Innate and Conditioned Responses to Chemosensory and Visual Cues in Asian Citrus Psyllid, Diaphorina citri (Hemiptera: Liviidae), Vector of Huanglongbing Pathogens
Abstract
:1. Introduction
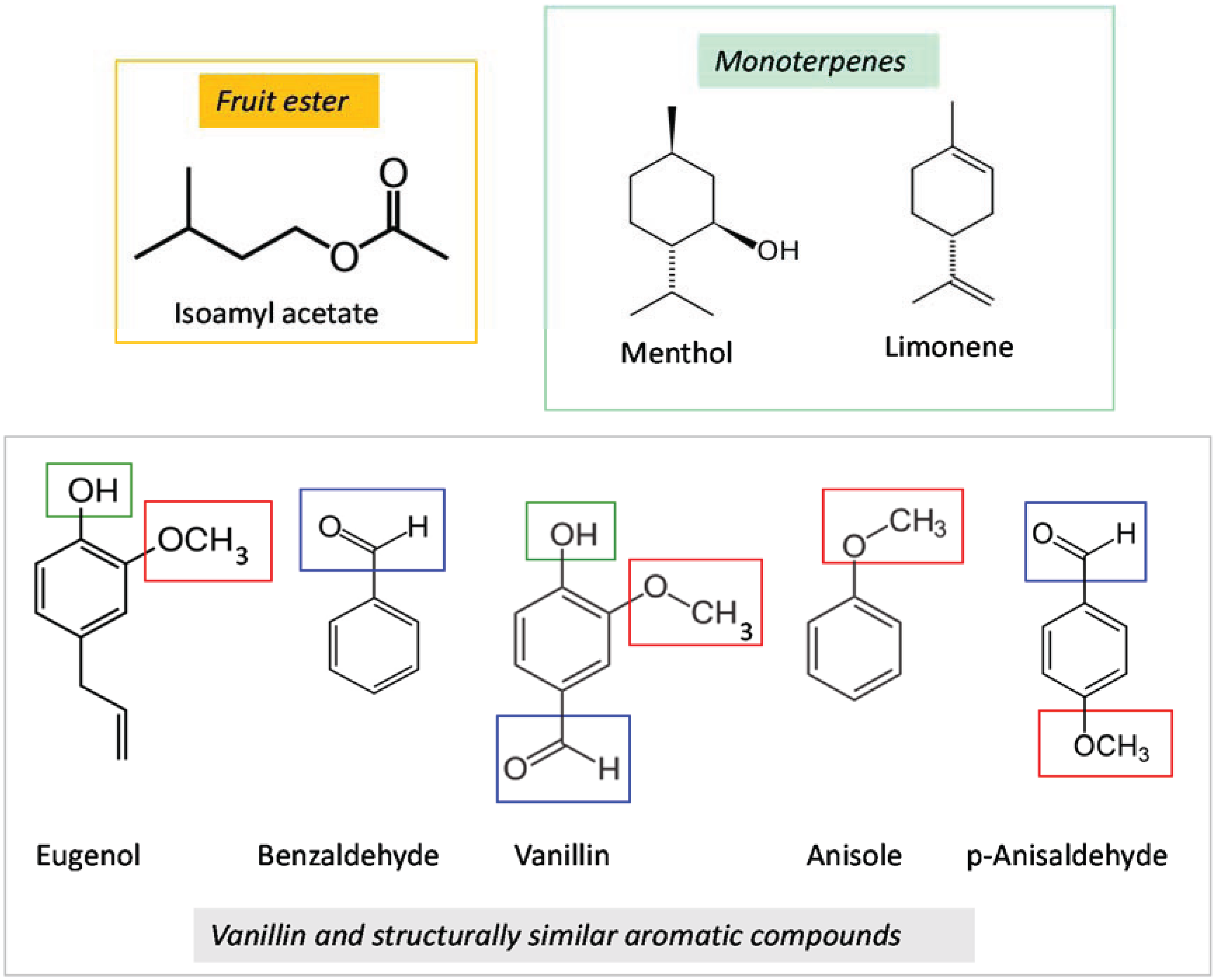
2. Materials and Methods
2.1. Experiment Overview
| Behavior test | Stimuli tested |
|---|---|
| Innate response tests (Dose-response) | Monoterpenes: Limonene, Menthol |
| Fruit Ester: Isoamyl acetate | |
| Aromatics: Vanillin, Anisole, Anisaldehyde, Eugenol, Benzaldehyde | |
| Conditioned response to individual compounds | |
| Trained on: US blank or US + CS | US:0.3 M Sucrose solution |
| Tested on: US blank or US + CS | CS: Vanillin, Limonene, Isoamyl acetate, Eugenol |
| Conditioned response to concentration | US: 0.3 M Sucrose solution |
| CS: Benzaldehyde (3 µL or 10 µL/10 mL SPLAT) | |
| Conditioned response to binary mixtures | US: 0.3 M Sucrose solution |
| CS: 1:1 Vanillin: Isoamyl acetate, 1:2 Vanillin: Isoamyl acetate, 2:1 Vanillin: Isoamyl acetate | |
| Conditioned response to blue-colored feeding response with and without generic citrus chemosensory cue | US: 0.3 M Sucrose solution |
| CS: Blue food coloring ± Generic citrus scent | |
2.2. Test Compounds
| Test Compound | Conditioning Phase Concentration (µL/100 mL Sucrose Solution) | Probing Test Concentration (µL/10 mL Emulsified Wax) |
|---|---|---|
| Vanillin | 100 | 40 |
| Isoamyl acetate | 100 | 20 |
| Benzaldehyde | 100 | 3 and 30 |
| Limonene | 100 | 20 |
| Eugenol | 20 | 20 |
| Anisaldehyde | 3 | 20 |
| Anisole | 20 | 20 |
2.3. Study Insects
2.4. Innate Response Tests

2.5. Learning Test
2.5.1. Learning Test Part 1: Conditioning Procedure
- (1)
- Fed blank sucrose solution (unconditioned stimulus (US)), then tested on blank SPLAT (US → blank); this treatment provided a baseline level of probing by naïve psyllids exposed only to the US.
