, periodicity, and potential role in cotton aphid (Homoptera: Aphididae) epizootics. Environ. Entomol. 1996, 25, 48–57. [Google Scholar] [CrossRef]
- Steinkraus, D.C.; Howard, M.N.; Hollingsworth, R.G.; Boys, G.O. Infection of sentinel cotton aphids (Homoptera: Aphididae) by aerial conidia of Neozygites fresenii (Entomophthorales: Neozygitaceae). Biol. Contr. 1999, 14, 181–185. [Google Scholar] [CrossRef]
- Ben-Ze’ev, I.S.; Bitton, S.; Kenneth, R.G. Induction and inhibition in Neozygites fresenii (Entomophthorales: Neozygitaceae) zygospores by various time-temperature stimuli. J. Invertebr. Pathol. 1990, 55, 1–10. [Google Scholar] [CrossRef]
- Bitton, S.; Kenneth, R.G.; Ben-Ze’ev, I. Zygospore overwintering and sporulative germination in Triplosporium fresenii (Entomoiphthoraceae) attacking Aphis spiraecola on citrus in Israel. J. Invertebr. Pathol. 1979, 34, 295–302. [Google Scholar] [CrossRef]
- Steinkraus, D.C.; Mueller, A.J.; Humber, R.A. Furia virescens (Thaxter) Humber (Zygomycetes: Entomophthoraceae) infections in the armyworm, Pseudaletia unipuncta (Haworth) (Lepidoptera: Noctuidae) with notes on other natural enemies. J. Entomol. Sci. 1993, 28, 376–386. [Google Scholar] [CrossRef]
- Steinkraus, D.C.; Hajek, A.E.; Liebherr, J.K. Zombie soldier beetles: Epizootics in the goldenrod soldier beetle, Chauliognathus pensylvanicus (Coleoptera: Cantharidae) caused by Eryniopsis lampyridarum (Entomophthoromycotina: Entomophthoraceae). J. Invertebr. Pathol. 2017, 148, 51–59. [Google Scholar] [CrossRef] [PubMed]
- De Bekker, C.; Quevillon, L.; Smith, P.; Patterson, A.D.; Flemming, K.; Ghosh, D.; Hughes, D.P. Species- specific ant brain manipulation by a specialized fungal parasite. BMC Evol. Biol. 2014, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Carner, G.R. Entomophthora lampyridarum, a fungal pathogen of the soldier beetle, Chauliognathus pennsylvanicus. J. Invertebr. Pathol. 1980, 36, 394–398. [Google Scholar] [CrossRef]
- Hajek, A.E.; Soper, R.S. Within-tree location of gypsy moth, Lymantria dispar, larvae killed by Entomophaga maimaiga (Zygomycetes: Entomophthorales). J. Invertebr. Pathol. 1991, 58, 468–469. [Google Scholar] [CrossRef]
- Hajek, A.E.; Tatman, K.M.; Wanner, P.H.; Wheeler, M.M. Location and persistence of cadavers of gypsy moth, Lymantria dispar, containing Entomophaga maimaiga azygospores. Mycologia 1998, 90, 754–760. [Google Scholar] [CrossRef]
- Hajek, A.E. Larval behavior in Lymantria dispar increases risk of fungal infection. Oecologia 2001, 126, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Hajek, A.E.; Roberts, D.W. Field diagnosis of gypsy moth (Lepidoptera: Lymantriidae) larval mortality caused by Entomophaga maimaiga and the gypsy moth nuclear polyhedrosis virus. Environ. Entomol. 1992, 2, 706–713. [Google Scholar] [CrossRef]
- Hajek, A.E.; Wheeler, M.M. Application of techniques for quantification of soil-borne entomophthoralean resting spores. J. Invertebr. Pathol. 1994, 64, 71–73. [Google Scholar] [CrossRef]
- MacDonald, R.M.; Spokes, J.R. Conidiobolus obscurus in arable soil: A method for extracting and counting azygospores. Soil. Biol. Biochem. 1981, 13, 551–553. [Google Scholar] [CrossRef]
- Weseloh, R.M.; Andreadis, T.E. Mechanisms of transmission of the gypsy moth (Lepidoptera: Lymantriidae) fungus, Entomophaga maimaiga (Entomophthorales: Entomophthoraceae) and effects of site conditions on its prevalence. Environ. Entomol. 1992, 21, 901–906. [Google Scholar] [CrossRef]
- Hajek, A.E.; Plymale, R.C.; Reilly, J.R. Comparing two methods for quantifying soil-borne Entomophaga maimaiga resting spores. J. Invertebr. Pathol. 2012, 111, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Soper, R.S.; Hajek, A.E. A method for detecting resting spores of Entomophthorales (Zygomycetes) in soil. J. Invertebr. Pathol. 1988, 52, 18–26. [Google Scholar] [CrossRef]
