Aggression in Tephritidae Flies: Where, When, Why? Future Directions for Research in Integrated Pest Management
Abstract
:1. Introduction
2. Male-Male Fighting Behavior
2.1. Why Males Fight
2.2. How Males Fight
2.3. Where Males Fight
2.4. When Males Fight
3. Female-Female Fighting Behavior
3.1. Why Females Fight

3.2. How Females Fight
3.3. Where Females Fight
3.4. When Females Fight
4. Interspecific Contests in Tephritidae
5. Knowledge on Aggression in Tephritidae: Future Directions and Potential Implications for Integrated Pest Management
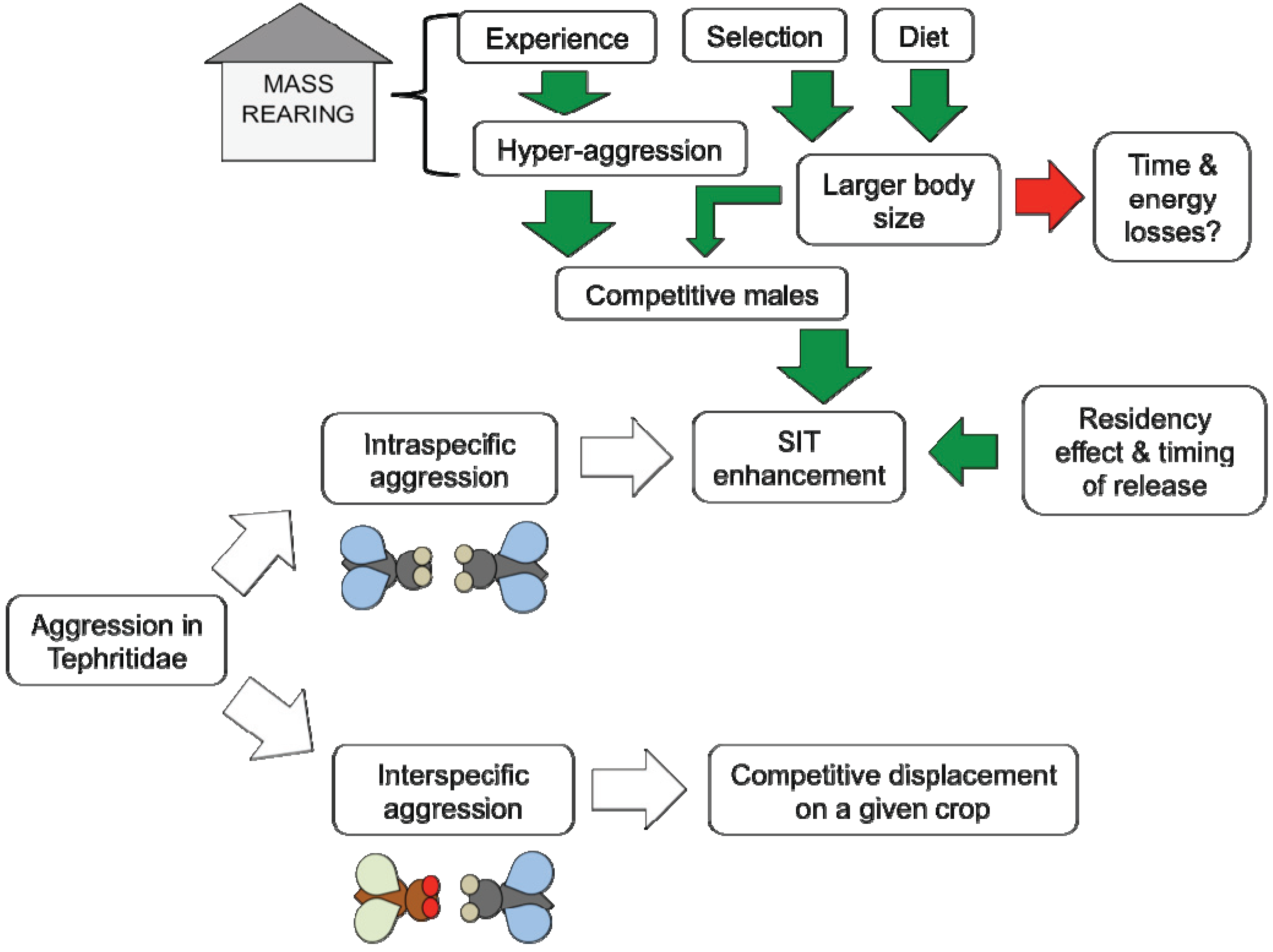
5.1. Experience
5.2. Selection
5.3. Diet
5.4. Residency Effect
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Ekesi, S.; Billah, M.K. A Field Guide to the Management of Economically Important Tephritid Fruit Flies in Africa; ICIPE Science Press: Nairobi, Kenya, 2007. [Google Scholar]
- White, I.M.; Elson-Harris, M.M. Fruit Flies of Economic Significance: Their Identification and Bionomics; CAB International: Wallingford, UK, 1992. [Google Scholar]
- Liu, X.; Jin, Y.; Ye, H. Recent spread and climatic ecological niche of the invasive guava fruit fly, Bactrocera correcta, in mainland China. J. Pest Sci. 2013, 86, 449–458. [Google Scholar] [CrossRef]
- Daane, K.M.; Johnson, M.W. Olive fruit fly: Managing an ancient pest in modern times. Annu. Rev. Entomol. 2010, 55, 151–169. [Google Scholar] [CrossRef]
- Ekesi, S.; Mohamed, S.A. Mass rearing and quality control parameters for tephritid fruit flies of economic importance in Africa. In Wide Spectra of Quality Control; Akyar, I., Ed.; InTech: Rijeka, Croatia, 2011; pp. 387–410. [Google Scholar]
- Vargas, R.I.; Shelly, T.E.; Leblanc, L.; Piñero, J.C. Recent advances in methyl eugenol and cue lure technologies for fruit fly detection, monitoring and control in Hawaii. In Vitamins and Hormones; Liwack, G., Ed.; Elsevier Inc., Academic Press: Burlington, UK, 2010; Volume 83, pp. 575–596. [Google Scholar]
- Estes, A.M.; Nestel, D.; Belcari, A.; Jessup, A.; Rempoulakis, P.; Economopoulos, A.P. A basis for the renewal of sterile insect technique for the olive fly, Bactrocera oleae (Rossi). J. Appl. Entomol. 2012, 136, 1–16. [Google Scholar] [CrossRef]
- Benelli, G.; Canale, A.; Flamini, G.; Cioni, P.L.; Demi, F.; Ceccarini, L.; Macchia, M.; Conti, B. Biotoxicity of Melaleuca alternifolia (Myrtaceae) essential oil against the Mediterranean fruit fly, Ceratitis capitata (Diptera: Tephritidae), and its parasitoid Psyttalia concolor (Hymenoptera: Braconidae). Ind. Crops Prod. 2013, 50, 596–603. [Google Scholar] [CrossRef]
- Canale, A.; Benelli, G.; Conti, B.; Lenzi, G.; Flamini, G.; Francini, A.; Cioni, P.L. Ingestion toxicity of three Lamiaceae essential oils incorporated in protein baits against the olive fruit fly, Bactrocera oleae (Rossi) (Diptera Tephritidae). Nat. Prod. Res. 2013, 27, 2091–2099. [Google Scholar] [CrossRef]
