Predation of the Peach Aphid Myzus persicae by the mirid Predator Macrolophus pygmaeus on Sweet Peppers: Effect of Prey and Predator Density
Abstract
:1. Introduction
2. Material and Method
2.1. Plant and Insect Rearings
2.2. Effect of Predator Density
2.3. Predation Efficiency on Large Aphid Colonies
2.4. Impact of Alternative Prey on M. pygmaeus Predation
2.5. Statistical Analyses
3. Results
3.1. Effect of Predator Density on Initially Low Aphid Densities
3.2. Predation Efficiency on Initially Large Aphid Colonies
3.3. Impact of Alternative Prey on M. pygmaeus Predation
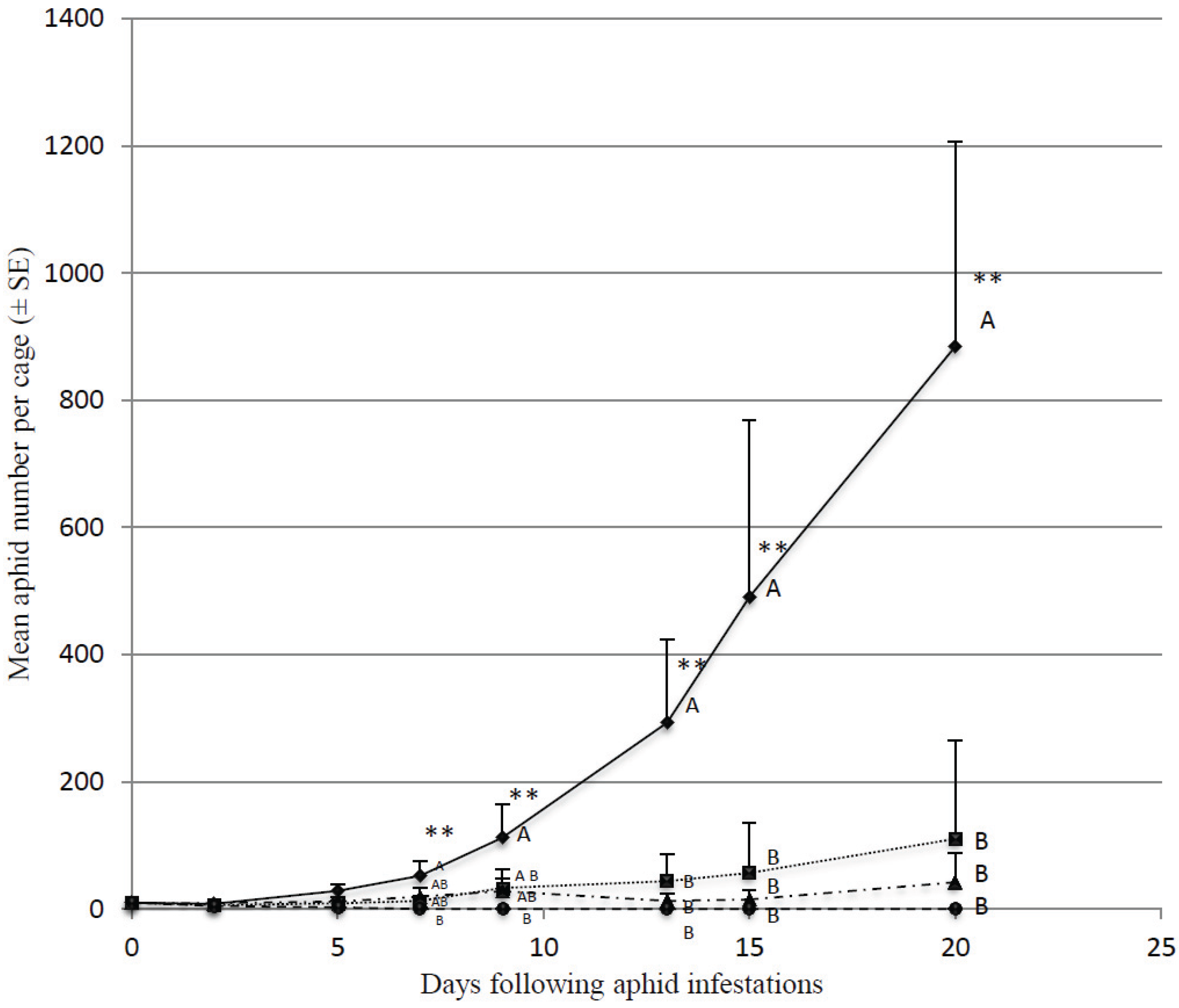
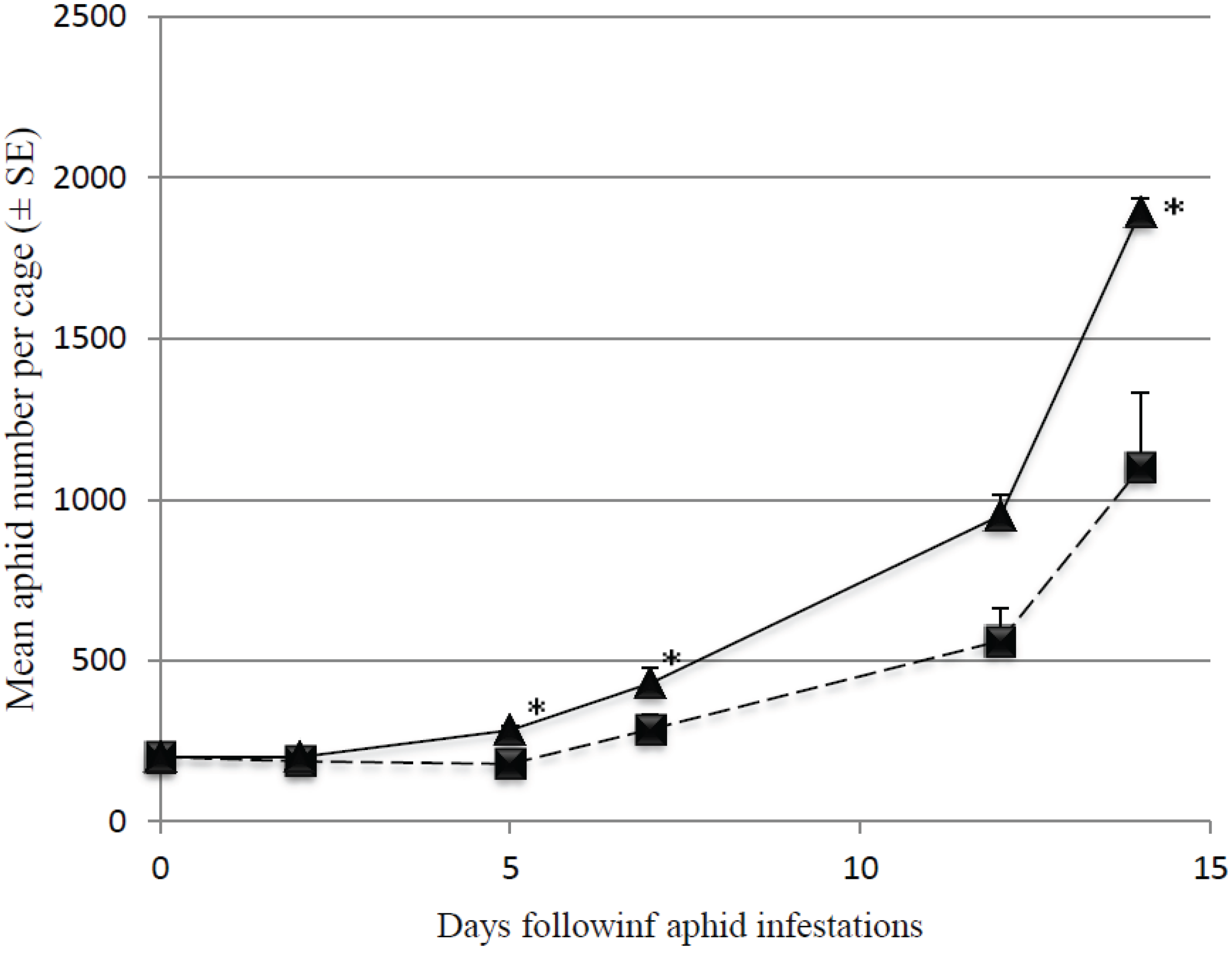

4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Stansly, P.A.; Calvo, F.J.; Urbaneja, A. Biological control of Bemisia tabaci (Homoptera, Aleyrodidae) in protected tomato and pepper culture in southern Spain. Acta Hort. 2004, 659, 383–394. [Google Scholar]
- Van Lenteren, J.C. A greenhouse without pesticides: Fact or fantasy? Crop. Protect. 2000, 19, 375–384. [Google Scholar] [CrossRef]
- Calvo, F.J.; Lorente, M.J.; Stansly, P.A.; Belda, J.E. Preplant release of Nesidiocoris tenuis and supplementary tactics for control of Tuta absoluta and Bemisa tabaci in greenhouse tomato. Entomol. Exp. Appl. 2012, 143, 111–119. [Google Scholar] [CrossRef]
