Growing Industries, Growing Invasions? The Case of the Argentine Ant in Vineyards of Northern Argentina
Abstract
:1. Introduction
2. Materials and Methods
3. Results
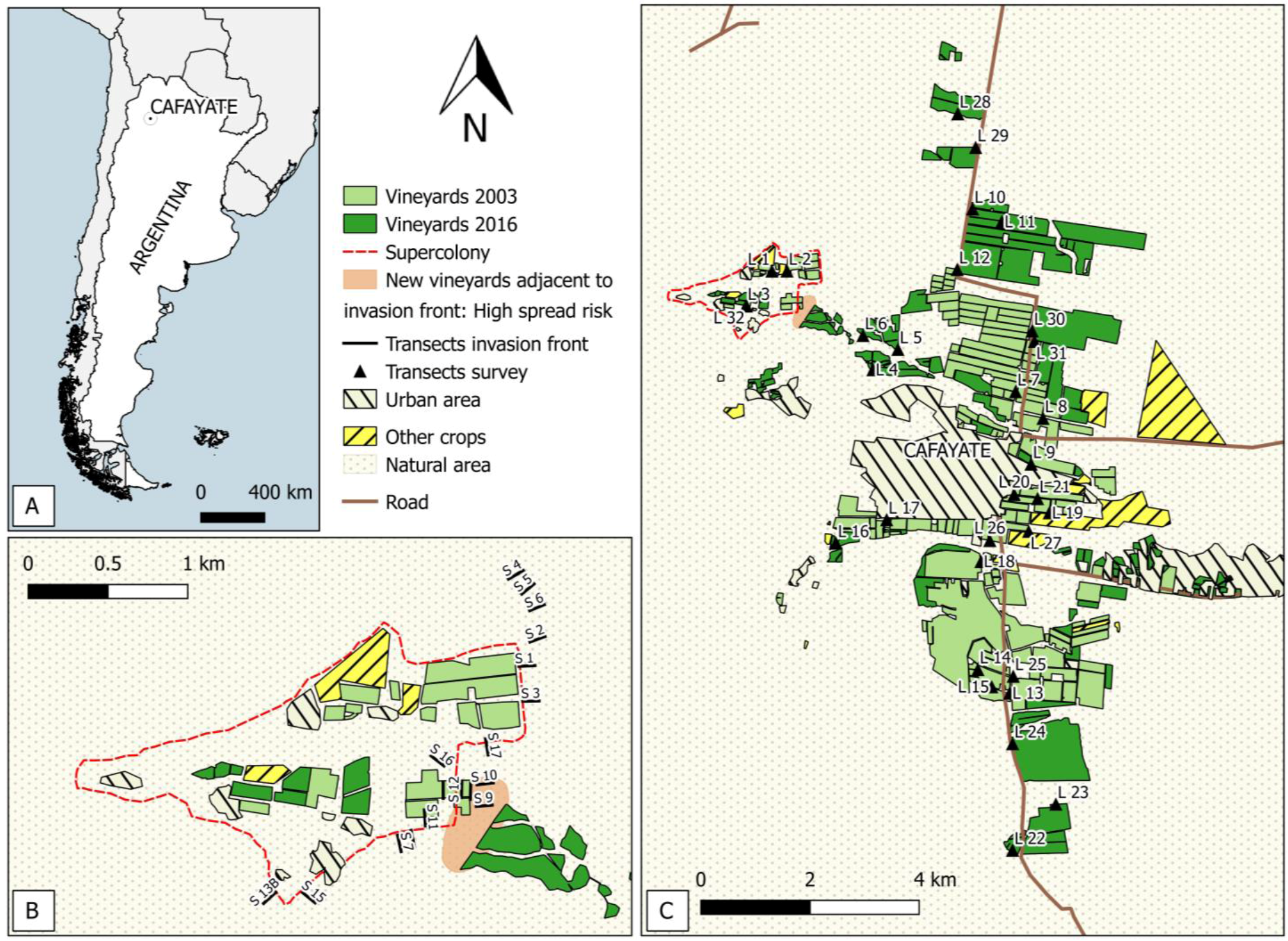
3.1. Vineyard Surface
3.2. Spatio-Temporal Development of the Invasion Front
3.3. Survey of L. humile around Cafayate
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wild, A. Taxonomy and distribution of the argentine ant, Linepithema humile (Hymenoptera: Formicidae). Ann. Entomol. Soc. Am. 2004, 97, 1204–1215. [Google Scholar] [CrossRef]
- Wetterer, J.K.; Wild, A.L.; Suarez, A.V.; Roura-Pascual, N.; Espadaler, X. Worldwide spread of the argentine ant, Linepithema humile (Hymenoptera: Formicidae). Myrmecol. News 2009, 12, 187–194. [Google Scholar]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species: A Selection from the Global Invasive Species Database; The Invasive Species Specialist Group (ISSG) a Specialist Group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN): Auckland, New Zealand, 2000; p. 12. [Google Scholar]
- Human, K.G.; Gordon, D.M. Exploitation and interference competition between the invasive argentine ant, Linepithema humile, and native ant species. Oecologia 1996, 105, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Holway, D.A.; Lach, L.; Suarez, A.V.; Tsutsui, N.D.; Case, T.J. The causes and consequences of ant invasions. Ann. Rev. Ecol. Syst. 2002, 33, 181–233. [Google Scholar] [CrossRef]
- Daane, K.M.; Sime, K.R.; Fallon, J.; Cooper, M.L. Impacts of argentine ants on mealybugs and their natural enemies in California’s coastal vineyards. Ecol. Entomol. 2007, 32, 583–596. [Google Scholar] [CrossRef]
- Phillips, P.; Sherk, C. To control mealybugs, stop honeydew-seeking ants. Calif. Agric. 1991, 45, 26–28. [Google Scholar]
- Addison, P.; Samways, M.J. A survey of ants (Hymenoptera: Formicidae) that forage in vineyards in the Western Cape province, South Africa. Afr. Entomol. 2000, 8, 251–260. [Google Scholar]
- Mgocheki, N.; Addison, P. Interference of ants (Hymenoptera: Formicidae) with biological control of the vine mealybug Planococcus ficus (Signoret) (Hemiptera: Pseudococcidae). Biol. Control 2009, 49, 180–185. [Google Scholar] [CrossRef]
- Westermann, F.L.; Bell, V.A.; Suckling, D.M.; Lester, P.J. Synthetic pheromones as a management technique—Dispensers reduce Linepithema humile activity in a commercial vineyard. Pest Manag. Sci. 2016, 72, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Suarez, A.V.; Holway, D.A.; Case, T.J. Patterns of spread in biological invasions dominated by long-distance jump dispersal: Insights from argentine ants. Proc. Natl. Acad. Sci. USA 2001, 98, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Holway, D.A.; Suarez, A.V.; Case, T.J. Role of abiotic factors in governing susceptibility to invasion: A test with argentine ants. Ecology 2002, 83, 1610–1619. [Google Scholar] [CrossRef]
- Human, K.G.; Weiss, S.; Weiss, A.; Sandler, B.; Gordon, D.M. Effects of abiotic factors on the distribution and activity of the invasive argentine ant (Hymenoptera: Formicidae). Environ. Entomol. 1998, 27, 822–833. [Google Scholar] [CrossRef]