- Lauzon, C.R.; Potter, S.E. Description of the irradiated and nonirradiated midgut of Ceratitis capitata Wiedemann (Diptera: Tephritidae) and Anastrepha ludens Loew (Diptera: Tephritidae) used for sterile insect technique. J. Pest Sci. 2012, 85, 217–226. [Google Scholar] [CrossRef]
- Benelli, G.; Daane, K.M.; Canale, A.; Niu, C.Y.; Messing, R.H.; Vargas, R.I. Sexual communication and related behaviours in Tephritidae—Current knowledge and potential applications for Integrated Pest Management. J. Pest Sci. 2014, 87, 385–405. [Google Scholar]
- Benelli, G.; Giunti, G.; Canale, A.; Messing, R.H. Lek dynamics and cues evoking mating behavior in tephritid flies infesting soft fruits: Implications for behavior-based control tools. Appl. Zool. Entomol. 2014, 49, 363–373. [Google Scholar] [CrossRef]
- Canale, A.; Benelli, G. Impact of mass-rearing on the host-seeking behaviour and parasitism by the fruit fly parasitoid Psyttalia concolor (Szépligeti) (Hymenoptera: Braconidae). J. Pest Sci. 2012, 85, 65–74. [Google Scholar] [CrossRef]
- Sivinski, J.; Aluja, M. The roles of parasitoid foraging for hosts, food and mates in the augmentative control of Tephritidae. Insects 2012, 3, 668–691. [Google Scholar] [CrossRef]
- Vargas, R.I.; Leblanc, L.; Harris, E.J.; Manoukis, N.C. Regional suppression of Bactrocera fruit flies (Diptera: Tephritidae) in the Pacific through biological control and prospects for future introductions into other areas of the world. Insects 2012, 3, 727–742. [Google Scholar] [CrossRef]
- Benelli, G.; Revadi, S.; Carpita, A.; Giunti, G.; Raspi, A.; Anfora, G.; Canale, A. Behavioral and electrophysiological responses of the parasitic wasp Psyttalia concolor (Szépligeti) (Hymenoptera: Braconidae) to Ceratitis capitata-induced fruit volatiles. Biol. Control 2013, 64, 116–124. [Google Scholar] [CrossRef]
- Kuba, H.; Koyama, J. Mating behavior of wild melon flies, Dacus cucurbitae Coquillett (Diptera: Tephritidae) in a field cage: Courtship behavior. Appl. Entomol. Zool. 1985, 20, 365–372. [Google Scholar]
- Robacker, D.C.; Hart, W.G. Courtship territoriality of laboratory-reared Mexican fruit flies, Anastrepha ludens (Diptera: Tephritidae), in cages containing host and nonhost trees. Ann. Entomol. Soc. Am. 1985, 78, 488–494. [Google Scholar]
- Arita, L.H.; Kaneshiro, K.Y. Sexual selection and lek behavior in the Mediterranean fruit fly, Ceratitis capitata (Diptera: Tephritidae). Pacif. Sci. 1989, 43, 135–143. [Google Scholar]
- Whittier, T.S.; Kaneshiro, K.Y.; Prescott, L.D. Mating behavior of Mediterranean fruit flies (Diptera: Tephritidae) in a natural environment. Ann. Entomol. Soc. Am. 1992, 85, 214–218. [Google Scholar]
- Benelli, G. Aggressive behavior and territoriality in the olive fruit fly, Bactrocera oleae (Rossi) (Diptera: Tephritidae): Role of residence and time of day. J. Insect Behav. 2014, 27, 145–161. [Google Scholar]
- Aluja, M.; Birke, A. Habitat use by Anastrepha obliqua flies (Diptera: Tephritidae) in a mixed mango (Mangifera indica) and tropical plum (Spondias purpurea) orchard. Ann. Entomol. Soc. Am. 1993, 86, 799–812. [Google Scholar]