- Calvo, F.J.; Bolckmans, K.; Belda, J.E. Development of a biological control-based integrated pest management method for Bemisia tabaci for protected sweet pepper crops. Entomol. Exp. Appl. 2009, 133, 9–18. [Google Scholar] [CrossRef]
- Calvo, F.J.; Soriano, J.; Bolckmans, K.; Belda, J.E. A successful method for whitefly and Tuta absoluta control in tomato. Evaluation after two years of application in practice. IOBC/WPRS Bull. 2012, 80, 237–244. [Google Scholar]
- Van Lenteren, J.C. The state of commercial augmentative biological control: Plenty of natural enemies, but a frustrating lack of uptake. BioControl 2012, 57, 1–20. [Google Scholar] [CrossRef]
- Calvo, F.J.; Bolckmans, K.; Belda, J.E. Control of Bemisia tabaci and Frankliniella occidentalis in cucumber by Amblyseius swirskii. BioControl 2011, 56, 185–192. [Google Scholar] [CrossRef]
- Calvo, F.J.; Knapp, M.; van Houten, Y.M.; Hoogerbrugge, H.; Belda, J.E. Amblyseius swirskii: What made this predatory mite such a successful biocontrol agent? Exp. Appl. Acarol. 2014, 65, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S. Are Outbreaks of Tuta Absoluta Mitigated by the Cultivation Calendar of Swiss Tomatoes? In Proceedings of the EPPO/IOBC/FAO/NEPPO Joint International Symposium on management of Tuta absoluta, Agadir, Morocco, 16–18 November 2011.