- (2)
- Fed blank sucrose solution then tested on SPLAT + test CS (US → CS); this treatment measured the level of probing by naïve psyllids exposed to the CS only during the testing phase.
- (3)
- Fed sucrose solution + test CS then tested on blank SPLAT (US + CS → blank); this treatment measured the level of probing by psyllids that had been exposed to both US + CS during the conditioning phase.
- (4)
- Fed sucrose solution + test CS then tested on SPLAT + CS (US + CS → CS); this treatment measured the response of psyllids to the CS following exposure to it during the conditioning phase.
2.5.2. Learning Test Part 2: Probing Response Procedure
2.6. Conditioned Response to the Same CS at Two Concentrations
2.7. Effect of Mixing on Conditioned Response
2.8. Conditioned Response to Visual Cues
2.9. Data Analysis
3. Results
3.1. Innate Response
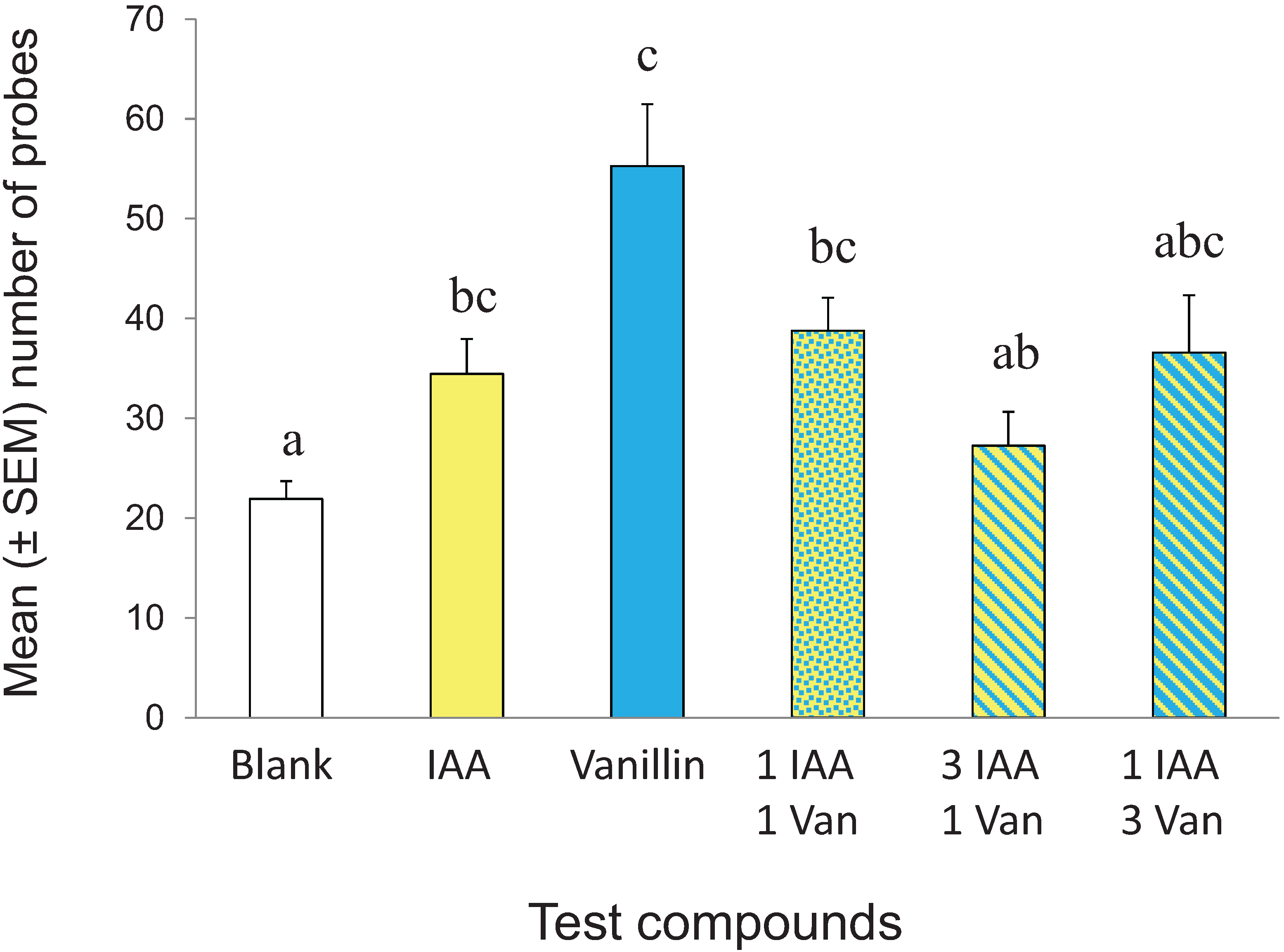
3.2. Conditioned Response
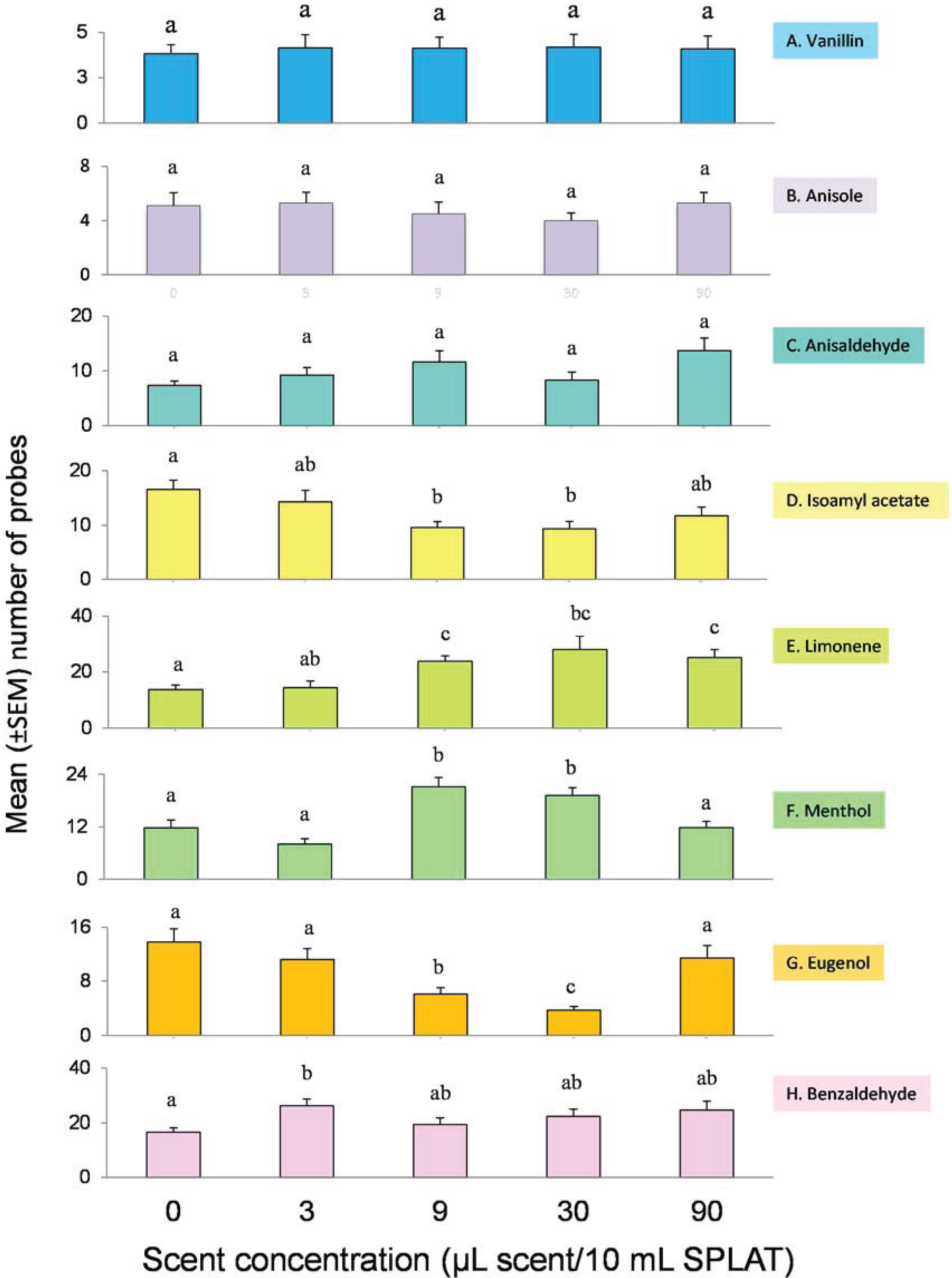

3.3. Effect of Concentration on Conditioned Response
3.4. Effect of CS Mixing on Conditioned Response
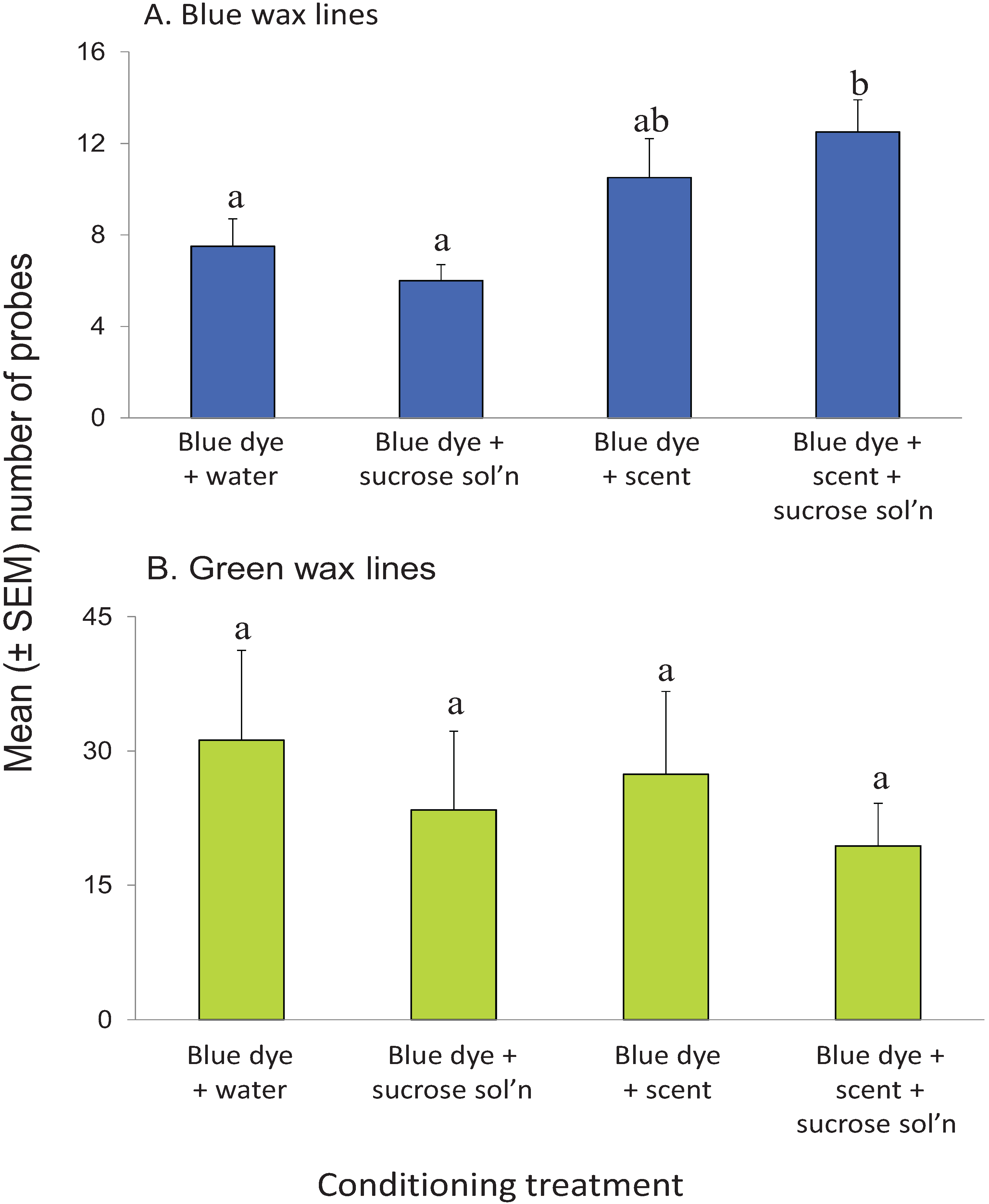
3.5. Visual Conditioning
4. Discussion
4.1. Innate Response
4.2. Conditioning
4.3. Mixing Test
4.4. Visual Learning
5. Conclusions
Acknowledgements
Author Contributions
Conflict of Interest
References
- Grafton-Cardwell, E.E.; Stelinski, L.L.; Stansly, P.A. Biology and management of Asian citrus psyllid, vector of the Huanglongbing pathogens. Annu. Rev. Entomol. 2013, 58, 413–432. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.G.; Richardson, M.L.; El-Desouky, A.; Halbert, S.E. Asian citrus psyllid, Diaphorina citri, vector of citrus Huanglongbing disease. Entomol. Exp. Appl. 2013, 146, 207–223. [Google Scholar] [CrossRef]