- Landolt, P.J.; Heath, R.R. Effects of age, mating, and time of day on behavioral responses of female papaya fruit fly, Toxotrypana curvicauda Gerstaecker (Diptera: Tephritidae), to synthetic sex pheromone. Environ. Entomol. 1988, 17, 47–51. [Google Scholar]
- Landolt, P.J.; Heath, R.R.; Agee, H.R.; Tumlinson, J.H.; Calkins, C.O. Sex pheromone-based trapping system for papaya fruit fly (Diptera: Tephritidae). J. Econ. Entomol. 1988, 81, 1163–1169. [Google Scholar]
- Landolt, P.J.; Reed, H.C.; Heath, R.R. Attraction of female papaya fruit fly (Diptera: Tephritidae) to male pheromone and host fruit. Environ. Entomol. 1992, 21, 1154–1159. [Google Scholar]
- Shelly, T.E. Feeding on methyl eugenol and Fagraea berteriana flowers increases long-range female attraction by males of the oriental fruit fly (Diptera: Tephritidae). Fla. Entomol. 2001, 84, 634–640. [Google Scholar] [CrossRef]
- Opp, S.B.; Spisak, S.A.; Telang, A.; Hammond, S.S. Comparative mating systems of two Rhagoletis species: The adaptive significance of mate guarding. In Fruit Fly Pests: A World Assessment of Their Biology and Management; McPheron, B.A., Steck, G.J., Eds.; CRC Press: Boca Raton, FL, USA, 1996; pp. 43–49. [Google Scholar]
- Wilkinson, G.S.; Johns, P.M. Sexual selection and the evolution of mating systems in flies. In The Biology of the Diptera; Yeates, D.K., Weigmann, B.M., Eds.; Columbia University Press: New York, NY, USA, 2005; pp. 312–339. [Google Scholar]
- Yurkovic, A.; Wang, O.; Basu, A.C.; Kravitz, E.A. Learning and memory associated with aggression in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2010, 103, 17519–17524. [Google Scholar]
- Yuval, B.; Hendrichs, J. Behavior of flies in the Genus Ceratitis (Dacinae: Ceratitidini). In Fruit Flies (Tephritidae): Phylogeny and Evolution of Behavior; Aluja, M., Norrbom, A.L., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 429–457. [Google Scholar]
- Benelli, G.; Canale, A.; Bonsignori, G.; Ragni, G.; Stefanini, C.; Raspi, A. Male wing vibration in the mating behavior of the olive fruit fly Bactrocera oleae (Rossi) (Diptera: Tephritidae). J. Insect Behav. 2012, 25, 590–603. [Google Scholar] [CrossRef]
- Câmara de Aquino, J.; Joachim-Bravo, I.S. Relevance of male size to female mate choice in Ceratitis capitata (Diptera: Tephritidae): Investigations with wild and laboratory-reared flies. J. Insect Behav. 2013, 27, 162–176. [Google Scholar]
- Díaz-Fleischer, F.; Arredondo, J. Light conditions affect sexual performance in a lekking tephritid fruit fly. J. Exp. Biol. 2011, 214, 2595–2602. [Google Scholar] [CrossRef] [PubMed]
- Malavasi, A.; Morgante, J.S.; Prokopy, R.J. Distribution and activities of Anastrepha fraterculus (Diptera: Tephritidae) flies on host and non-host trees. Ann. Entomol. Soc. Am. 1983, 76, 286–292. [Google Scholar]
