Evaluation of Liquid and Bait Insecticides against the Dark Rover Ant (Brachymyrmex patagonicus)
Abstract
:1. Introduction
2. Experimental Section
2.1. Liquid Insecticide Study
2.1.1. Experiment Set Up
| Active ingredient | Manufacturer | Brand name | Chemical class | Concentration applied (%) | Application rate |
|---|---|---|---|---|---|
| Liquid insecticides | |||||
| Fipronil | BASF, Florham Park, NJ, USA | Termidor® SC | Phenylpyrazole | 0.06 | 61 mL/m2 |
| Lambda-cyhalothrin | Syngenta, Greensboro, NC, USA | Demand® CS | Pyrethroid | 0.03 | 41 mL/m2 |
| Indoxacarb (1X) | Arilon® | Oxadiazine | 0.06 | 41 mL/m2 | |
| Indoxacarb (2X) | 82 mL/m2 | ||||
| Indoxacarb (4X) | 164 mL/m2 | ||||
| Gel and liquid baits | |||||
| Imidacloprid | Bayer CropScience, Research Triangle Park, NC, USA | Maxforce® Quantum ant bait | Neonicotinoid | 0.03 | 0.1 g replenished as necessary |
| Sodium tetraborate | Rockwell labs Ltd., North Kansas City, MO, USA | Intice ® rover ant bait | Boron compound | 5 | |
| Indoxacarb | Syngenta, Greensboro, NC, USA | Advion® ant gel | Oxadiazine | 0.05 | |
2.1.2. Statistical Analysis
2.2. Bait Study
2.2.1. Experiment Set Up
2.2.2. Statistical Analysis
3. Results
3.1. Liquid Insecticides
| Model parameters | Parameter estimates (± SE) | p > t | |
|---|---|---|---|
| Intercept | 33.25 ± 1.03 | <0.0001* | |
| Treatment age | 5.4 ± 1.39 | 0.0001* | |
| Treatment | |||
| Control | 39.53 ± 2.3 | <0.0001* | |
| Indoxacarb (1X) | −8.02 ± 2.3 | 0.0006* | |
| Indoxacarb (2X) | −17.91 ± 2.3 | <0.0001* | |
| Indoxacarb (4X) | −21.91 ± 2.3 | <0.0001* | |
| Lambda-cyhalothrin | −20.55 ± 2.3 | <0.0001* | |
| Fipronil | 28.86 ± 2.3 | <0.0001* | |
| Treatment age × Treatment | |||
| Treatment age × Control | −2.32 ± 3.1 | 0.4552 | |