- Blackman, R.L.; Eastop, V.F. Taxonomic Issues. In Aphids as Crop Pests; van Emden, H.F., Harrington, R., Eds.; CABI: Wallingford, UK, 2007; pp. 1–29. [Google Scholar]
- Sanchez, J.A.; La-Spina, M.; Michelena, J.M.; Lacasa, A.; de Mendoza, A.H. Ecology of the aphid pests of protected pepper crops and their parasitoids. BioControl 2011, 21, 171–188. [Google Scholar] [CrossRef]
- Blümel, S. Biological Control of Aphids on Vegetable Crops. In Biocontrol in Protected Culture; Heinz, K.M., van Driesche, R.G., Parrella, M.P., Eds.; Ball Publishing: Batavia, IL, USA, 2004; pp. 297–312. [Google Scholar]
- Andorno, A.V.; López, S.N. Biological control of Myzus persicae (Hemiptera: Aphididae) through banker plant system in protected crops. Biol. Control 2014, 78, 9–14. [Google Scholar] [CrossRef]
- Huang, N.X.; Enkegaard, A.; Osborne, L.S.; Ramakers, P.M.J.; Messelink, G.J.; Pijnakker, J.; Murphy, G. The banker plant method in biological control. Crit. Rev. Plant. Sci. 2011, 30, 259–278. [Google Scholar] [CrossRef]
- Gavkare, O.; Kumar, S.; Japoshvili, G. Effectiveness of native parasitoids of Myzus persicae in greenhouse environments in India. Phytoparasitica 2014, 42, 141–144. [Google Scholar] [CrossRef]
- Bompard, A.; Jaworski, C.C.; Bearez, P.; Desneux, N. Sharing a predator: Can an invasive alien pest affect the predation on a local pest? Popul. Ecol. 2013, 55, 433–440. [Google Scholar] [CrossRef]
- Messelink, G.J.; Maanen, R.v.; van Steenpaal, S.E.F.; Janssen, A. Biological control of thrips and whiteflies by a shared predator: Two pests are better than one. Biol. Control 2008, 44, 372–379. [Google Scholar] [CrossRef]
- Messelink, G.J.; Bloemhard, C.M.J.; Kok, L.W.; Janssen, A. Generalist predatory bugs control aphids in sweet pepper. IOBC/WPRS Bull. 2011, 68, 115–118. [Google Scholar]
- Limburg, D.D.; Rosenheim, J.A. Extrafloral Nectar Consumption and Its Influence on Survival and Development of an Omnivorous Predator, Larval Chrysoperla plorabunda (Neuroptera: Chrysopidae). Environ. Entomol. 2001, 30, 595–604. [Google Scholar] [CrossRef]
- Nomikou, M.; Janssen, A.; Sabelis, M. Phytoseiid predators of whiteflies feed and reproduce on non-prey food sources. Exp. Appl. Acarol. 2003, 31, 15–26. [Google Scholar] [CrossRef] [PubMed]
- van Rijn, P.; Tanigoshi, L. The contribution of extrafloral nectar to survival and reproduction of the predatory mite Iphiseius degenerans on Ricinus communis. Exp. Appl. Acarol. 1999, 23, 281–296. [Google Scholar] [CrossRef]
- Messelink, G.J.; Bennison, J.; Alomar, O.; Ingegno, B.L.; Tavella, L.; Shipp, L.; Palevsky, E.; Wäckers, F.L. Approaches to conserving natural enemy populations in greenhouse crops: Current methods and future prospects. BioControl 2014, 59, 377–393. [Google Scholar] [CrossRef]
- Castañé, C.; Agustí, N.; Arnó, J.; Gabarra, R.; Riudavets, J.; Comas, J.; Alomar, Ó. Taxonomic identification of Macrolophus pygmaeus and Macrolophus melanotoma based on morphometry and molecular markers. Bull. Entomol. Res. 2013, 103, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Messelink, G.J.; Bloemhard, C.M.J.; Hoogerbrugge, H.; van Schelt, J.; Ingegno, B.L.; Tavella, L. Evaluation of mirid predatory bugs and release strategy for aphid control in sweet pepper. J. Appl. Entomol. 2014, 139, 333–341. [Google Scholar] [CrossRef]