- Thomsen, L.; Jensen, A.B. Application of nested-PCR technique to resting spores from the Entomophthora muscae species complex: Implications for analyses of host-pathogen population interactions. Mycologia 2002, 94, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Castrillo, L.A.; Thomsen, L.; Juneja, P.; Hajek, A.E. Detection and quantification of Entomophaga maimaiga resting spores in forest soil using real-time PCR. Mycol. Res. 2007, 111, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Hajek, A.E.; Jensen, A.B.; Thomsen, L.; Hodge, K.T.; Eilenberg, J. PCR-RFLP is used to investigate relations among species in the entomopathogenic genera Eryniopsis and Entomophaga. Mycologia 2003, 95, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Hajek, A.E.; Bauer, L.; McManus, M.L.; Wheeler, M.M. Distribution of resting spores of the Lymantria dispar pathogen Entomophaga maimaiga in soil and on bark. BioControl 1998, 43, 189–200. [Google Scholar] [CrossRef]
- Jikamaru, S.; Sano, T. Distribution of late instar Lymantria dispar cadavers killed by Entomophaga maimaiga on trunks of several tree species in southwestern Japan. Can. J. Bot. 2007, 85, 25–30. [Google Scholar] [CrossRef]
- Hajek, A.E. Ecology of terrestrial fungal entomopathogens. Adv. Microb. Ecol. 1997, 15, 193–249. [Google Scholar]
- Perry, D.F. Germination of Erynia bullata resting spores. J. Invertebr. Pathol. 1988, 51, 161–162. [Google Scholar] [CrossRef]
- Hajek, A.E.; Eastburn, C.C. Effect of host insects on activation of Entomophaga maimaiga resting spores. J. Invertebr. Pathol. 2001, 77, 290–291. [Google Scholar] [CrossRef] [PubMed]
- Perry, D.F.; Fleming, R.A. The timing of Erynia radicans resting spore germination in relation to mycosis of Choristoneura fumiferana. Can. J. Bot. 1989, 67, 1657–1663. [Google Scholar] [CrossRef]
- Filotas, M.J.; Hajek, A.E. Influence of temperature and moisture on infection of forest tent caterpillars (Lepidoptera: Lasiocampidae) exposed to resting spores of the entomopathogenic fungus Furia gastropachae (Zygomycetes: Entomophthorales). Environ. Entomol. 2004, 33, 1127–1136. [Google Scholar] [CrossRef]
- Siegert, N.W.; McCullough, D.G.; Hajek, A.E.; Andresen, J.A. Effect of microclimatic conditions on primary transmission of the gypsy moth fungal pathogen Entomophaga maimaiga (Zygomycetes: Entomophthorales) in Michigan. Grt. Lks. Entomol. 2008, 41, 111–128. [Google Scholar]
- Siegert, N.W.; McCullough, D.G.; Wheeler, M.M.; Hajek, A.E. Evaluation of potential versus realized primary infection of gypsy moth (Lepidoptera: Lymantriidae) by Entomophaga maimaiga (Zygomycetes: Entomophthorales). Environ. Entomol. 2012, 41, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Weseloh, R.M. Entomophaga maimaiga (Zygomycete: Entomophthorales) resting spores and biological control of the gypsy moth (Lepidoptera: Lymantriidae). Environ. Entomol. 1999, 28, 1162–1171. [Google Scholar] [CrossRef]
- Hajek, A.E.; Wheeler, M.M.; Eastburn, C.C.; Bauer, L.S. Storage of resting spores of the gypsy moth fungal pathogen, Entomophaga maimaiga. Biocontrol Sci. Technol. 2001, 11, 637–647. [Google Scholar] [CrossRef]
- Hajek, A.E.; Shimazu, M.; Knoblauch, B. Isolating a species of Entomophthorales using resting spore-bearing soil. J. Invertebr. Pathol. 2000, 75, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Hajek, A.E.; Siegert, N.W.; Wheeler, M.M.; McCullough, D.G. Using bioassays to predict abundance of Entomophaga maimaiga resting spores in soil. J. Invertebr. Pathol. 2004, 86, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.R.; Hajek, A.E.; Liebhold, A.M.; Plymale, R.S. The impact of Entomophaga maimaiga on outbreak gypsy moth population: The role of weather. Environ. Entomol. 2014, 43, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Weseloh, R.M.; Andreadis, T.G. Persistence of resting spores of Entomophaga maimaiga, a fungal pathogen of the gypsy moth, Lymantria dispar. J. Invertebr. Pathol. 1997, 69, 195–196. [Google Scholar] [CrossRef]