- Halbert, S.E.; Munjunath, K.L. Asian citrus psyllid (Sternorrhyncha: Psyllidae) and greening disease of citrus: A literature review and assessment of risk in Florida. Fla. Entomol. 2004, 87, 330–353. [Google Scholar] [CrossRef]
- Bové, J.M. Huanglongbing: A destructive, newly emerging, century-old disease of citrus. J. Plant Path. 2006, 88, 7–37. [Google Scholar]
- Aubert, B.; Hua, X.-Y. Monitoring flight activity of Diaphorina citri on citrus and Murraya canopies. In Rehabilitation of Citrus Industry in the Asia Pacific Region; Aubert, B., Tontyaporn, S., Buangsuwon, D., Eds.; UNDP-FAO: Rome, Italy, 1990; pp. 181–187. [Google Scholar]
- Boina, D.R.; Meyer, W.L.; Onagbola, E.O.; Stelinski, L.L. Quantifying dispersal of Diaphorina citri (Hemiptera: Psyllidae) by immunomarking and potential impact of unmanaged groves on commercial citrus management. Environ. Entomol. 2009, 38, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Lewis-Rosenblum, H.; Pelz-Stelinski, K.; Stelinski, L.L. Incidence of Candidatus Liberibacter asiaticus infection in abandoned citrus occurring in proximity to commercially managed groves. J. Econ. Entomol. 2010, 103, 1972–1978. [Google Scholar] [CrossRef] [PubMed]
- Gottwald, T.R. Current epidemiological understanding of citrus Huanglongbing. Annu. Rev. Phytopathol. 2010, 48, 119–139. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.G.; Albrigo, L.G. Estimating the relative abundance of flush shoots in citrus with implications on monitoring insects associated with flush. HortScience 2007, 42, 364–368. [Google Scholar]