- Quilici, S.; Frank, A.; Peppuy, A.; Dos Reis Correira, E.; Mouniama, C.; Blard, F. Comparative studies of courtship behavior of Ceratitis spp. (Diptera: Tephritidae) in Reunion Island. Fla. Entomol. 2002, 85, 138–142. [Google Scholar]
- Segura, D.; Petit-Marty, N.; Sciurano, R.; Vera, T.; Calcagno, G.; Allinghi, A.; Cendra, P.G.; Cladera, J.; Vilardi, J. Lekking behavior of Anastrepha fraterculus (Diptera: Tephritidae). Fla. Entomol. 2007, 90, 154–162. [Google Scholar] [CrossRef]
- Whittier, T.S.; Kaneshiro, K.Y. Intersexual selection in the Mediterranean fruit fly: Does female choice enhance fitness? Evolution 1995, 49, 990–996. [Google Scholar] [CrossRef]
- Shelly, T.E. Flower-feeding affects mating performance in male oriental fruit flies Bactrocera dorsalis. Ecol. Entomol. 2000, 25, 109–114. [Google Scholar] [CrossRef]
- Kumaran, N.; Balagawi, S.; Mark, K.; Schutze, M.K.; Clarke, A.R. Evolution of lure response in tephritid fruit flies: Phytochemicals as drivers of sexual selection. Anim. Behav. 2013, 85, 781–789. [Google Scholar] [CrossRef]
- Reynolds, J.D.; Gross, M.R. Costs and benefits of female mate choice: Is there a lek paradox? Am. Nat. 1990, 136, 230–243. [Google Scholar] [CrossRef]
- Kirkpatrick, M.; Ryan, M.J. The evolution of mating preferences and the paradox of the lek. Nature 1991, 350, 33–38. [Google Scholar] [CrossRef]
- Jones, T.M.; Quinnell, R.J.; Balmford, A. Fisherian flies: Benefits of female choice in a lekking sandfly. Proc. R. Soc. Ser. B 1998, 265, 1651–1657. [Google Scholar]
- Janetos, A.C. Strategies of female mate choice: A theorical analysis. Behav. Ecol. Sociobiol. 1980, 7, 107–112. [Google Scholar] [CrossRef]
- Anjos-Duarte, C.S.; Moreira Costa, A.; Joachim-Bravo, I.S. Influence of female age on variation of mate choice behavior in Mediterranean fruit fly (Diptera: Tephritidae). J. Insect Behav. 2011, 24, 11–21. [Google Scholar] [CrossRef]
- Burk, T. Signalling and sex in acalyptrate flies. Fla. Entomol. 1981, 64, 30–43. [Google Scholar] [CrossRef]
- Biggs, J.D. Aggressive behavior in the adult apple maggot (Diptera: Tephritidae). Can. Entomol. 1972, 104, 349–353. [Google Scholar]
- AliNiazee, M.T. The Western cherry fruit fly, Rhagoletis indifferens (Diptera: Tephritidae): 2. Aggressive behavior. Can. Entomol. 1974, 106, 1201–1204. [Google Scholar]
- Messina, F.J.; Subler, J.K. Conspecific and heterospecific interactions of male Rhagoletis flies (Diptera: Tephritidae) on a shared host. J. Kans. Entomol. Soc. 1995, 68, 206–213. [Google Scholar]
- Boyce, A.M. Bionomics of the walnut husk fly, Rhagoletis completa. Hilgardia 1934, 8, 363–579. [Google Scholar] [CrossRef]
- Brooks, F.E. Walnut husk maggot. U.S. Dep. Agric. Bull. 1921, 992, 1–8. [Google Scholar]
- Smith, D.C.; Prokopy, R.J. Seasonal and diurnal activity of Rhagoletis mendax flies in nature. Ann. Entomol. Soc. Am. 1981, 74, 462–466. [Google Scholar]