- Perdikis, D.C.; Lykouressis, D.P. Myzus persicae (Homoptera: Aphididae) as Suitable Prey for Macrolophus pygmaeus (Hemiptera: Miridae) Population Increase on Pepper Plants. Environ. Entomol. 2004, 33, 499–505. [Google Scholar] [CrossRef]
- Perdikis, D.; Lykouressis, D. Effects of various items, host plants, and temperatures on the development and survival of Macrolophus pygmaeus Rambur (Hemiptera: Miridae). Biol. Control 2000, 17, 55–60. [Google Scholar] [CrossRef]
- Lykouressis, D.P.; Perdikis, D.C.; Gaspari, M.D. Prey preference and biomass consumption of Macrolophus pygmaeus (Hemiptera: Miridae) fed Myzus persicae and Macrosiphum euphorbiae (Hemiptera: Aphididae). Eur. J. Entomol. 2007, 104, 199–204. [Google Scholar] [CrossRef]
- Castañé, C.; Arnó, J.; Gabarra, R.; Alomar, O. Plant damage to vegetable crops by zoophytophagous mirid predators. Biol. Control 2011, 59, 22–29. [Google Scholar] [CrossRef]
- Urbaneja, A.; Gonzalez-Cabrera, J.; Arno, J.; Gabarra, R. Prospects for the biological control of Tuta absoluta in tomatoes of the Mediterranean basin. Pest. Manag. Sci. 2012, 68, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Lykouressis, D.; Perdikis, D.; Charalampous, P. Plant food effects on prey consumption by the omnivorous predator Macrolophus pygmaeus. Phytoparasitica 2014, 42, 303–309. [Google Scholar] [CrossRef]
- Fantinou, A.A.; Perdikis, D.C.; Labropoulos, P.D.; Maselou, D.A. Preference and consumption of Macrolophus pygmaeus preying on mixed instar assemblages of Myzus persicae. Biol. Control 2009, 51, 76–80. [Google Scholar] [CrossRef]
- Foglar, H.; Malausa, J.C.; Wajnberg, E. The functional response and preference of Macrolophus caliginosus [Heteroptera: Miridae] for two of its prey: Myzus persicae and Tetranychus urticae. Entomophaga 1990, 35, 465–474. [Google Scholar] [CrossRef]
- Fantinou, A.A.; Perdikis, D.C.; Maselou, D.A.; Lambropoulos, P.D. Prey killing without consumption: Does Macrolophus pygmaeus show adaptive foraging behaviour? Biol. Control 2008, 47, 187–193. [Google Scholar] [CrossRef]
- Maselou, D.A.; Perdikis, D.; Sabelis, M.W.; Fantinou, A.A. Use of plant resources by an omnivorous predator and the consequences for effective predation. Biol. Control 2014, 79, 92–100. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Backer, L.; Wäckers, F.L.; Francis, F.; Verheggen, F.J. Predation of the Peach Aphid Myzus persicae by the mirid Predator Macrolophus pygmaeus on Sweet Peppers: Effect of Prey and Predator Density. Insects 2015, 6, 514-523. https://doi.org/10.3390/insects6020514
De Backer L, Wäckers FL, Francis F, Verheggen FJ. Predation of the Peach Aphid Myzus persicae by the mirid Predator Macrolophus pygmaeus on Sweet Peppers: Effect of Prey and Predator Density. Insects. 2015; 6(2):514-523. https://doi.org/10.3390/insects6020514
Chicago/Turabian StyleDe Backer, Lara, Felix L. Wäckers, Frédéric Francis, and François J. Verheggen. 2015. "Predation of the Peach Aphid Myzus persicae by the mirid Predator Macrolophus pygmaeus on Sweet Peppers: Effect of Prey and Predator Density" Insects 6, no. 2: 514-523. https://doi.org/10.3390/insects6020514