- Sétamou, M.; Flores, D.; French, J.V.; Hall, D.G. Dispersion patterns and sampling plans for Diaphorina citri (Hemiptera: Psyllidae) in citrus. J. Econ. Entomol. 2008, 101, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.G. An assessment of yellow sticky card traps as indicators of the abundance of adult Diaphorina citri (Hemiptera: Psyllidae) in citrus. J. Econ. Entomol. 2009, 102, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, K.E.; Galindo, C.; Patt, J.M.; Luque-Williams, M. Evaluation of color and scent attractants used to trap and detect Asian citrus psyllid (Hemiptera: Liviidae) in urban environments. Fla. Entomologist. 2013, 96, 1406–1416. [Google Scholar] [CrossRef]
- Sétamou, M.; Sanchez, A.; Saldaña, R.R.; Patt, J.M.; Summy, R. Visual responses of adult Asian citrus psyllid (Hemiptera: Liviidae) to colored sticky traps on citrus trees. J. Insect Behav. 2014, 27, 540–553. [Google Scholar] [CrossRef]
- Wenninger, E.J.; Stelinski, L.L.; Hall, D.G. Role of olfactory cues, visual cues, and mating status in orientation of Diaphorina citri Kuwayama (Hemiptera: Psyllidae) to four different host-plants. Environ. Entomol. 2009, 38, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Patt, J.M.; Sétamou, M. Responses of the Asian citrus psyllid to volatiles emitted by the flushing shoots of its rutaceous host-plants. Environ. Entomol. 2010, 39, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Patt, J.M.; Meikle, W.G.; Mafra-Neto, A.; Setamou, M.; Mangan, R.; Yang, C.; Malik, N.; Adamczyk, J.J. Multimodal cues drive host-plant assessment in Asian citrus psyllid (Diaphorina citri). Environ. Entomol. 2011, 40, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Sule, H.; Muhamad, R.; Dzolkhifli, O.; Kah-Wei Hee, A. Response of Diaphorina citri Kuwayama (Hemiptera: Psyllidae) to volatiles emitted from leaves of two rutaceous plants. J. Agric. Sci. 2012. [Google Scholar] [CrossRef]