- Menke, S.B.; Holway, D.A. Abiotic factors control invasion by argentine ants at the community scale. J. Anim. Ecol. 2006, 75, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Paris, C.I. La hormiga argentina en viñedos cafayateños: Las dos caras de una invasión biológica. Bol. Soc. Entomol. Argent. 2011, 22, 1–3. [Google Scholar]
- Miano, J.L.; Lannoy, M.; Rolfi, M.R. Manejo integrado de plagas en viñedos, desafío sólo para amantes de la sustentabilidad. In Proceedings of the 37th World Congress of Vine and Wine and 12th General Assembly of the OIV (Part 2), Mendoza, Argentina, 9–14 November 2014; pp. 1–5. [Google Scholar]
- Cole, F.R.; Medeiros, A.C.; Loope, L.L.; Zuehlke, W.W. Effects of the argentine ant on arthropod fauna of Hawaiian high-elevation shrubland. Ecology 1992, 73, 1313–1322. [Google Scholar] [CrossRef]
- Perrings, C. The Socioeconomic Links between Invasive Alien Species and Poverty; Report to the Global Invasive Species Program; Global Invasive Species Program (GISP): Surrey, UK, 2005; Volume 40, pp. 1–36. [Google Scholar]
- Farji-Brener, A.G.; Corley, J.C. Successful invasions of hymenopteran insects into NW Patagonia. Ecol. Austral 1998, 8, 237–249. [Google Scholar]
- Wyckhuys, K.A.G.; Kondo, T.; Herrera, B.V.; Miller, D.R.; Naranjo, N.; Hyman, G. Invasion of exotic arthropods in South America’s biodiversity hotspots and agro-production systems. In Potential Invasive Pests of Agricultural Crops; Peña, J.E., Ed.; Centre for Agriculture and Biosciences International (CABI): Oxfordshire, UK, 2013; pp. 373–400. [Google Scholar]
- Crespo-Pérez, V.; Rebaudo, F.; Silvain, J.-F.; Dangles, O. Modeling invasive species spread in complex landscapes: The case of potato moth in Ecuador. Landsc. Ecol. 2011, 26, 1447–1461. [Google Scholar] [CrossRef]
- Pauchard, A.; Kueffer, C.; Dietz, H.; Daehler, C.C.; Alexander, J.; Edwards, P.J.; Arévalo, J.R.; Cavieres, L.A.; Guisan, A.; Haider, S. Ain’t no mountain high enough: Plant invasions reaching new elevations. Front. Ecol. Environ. 2009, 7, 479–486. [Google Scholar] [CrossRef]
- Becker, T.; Dietz, H.; Billeter, R.; Buschmann, H.; Edwards, P.J. Altitudinal distribution of alien plant species in the Swiss Alps. Perspect. Plant Ecol. Evol. Syst. 2005, 7, 173–183. [Google Scholar] [CrossRef]
- Brush, S.B. The natural and human environment of the central Andes. Mt. Res. Dev. 1982, 2, 19–38. [Google Scholar] [CrossRef]
- Nyssen, J.; Poesen, J.; Deckers, J. Land degradation and soil and water conservation in tropical highlands. Soil. Tillage Res. 2009, 103, 197–202. [Google Scholar] [CrossRef]
- Colautti, R.I.; Grigorovich, I.A.; MacIsaac, H.J. Propagule pressure: A null model for biological invasions. Biol. Invasions 2006, 8, 1023–1037. [Google Scholar] [CrossRef]
- Quantum GIS Development Team 2016; QGIS Geographic Information System. Open Source Geospatial Foundation Project. Available online: http://qgis.osgeo.org (accessed on 30 May 2016).