- Smith, D.C.; Prokopy, R.J. Mating behaviour of Rhagoletis mendax (Diptera: Tephritidae) flies in nature. Ann. Entomol. Soc. Am. 1982, 75, 388–392. [Google Scholar]
- Headrick, D.; Goeden, R.D. Life history of Paracantha gentilis (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 1990, 83, 776–785. [Google Scholar]
- Sivinski, J.; Aluja, M.; Dodson, G.; Freidberg, A.; Headrick, D.; Kaneshiro, K.; Landolt, P. Topics in the evolution of sexual behavior in the Tephritidae. In Fruit Flies (Tephritidae): Phylogeny and Evolution of Behavior; Aluja, M., Norrbom, A.L., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 751–792. [Google Scholar]
- Landolt, P.J.; Hendrichs, J. Reproductive behavior of the papaya fruit fly, Toxotrypana curvicauda Gerstaecker (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 1983, 76, 413–417. [Google Scholar]
- Aluja, M. Unusual calling behavior of Anastrepha robusta (Diptera: Tephritidae) flies in nature. Fla. Entomol. 1993, 76, 391–395. [Google Scholar] [CrossRef]
- Burk, T. Behavioral ecology of mating in the Caribbean fruit fly, Anastrepha suspensa (Loew) (Diptera: Tephritidae): Territorial fights and signaling stimulation. Fla. Entomol. 1983, 66, 330–344. [Google Scholar]
- Prokopy, R.; Hendrichs, J. Mating behavior of Ceratitis capitata on a field caged host tree. Ann. Entomol. Soc. Am. 1979, 72, 642–648. [Google Scholar]
- Hendrichs, M.A.; Hendrichs, J. Perfumed to be killed: Interception of Mediterranean fruit fly (Diptera: Tephritidae) sexual signalling by predatory foraging wasps (Hymenoptera: Vespidae). Ann. Entomol. Soc. Am. 1998, 91, 228–234. [Google Scholar]
- Smith, D.C.; Prokopy, R.J. Mating behavior of Rhagoletis pomonella. VI. Site of early season encounters. Can. Entomol. 1980, 112, 585–590. [Google Scholar]
- Burk, T. Male-male interactions in the Caribbean fruit flies, Anastrepha suspensa (Loew) (Diptera: Tephritidae): Territorial fights and signaling stimulation. Fla. Entomol. 1984, 67, 542–547. [Google Scholar] [CrossRef]
- Ekesi, S.; Billah, M.K.; Peterson, W.; Nderitu, W.; Lux, S.A.; Rwomushana, I. Evidence for competitive displacement of Ceratitis cosyra by the invasive fruit fly Bactrocera invadens (Diptera: Tephritidae) on mango and mechanisms contributing to the displacement. J. Econ. Entomol. 2009, 102, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Dodson, G. Biological observation on Aciurina trixa and Valentibulla dodsoni (Diptera: Tephritidae) in New Mexico. Ann. Entomol. Soc. Am. 1987, 80, 494–500. [Google Scholar]
- Dodson, G. Lek mating system and large male aggressive advantage in a gall-forming tephritid fly (Diptera: Tephritidae). Ethology 1986, 72, 99–108. [Google Scholar]
- Briceño, R.D.; Ramos, D.; Eberhard, W.G. Aggressive behavior in medflies (Ceratitis capitata) and its modification by mass rearing (Diptera: Tephritidae). J. Kans. Entomol. Soc. 1999, 72, 17–27. [Google Scholar]
- Benelli, G.; Donati, E.; Romano, D.; Stefanini, C.; Messing, R.H.; Canale, A. Lateralisation of aggressive displays in a tephritid fly. Sci. Nat. Naturwissenschaften 2015. [Google Scholar] [CrossRef]