- Moghbeli Gharaei1, A.; Ziaaddini, M.; Jalali, M.A.; Michaud, J.P. Sex-specific responses of Asian citrus psyllid to volatiles of conspecific and host-plant origin. J. Appl. Entomol. 2013. [Google Scholar] [CrossRef]
- Mann, R.S.; Ali, J.G.; Hermann, S.L.; Tiwari, S.; Pelz-Stelinski, K.S.; Hans, T.; Alborn, H.T.; Stelinski, L.L. Induced release of a plant-defense volatile “deceptively” attracts insect vectors to plants infected with a bacterial pathogen. PLoS Pathog. 2012, 8. [Google Scholar] [CrossRef]
- Mayer, C.J.; Vilcinskas, A.; Gross, J. Phytopathogen lures its insect vector by altering host-plant odor. J. Chem. Ecol. 2008, 34, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.J.; Vilcinskas, A.; Gross, J. Pathogen-induced release of plant allomone manipulates vector insect behavior. J. Chem. Ecol. 2008, 34, 1518–1522. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.S.; Horton, D.R.; Munyaneza, J.E.; Landolt, P.J. Experimental infection of plants with herbivore-associated bacterial endosymbiont influences herbivore host selection behavior. PLoS One 2012, 7. [Google Scholar] [CrossRef]
- Attaway, J.; Pieringer, A.P.; Barabas, L.J. The origin of citrus flavor components-I. The analysis of citrus leaf oils using gas-liquid chromatography, thin-layer chromatography, and mass spectrometry. Phytochemistry 1966, 5, 141–151. [Google Scholar] [CrossRef]
- Dugo, G.; Mondello, L.; Bonaccorsi, I. Composition of the petigrain oils. In Citrus: The Genus Citrus; Dugo, D., di Giacomo, A., Eds.; Taylor and Francis: Boca Raton, FL, USA, 2002; pp. 425–460. [Google Scholar]
- Niinemets, Ü.; Reichstein, M.; Staudt, M.; Seufert, G.; Tenhunen, J.D. Stomatal constraints may affect emission of oxygenated monoterpenoids from the foliage of Pinus pinea. Plant Physiol. 2002, 130, 1371–1385. [Google Scholar]