- Hajek, A.E.; Strazanac, J.S.; Wheeler, M.M.; Vermeylen, F.; Butler, L. Persistence of the fungal pathogen Entomophaga maimaiga and its impact on native Lymantriidae. Biol. Contr. 2004, 30, 466–471. [Google Scholar] [CrossRef]
- Hajek, A.E.; Longcore, J.E.; Simmons, D.R.; Peters, K.; Humber, R.A. Chytrid mycoparasitism of entomophthoralean azygospores. J. Invertebr. Pathol. 2013, 114, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Castrillo, L.A.; Hajek, A.E. Detection of presumptive mycoparasites associated with Entomophaga maimaiga resting spores in forest soils. J. Invertebr. Pathol. 2015, 124, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Boddy, L. Interactions between fungi and other microbes. In The Fungi; Watkinson, S.C., Boddy, L., Money, J.P., Eds.; Elsevier: Waltham, MA, USA, 2016; pp. 337–360. [Google Scholar]
- Jeffries, P.; Young, T.W.K. Interfungal Parasitic Relationships; CAB International: Wallingford, UK, 1994. [Google Scholar]
- Kogan, P.H.; Hajek, A.E. Formation of azygospores by the insect pathogenic fungus Entomophaga maimaiga in cell culture. J. Invertebr. Pathol. 2000, 75, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Hajek, A.E.; Elkinton, J.S.; Witcosky, J.J. Introduction and spread of the fungal pathogen Entomophaga maimaiga along the leading edge of gypsy moth spread. Environ. Entomol. 1996, 25, 1235–1247. [Google Scholar] [CrossRef]
- Hajek, A.E.; Webb, R.E. Inoculative augmentation of the fungal entomopathogen Entomophaga maimaiga as a homeowner tactic to control gypsy moth (Lepidoptera: Lymantriidae). Biol. Contr. 1999, 14, 11–18. [Google Scholar] [CrossRef]
- Pilarska, D.; McManus, M.; Hajek, A.E.; Hérard, F.; Vega, F.E.; Pilarska, P.; Markova, G. Introduction of the entomopathogenic fungus Entomophaga maimaiga Hum., Shim. & Sop. (Zygomycetes: Entomophthorales) to a Lymantria dispar (L.) (Lepidoptera: Lymantriidae) population in Bulgaria. Anz. Schadlingsk. 2000, 73, 125–126. [Google Scholar]




| Class | Order | Family | Genus (No. of Species) ** | Examples (Habit/Hosts) [Reference] |
|---|---|---|---|---|
| Basidiobolomycetes | Basidiobolales | Basidiobolaceae | Basidiobolus (10) | B. ranarum (commensal in gut of frogs and toads) [22] |
| Neozygitomycetes | Neozygitales | Neozygitaceae | Apterivorax (2) | A. acaricida (mites) [23] |
| Neozygites (20) | N. fresenii (aphids) [24] N. floridana (mites) [25] | |||
| Thaxterosporium (1) | T. turbinatum (aphids) [26] | |||
| Entomophthoromycetes | Entomophthorales | Ancylistaceae | Ancylistes (5) | A. closterii (green algae) [27] |
| Conidiobolus (55) | C. macrosporus (mosquitoes) [28] C. obscurus (aphids) [19] | |||
| Macrobiotophthora (2) | M. vermicola (nematodes) [29] | |||
| Completoriaceae | Completoria (1) | C. complens (plants) [30] | ||
| Entomophthoraceae | Batkoa (10) | B. apiculata (lepidopterans, dipterans and homopterans) [16] B. major (coleopterans, dipterans, and homopterans) [16] | ||
| Entomophaga (20) | E. maimaiga (gypsy moth) [31] E. grylli (grasshoppers) [32] | |||
| Entomophthora (59) | E. muscae (flies) [33] E. thripidum (thrips) [34] | |||
| Erynia (22) | E. aquatica (mosquitoes) [35] E. conica (blackflies) [36] | |||
| Furia (17) | F. gastropachae (lepidopterans) [37] | |||
| Massospora (14) | M. cicadina (cicadas) [38] | |||
| Orthomyces (1) | O. aleyrodis (whiteflies) [39] | |||
| Pandora (34) | P. bullata (flies) [40] P. neoaphidis (aphids) [41] | |||
| Strongwellsea (3) | S. castrans (flies) [21] | |||
| Zoophthora (41) | Z. radicans (lepidopterans and homopterans) [5] | |||
| Meristacraceae | Meristacrum (2) | M. milkoi (flies) [30] |
| Species | Host | Factors Associated with Resting Spore Formation (Type of Association) | Reference(s) |
|---|---|---|---|
| Entomophaga maimaiga | Lymantria dispar | Larval age (+), temperature (+), humidity (+), dose of inoculum (+), fungal isolate (variable) | [49,50] |