- Shelly, T.E.; Kaneshiro, K.Y. Lek behavior of the Oriental fruit fly, Dacus dorsalis, in Hawaii (Diptera: Tephritidae). J. Insect Behav. 1991, 4, 235–241. [Google Scholar] [CrossRef]
- Headrick, D.H.; Goeden, R.D. Reproductive behavior of California fruit flies and the classification and evolution of Tephritidae (Diptera) mating systems. Stud. Dipterol. 1994, 1, 194–252. [Google Scholar]
- Koyama, J.; Kakinohana, H.; Miyatake, T. Eradication of the melon fly, Bactrocera cucurbitae, in Japan: Importance of behavior, ecology, genetics, and evolution. Annu. Rev. Entomol. 2004, 49, 331–349. [Google Scholar] [CrossRef]
- Cavender, G.L.; Goeden, R.D. Life history of Trupanea bisetosa (Diptera: Tephritidae) on wild sunflower in Southern California. Ann. Entomol. Soc. Am. 1982, 75, 400–406. [Google Scholar]
- Tauber, M.J.; Toschi, C.A. Bionomics of Euleia fratria. I. Life history and mating behavior. Can. J. Zool. 1965, 43, 369–379. [Google Scholar]
- Tauber, M.J.; Toschi, C.A. Life history and mating behavior of Tephritis stigmatica. Pan-Pacific Entomol. 1965, 41, 73–79. [Google Scholar]
- Whittier, T.S.; Nam, F.Y.; Shelly, T.E.; Kaneshiro, K.Y. Male courtship success and female discrimination in the Mediterranean fruit fly (Diptera: Tephritidae). J. Insect Behav. 1994, 7, 159–170. [Google Scholar] [CrossRef]
- Iwahashi, O.; Majima, T. Lek formation and male-male competition in the melon fly, Dacus cucurbitae Coquillett (Diptera: Tephritidae). Appl. Zool. Entomol. 1986, 21, 70–75. [Google Scholar]
- Aluja, M.; Norrbom, A.L. Fruit Flies (Tephritidae): Phylogeny and Evolution of Behavior; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Arnott, G.; Ashton, C.; Elwood, R.W. Lateralization of lateral displays in convict cichlids. Biol. Lett. 2011, 7, 683–685. [Google Scholar] [CrossRef]
- Vallortigara, G.; Rogers, L.J. Survival with an asymmetrical brain: Advantages and disadvantages of cerebral lateralization. Behav. Brain Sci. 2005, 28, 575–633. [Google Scholar]
- Jennings, D.J. Information gathering during contests: The relationship between lateralisation and contestant behaviour during fallow deer fights. Behav. Proc. 2014, 103, 278–282. [Google Scholar] [CrossRef]
- Austin, N.P.; Rogers, L.J. Lateralization of agonistic and vigilance responses in Przewalski horses (Equus przewalskii). Appl. Anim. Behav. Sci. 2014, 151, 43–50. [Google Scholar] [CrossRef]
- Ades, C; Ramires, E.N. Asymmetry of leg use during prey handling in the spider Scytodes globula (Scytodidae). J. Insect Behav. 2002, 15, 563–570. [Google Scholar]
- Backwell, P.R.Y.; Matsumasa, M.; Double, M.; Roberts, A.; Murai, M.; Keogh, J.S.; Jennions, M.D. What are the consequences of being left-clawed in a predominantly right-clawed fiddler crab? Proc. R. Soc. B 2007, 274, 2723–2729. [Google Scholar] [CrossRef] [PubMed]