- Niinemets, Ü.; Loreto, F.; Reichstein, M. Physiological and physiochemical controls on foliar volatile organic compound emissions. Trends Plant Sci. 2004, 9, 180–186. [Google Scholar]
- Attaway, J.; Pieringer, A.P.; Barabas, L.J. The origin of citrus flavor components-III. A study of the percentage variations in peel and leaf oil terpenes during one season. Phytochemistry 1967, 6, 25–32. [Google Scholar] [CrossRef]
- Bergström, G.; Rothschild, M.; Groth, I.; Crighton, C. Oviposition by butterflies on young leaves: Investigation of leaf volatiles. Chemoecology 1994/1995, 5–6, 147–158. [Google Scholar]
- Flami, G.; Tebano, M.; Cioni, P.L. Volatile emission patterns of different plant organs and pollen of Citrus limon. Anal. Chim. Acta 2007, 589, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Marie-Laure Lota, M.-L.; de Rocca Serra, D.; Jacquemond, C.; Tomi, F.; Casanova, J. Chemical variability of peel and leaf essential oils of sour orange. Flavour Fragr. J. 2001, 16, 89–96. [Google Scholar]
- Azam, M.; Jiang, Q.; Zhang, B.; Xu, C.; Chen, K. Citrus leaf volatiles as affected by developmental stage and genetic type. Int. J. Mol. Sci. 2013, 14, 17744–17766. [Google Scholar] [CrossRef] [PubMed]
- Blackiston, D.J.; Casey, E.S.; Weiss, M.R. Retention of memory through metamorphosis: Can a moth remember what it learned as a caterpillar? PLoS One 2008, 3. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.; Sadek, M.M.; Larsson, M.; Hansson, B.S.; Thöming, G. Larval host-plant experience modulates both mate finding and oviposition choice in a moth. Anim. Behav. 2013, 85, 1169–1175. [Google Scholar] [CrossRef]
- Prager, S.M.; Esquivel, I.; Trumble, J.T. Factors influencing host-plant choice and larval performance in Bactericera cockerelli. PLoS One 2014, 9. [Google Scholar] [CrossRef]