| Pandora nouryi | Myzus persicae | Temperature (−), dose of inoculum (+), photoperiod (+) | [51,52,53] |
| Entomophthora muscae | Musca domestica | Temperature(−), fungal isolate (variable) | [54] |
| Entomophthora muscae | Delia radicum | Photoperiod after midsummer (−), sex (+female) | [55] |
| Zoophthora radicans | Therioaphis trifolii f. maculata | Temperature (−), humidity (+), dose of inoculum (+), fungal isolate (variable) | [56] |
| Neozygites floridana | Tetranychus urticae | Temperature (−), photoperiod (−), light intensity (−) | [57] |
| Conditions | Results | Reference |
|---|---|---|
| Caged on soil (1 day exposures; 29 April–30 June, 24–26 July) | Infections occurred throughout April–June but not during July exposure; infections correlated with precipitation | [80] |
| Caged on soil (3–4 day exposures of different instars; 18 April–5 August 1994, 24 April–30 June 1995). Cages covered while in the field/local gypsy moth population very low. | First infections one week before egg hatch. Infections ending in mid-late June. Infection levels associated with soil moisture for different instars. | [45] |
| Caged on soil (2–5 day exposures; 27 April–25 June 1998) | Proportions infected associated with 5 day running totals of precipitation. | [95] |
| Caged on soil vs. on tree trunks vs. in the air within the forest canopy | Highest levels of infection occurred among larvae on the soil; this agreed with high levels of infection in the field and litter-dwelling behavior of later instars. | [96] |
| Caged on soil or in the forest canopy, comparing L. dispar infection with Orgyia leucostigma (2 day exposures beginning 17, 19 June, and 9 July) | Much more infection from soil than canopy exposures and more infection for L. dispar than O. leucostigma. | [97] |
| Caged on soil (2 day intervals; 4 April–8 May 1997 in Virginia); watered versus unwatered soil | Earliest infection was low (4–6 April), with egg hatch approx. 10 day later. Much more infection when soil beneath cages was watered. | [98] |
| Cages on soil (2001, 2002; tested from end May–beginning July). Resident gypsy moth populations very low. | Infection rates associated with precipitation in 2002 and temperature between 15 and 25 °C, atmospheric vapor pressure and precipitation in 2001. | [93] |
| Caged on soil and compared with lab (1999–2001; 4 day exposures). Resident gypsy moth populations low. | Infection rates associated with resting spore density, gypsy moth density, canopy cover, and soil pH (all soils were acidic). Cardinal direction not significant. | [94] |
| Caged on soil (2007–2009; early to late June in Pennsylvania) | Infection levels associated with moisture levels. | [99] |
| Caged on soil (1 June–19 July, 2010, in central New York where L. dispar populations were low). | Infections decreased after 25 June, but continued through 3 July, but not after. | Hajek et al. unpublished data |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajek, A.E.; Steinkraus, D.C.; Castrillo, L.A. Sleeping Beauties: Horizontal Transmission via Resting Spores of Species in the Entomophthoromycotina. Insects 2018, 9, 102. https://doi.org/10.3390/insects9030102
Hajek AE, Steinkraus DC, Castrillo LA. Sleeping Beauties: Horizontal Transmission via Resting Spores of Species in the Entomophthoromycotina. Insects. 2018; 9(3):102. https://doi.org/10.3390/insects9030102
Chicago/Turabian StyleHajek, Ann E., Donald C. Steinkraus, and Louela A. Castrillo. 2018. "Sleeping Beauties: Horizontal Transmission via Resting Spores of Species in the Entomophthoromycotina" Insects 9, no. 3: 102. https://doi.org/10.3390/insects9030102
APA StyleHajek, A. E., Steinkraus, D. C., & Castrillo, L. A. (2018). Sleeping Beauties: Horizontal Transmission via Resting Spores of Species in the Entomophthoromycotina. Insects, 9(3), 102. https://doi.org/10.3390/insects9030102