- Frasnelli, E.; Vallortigara, G.; Rogers, L. Left-right asymmetries of behaviour and nervous system in invetebrates. Neurosci. Biobehav. Rev. 2012, 36, 1273–1291. [Google Scholar] [CrossRef]
- Ghirlanda, S.; Frasnelli, E.; Vallortigara, G. Intraspecific competition and coordination in the evolution of lateralization. Philos. Trans. R. Soc. Lond. B 2009, 364, 861–866. [Google Scholar] [CrossRef]
- Rogers, L.J.; Rigosi, E.; Frasnelli, E.; Vallortigara, G. A right antenna for social behaviour in honeybees. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Frasnelli, E.; Haase, A.; Rigosi, E.; Anfora, G.; Rogers, L.J.; Vallortigara, G. The bee as a model to investigate brain and behavioural asymmetries. Insects 2014, 5, 120–138. [Google Scholar] [CrossRef]
- Tadeo, E.; Aluja, M.; Rull, J. Alternative mating tactics as potential prezygotic barriers to gene flow between two sister species of frugivorous fruit flies. J. Insect Behav. 2013, 26, 708–720. [Google Scholar] [CrossRef]
- Ramos, D. Seleccion y Comportamiento de Apareamiento de la Mosca Mediterranea de la Fruta (Ceratitis capitata) en Laboratorio y la Comparacion Con Una Cepa Silvestre Bajo Condiciones Semi-Naturales. M.S. Thesis, Costa Rica University, Ciudad Universitaria Rodrigo Facio Brenes, Costa Rica, 1991. [Google Scholar]
- Shelly, T.E. Aggression between wild and laboratory-reared sterile males of the Mediterranean fruit fly in a natural habitat (Diptera: Tephritidae). Fla. Entomol. 2000, 83, 105–108. [Google Scholar]
- Loher, W.; Zervas, G. The mating rhythm of the olive fruit fly Dacus oleae (Gmelin). Zeit. Angew. Entomol. 1979, 88, 425–443. [Google Scholar]
- Wangberg, J.K. Biology of gall-formers of the genus Valentibulla (Diptera: Tephritidae) on rabbit bush in Idaho. J. Kans. Entomol. Soc. 1978, 53, 401–420. [Google Scholar]
- Batra, S.W.T. Reproductive behavior of Euaresta bella and E. festiva (Diptera: Tephritidae), potential agents for the biological control of adventive N. American rageweeds (Ambrosia spp.) in Eurasia. J. N. Y. Entomol. Soc. 1979, 87, 118–125. [Google Scholar]
- Freidberg, A. Mating behavior of Schistopterum moebiusi Becker (Diptera: Tephritidae). Isr. J. Entomol. 1981, 15, 89–95. [Google Scholar]
- Shelly, T.E. Defense of oviposition sites by female oriental fruit flies (Diptera: Tephritidae). Fla. Entomol. 1999, 82, 339–346. [Google Scholar] [CrossRef]
- Pritchard, G. The ecology of a natural population of Queensland fruit fly, Dacus tryoni. II. The distribution of eggs and its relation to behaviour. Austr. J. Zool. 1969, 17, 293–311. [Google Scholar]
- Papaj, D.R.; Messing, R.H. Functional shifts in the use of parasitized hosts by a tephritid fly: The role of host quality. Behav. Ecol. 1996, 3, 235–242. [Google Scholar]
- Debouzie, D. Biotic mortality factors in tephritid populations. In World Crop Pests, Fruit Flies: Their Biology, Natural Enemies and Control; Robinson, A.S., Hooper, G., Eds.; Elsevier: New York, USA, 1989; Volume 3B, pp. 221–227. [Google Scholar]