- Burbott, A.J.; Loomis, W.D. Effects of light and temperature on the monoterpenes of peppermint. Plant Physiol. 1967, 42, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Bonani, J.P.; Fereres, A.; Garzo, E.; Miranda, M.P.; Appezzato-da-Gloria, B.; Lopes, J.R.S. Characterization of electrical penetration graphs of the Asian citrus psyllid, Diaphorina citri, in sweet orange seedlings. Entomol. Exp. Appl. 2010, 134, 35–49. [Google Scholar] [CrossRef]
- Hall, D.G.; Shatters, R.G.; Carpenter, J.E.; Shapiro, J.P. Research toward an artificial diet for adult Asian citrus psyllid. Ann. Entomol. Soc. Am. 2010, 103, 611–617. [Google Scholar] [CrossRef]
- Powell, G.; Tosh, C.R.; Hardie, J. Host-plant selection by aphids: Behavioral, evolutionary, and applied perspectives. Annu. Rev. Entomol. 2006, 51, 309–330. [Google Scholar] [CrossRef] [PubMed]
- Stelinski, L.L.; Gut, L.G.; Mallinger, R.E.; Epstein, D.; Reed, T.P.; Miller, J.R. Small plot trials documenting effective mating disruption of oriental fruit moth by using high densities of wax-drop pheromone dispensers. J. Econ. Entomol. 2005, 98, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Vargas, R.I.; Piñero, J.C.; Mau, R.F.L.; Stark, J.D.; Hertlein, M.; Mafra-Neto, A.; Coler, R.; Getchell, A. Attraction and mortality of oriental fruit flies to SPLAT-MAT methyl eugenol with spinosad. Entomol. Exp. Appl. 2009, 131, 286–293. [Google Scholar] [CrossRef]
- Marascuilo, L.A.; Serlin, R.C. Statistical Methods for the Social and Behavioral Sciences; W.H. Freeman and Co.: New York, NY, USA, 1988. [Google Scholar]