- Underwood, E.C.; Fisher, B.L. The role of ants in conservation monitoring: If, when, and how. Biol. Conserv. 2006, 132, 166–182. [Google Scholar] [CrossRef]
- Way, M.J.; Cammell, M.E.; Paiva, M.R.; Collingwood, C.A. Distribution and dynamics of the argentine ant Linepithema (Iridomyrmex) humile (Mayr) in relation to vegetation, soil conditions, topography and native competitor ants in portugal. Insectes Soc. 1997, 44, 415–433. [Google Scholar] [CrossRef]
- Markin, G.P. Foraging behavior of the argentine ant in a California citrus grove. J. Econ. Entomol. 1970, 63, 740–744. [Google Scholar] [CrossRef]
- Fidel, G.; Barletta, G.; Alguacil, M. VIII Informe Nacional de Enoturismo República Argentina año 2013; Bodegas de Argentina, A.C., Ed.; Bodegas de Argentina, Asociacion Civil: Mendoza, Argentina, 2014; p. 60. [Google Scholar]
- Floerl, O.; Inglis, G.; Dey, K.; Smith, A. The importance of transport hubs in stepping-stone invasions. J. Appl. Ecol. 2009, 46, 37–45. [Google Scholar] [CrossRef]
- Sagata, K.; Lester, P.J. Behavioural plasticity associated with propagule size, resources, and the invasion success of the argentine ant Linepithema humile. J. Appl. Ecol. 2009, 46, 19–27. [Google Scholar] [CrossRef]
| Transect | Year | Trap 1 | Trap 2 | Trap 3 | Trap 4 | Trap 5 | Trap 6 | Trap 7 | Trap 8 | Trap 9 | Trap 10 | Trap 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 2013 | 11 | 20 | 3 | 1 | 21 | 10 | |||||
| 2014 | 2 | 2 | 1 | |||||||||
| S2 | 2013 | 1 | 2 | 37 | 2 | 3 | 10 | 3 | ||||
| 2014 | 16 | 24 | 17 | 4 | 3 | |||||||
| S3 | 2013 | |||||||||||
| 2014 | 22 | 9 | ||||||||||
| S8 | 2013 | 1 | ||||||||||
| 2014 | ||||||||||||
| S9 | 2013 | 2 | ||||||||||
| 2014 | ||||||||||||
| S11 | 2013 | 1 | 2 | |||||||||
| 2014 | ||||||||||||
| S14 | 2013 | |||||||||||
| 2014 | 1 | 2 | ||||||||||
| S16 | 2013 | 23 | 48 | 9 | 17 | 31 | ||||||
| 2014 | NA 1 | NA 1 | NA 1 | NA 1 | NA 1 | NA 1 | NA 1 | NA 1 | NA 1 | NA 1 | NA 1 |
| Genus/Species | Caught Individuals | Samples (Baits) | Transects | |||
|---|---|---|---|---|---|---|
| Number | Percent (%) | Number | Percent (%) | Number | Percent (%) | |
| Linepithema humile | 941 | 49.79 | 23 | 7.19 | 4 | 12.50 |
| Other ants | 949 | 50.21 | 164 | 51.25 | 31 | 96.88 |
| Total | 1890 | - | 320 | - | 32 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulze-Sylvester, M.; Corronca, J.A.; Paris, C.I. Growing Industries, Growing Invasions? The Case of the Argentine Ant in Vineyards of Northern Argentina. Insects 2018, 9, 11. https://doi.org/10.3390/insects9010011
Schulze-Sylvester M, Corronca JA, Paris CI. Growing Industries, Growing Invasions? The Case of the Argentine Ant in Vineyards of Northern Argentina. Insects. 2018; 9(1):11. https://doi.org/10.3390/insects9010011
Chicago/Turabian StyleSchulze-Sylvester, Maria, José A. Corronca, and Carolina I. Paris. 2018. "Growing Industries, Growing Invasions? The Case of the Argentine Ant in Vineyards of Northern Argentina" Insects 9, no. 1: 11. https://doi.org/10.3390/insects9010011
APA StyleSchulze-Sylvester, M., Corronca, J. A., & Paris, C. I. (2018). Growing Industries, Growing Invasions? The Case of the Argentine Ant in Vineyards of Northern Argentina. Insects, 9(1), 11. https://doi.org/10.3390/insects9010011