- Dukas, R.; Prokopy, R.J.; Papaj, D.R.; Duan, J.J. Egg laying behavior of Mediterranean fruit flies (Diptera: Tephritidae): Is social facilitation important? Fla. Entomol. 2001, 84, 665–671. [Google Scholar] [CrossRef]
- Duyck, P.-F.; David, P.; Quilici, S. A review of relationships between interspecific competition and invasions in fruit flies (Diptera: Tephritidae). Ecol. Entomol. 2004, 29, 511–520. [Google Scholar] [CrossRef]
- Greene, E.; Orsak, L.J.; Whitman, D.W. A tephritid fly mimics the territorial displays of its jumping spider predators. Science 1987, 236, 310–312. [Google Scholar] [CrossRef]
- Rao, D.; Diaz-Fischer, F. Characterization of predator-directed displays in tephritid flies. Ethology 2012, 118, 1–8. [Google Scholar] [CrossRef]
- Hendrichs, J.; Robinson, A.S.; Cayol, J.P.; Enkerlin, W. Medfly areawide sterile insect technique programmes for prevention, suppression or eradication: The importance of mating behavior studies. Fla. Entomol. 2002, 85, 1–13. [Google Scholar] [CrossRef]
- Pérez-Staples, D.; Todd, E.; Shelly, T.E.; Yuval, B. Female mating failure and the failure of “mating” in sterile insect programs. Entomol. Exp. Appl. 2012, 146, 66–78. [Google Scholar]
- Hsu, Y.; Earley, R.L.; Wolf, L.L. Modulation of aggressive behaviour by fighting experience: Mechanisms and contest outcomes. Biol. Rev. Cambr. Philos. Soc. 2006, 81, 33–74. [Google Scholar]
- Rutte, C.; Taborsky, M.; Brinkhof, M.W.G. What sets the odd of winning and losing? Trends Ecol. Evol. 2006, 21, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, P.A.; Schildberger, K. Mechanisms of experience dependent control of aggression in crickets. Curr. Opin. Neurobiol. 2013, 23, 318–323. [Google Scholar] [CrossRef]
- Benelli, G.; Desneux, N.; Romano, D.; Messing, R.H.; Canale, A. Experience-induced hyper-aggression in a fly: When losers learn to win. University of Pisa: Pisa, Italy, Unpublished data. 2015. [Google Scholar]
- Boake, C.; Shelly, T.E.; Kaneshiro, K.Y. Sexual selection in relation to pest-management strategies. Annu. Rev. Entomol. 1996, 41, 211–229. [Google Scholar] [CrossRef]
- Briceño, D.; Ramos, D.; Eberhard, W. Courtship behaviour of male Ceratitis capitata (Diptera: Tephritidae) in captivity. Fla. Entomol. 1996, 79, 130–143. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benelli, G. Aggression in Tephritidae Flies: Where, When, Why? Future Directions for Research in Integrated Pest Management. Insects 2015, 6, 38-53. https://doi.org/10.3390/insects6010038
Benelli G. Aggression in Tephritidae Flies: Where, When, Why? Future Directions for Research in Integrated Pest Management. Insects. 2015; 6(1):38-53. https://doi.org/10.3390/insects6010038
Chicago/Turabian StyleBenelli, Giovanni. 2015. "Aggression in Tephritidae Flies: Where, When, Why? Future Directions for Research in Integrated Pest Management" Insects 6, no. 1: 38-53. https://doi.org/10.3390/insects6010038