- Jabalpurwala, F.A.; Smoot, J.M.; Rouseff, R.L. A comparison of citrus blossom volatiles. Phytochemistry 2009, 70, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Mann, R.S.; Tiwari, S.; Smoot, J.M.; Rouseff, R.L.; Stelinski, L.L. Repellency and toxicity of plant-based essential oils and their constituents against Diaphorina citri Kuwayama (Hemiptera: Psyllidae). J. Appl. Entomol. 2012, 136, 87–96. [Google Scholar] [CrossRef]
- Snell-Rood, E.C.; Papaj, D.R. Patterns of phenotypic plasticity in common and rare environments: A study of host use and color learning in the cabbage white butterfly Pieris rapae. Am. Nat. 2009, 173, 615–631. [Google Scholar] [CrossRef] [PubMed]
- Patt, J.M.; Sétamou, M. Olfactory and visual stimuli affecting host-plant detection in Homalodisca coagulata (Hemiptera: Cicadellidae). Environ. Entomol. 2010, 36, 142–150. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patt, J.M.; Stockton, D.; Meikle, W.G.; Sétamou, M.; Mafra-Neto, A.; Adamczyk, J.J. Innate and Conditioned Responses to Chemosensory and Visual Cues in Asian Citrus Psyllid, Diaphorina citri (Hemiptera: Liviidae), Vector of Huanglongbing Pathogens. Insects 2014, 5, 921-941. https://doi.org/10.3390/insects5040921
Patt JM, Stockton D, Meikle WG, Sétamou M, Mafra-Neto A, Adamczyk JJ. Innate and Conditioned Responses to Chemosensory and Visual Cues in Asian Citrus Psyllid, Diaphorina citri (Hemiptera: Liviidae), Vector of Huanglongbing Pathogens. Insects. 2014; 5(4):921-941. https://doi.org/10.3390/insects5040921
Chicago/Turabian StylePatt, Joseph M., Dara Stockton, William G. Meikle, Mamoudou Sétamou, Agenor Mafra-Neto, and John J. Adamczyk. 2014. "Innate and Conditioned Responses to Chemosensory and Visual Cues in Asian Citrus Psyllid, Diaphorina citri (Hemiptera: Liviidae), Vector of Huanglongbing Pathogens" Insects 5, no. 4: 921-941. https://doi.org/10.3390/insects5040921