| Treatment age × Indoxacarb (1X) | 8.6 ± 3.1 | 0.0058* | |
| Treatment age × Indoxacarb (2X) | −2.02 ± 3.1 | 0.5149 | |
| Treatment age × Indoxacarb (4X) | 0.29 ± 3.1 | 0.9252 | |
| Treatment age × Lambda-cyhalothrin | 0.18 ± 3.1 | 0.9546 | |
| Treatment age × Fipronil | −4.73 ± 3.1 | 0.1284 | |
| Surface | |||
| Tile | −3.97 ± 1.03 | 0.0001* | |
| Surface × Treatment | |||
| Tile × Control | 10.07 ± 2.3 | <0.0001* | |
| Tile × Indoxacarb (1X) | −3.42 ± 2.3 | 0.1394 | |
| Tile × Indoxacarb (2X) | −7.11 ± 2.3 | 0.0022* | |
| Tile × Indoxacarb (4X) | −6.3 ± 2.3 | 0.0065* | |
| Tile × Lambda-cyhalothrin | −8.15 ± 2.3 | 0.0004* | |
| Tile × Fipronil | 14.91 ± 2.3 | <0.0001* | |
| Treatment age × Surface | |||
| Treatment age × Tile | −1.73 ± 1.39 | 0.213 | |
| Exposure time | |||
| 30 min | −2.97 ± 1.03 | 0.0041* | |
| Exposure time × Treatment | |||
| 30 min × Control | 1.01 ± 2.3 | 0.6623 | |
| 30 min × Indoxacarb (1X) | −3.74 ± 2.3 | 0.1063 | |
| 30 min × Indoxacarb (2X) | −1.9 ± 2.3 | 0.4112 | |
| 30 min × Indoxacarb (4X) | −0.08 ± 2.3 | 0.971 | |
| 30 min × Lambda-cyhalothrin | 2.06 ± 2.3 | 0.3717 | |
| 30 min × Fipronil | 2.64 ± 2.3 | 0.2525 | |
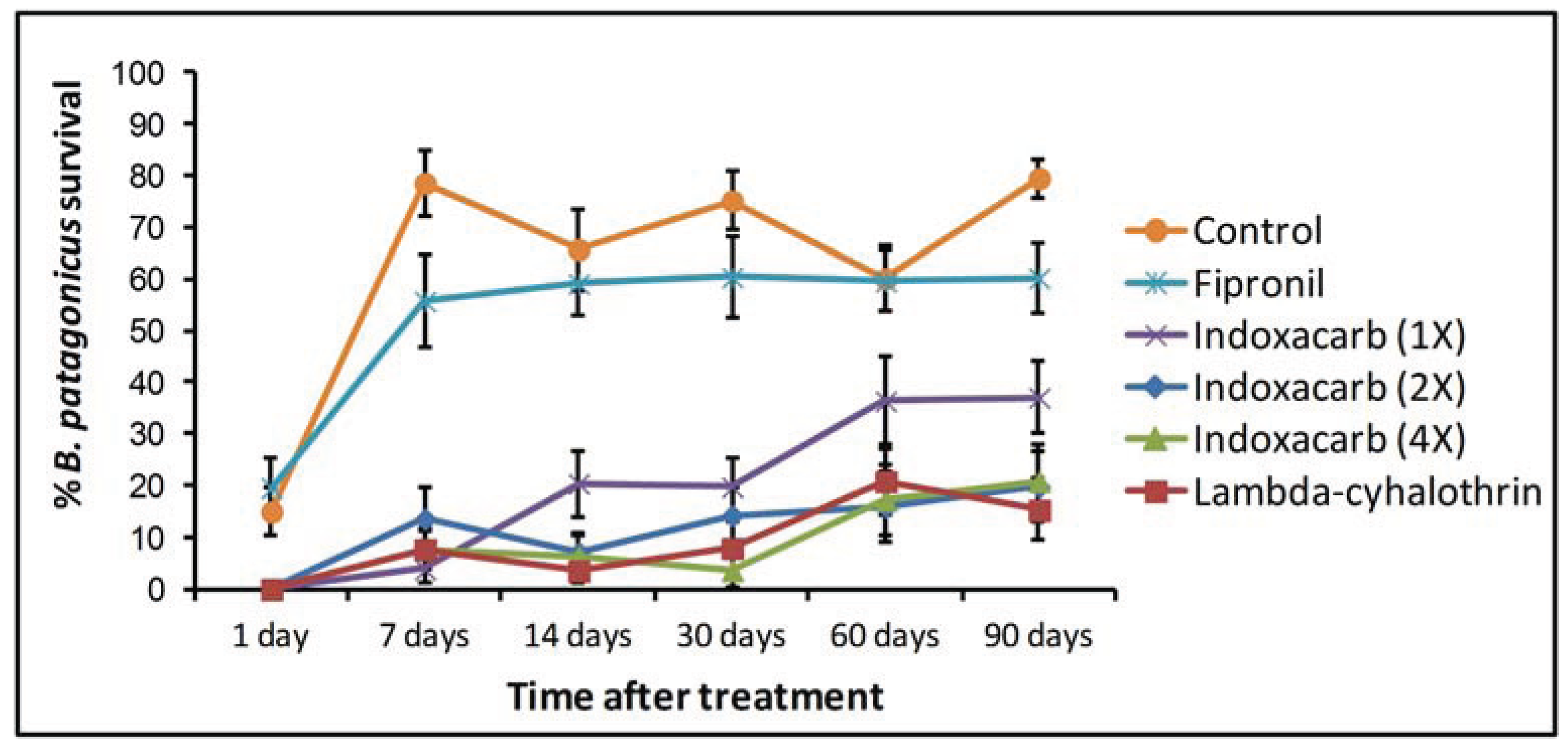
| Treatment | Surface × Treatment (interaction) | |||||||
|---|---|---|---|---|---|---|---|---|
| Level | Contrast | LS Means (95% CI) | Level | Contrast | LS Means (95% CI) | |||
| Control | A | 72.77 (67.8–77.74) | T × Control | A | 78.88 (71.85–85.9) | |||
| Fipronil | B | 62.11 (57.14–67.07) | T × Fipronil | A | 73.05 (66.02–80.08) | |||
| Indoxacarb (1X) | C | 25.23 (20.26–30.2) | W × Control | A B | 66.67 (59.64–73.69) | |||
| Indoxacarb (2X) | C | D | 15.33 (10.37–20.3) | W × Fipronil | B | 51.16 (44.14–58.19) | ||
| Lambda-cyhalothrin | D | 12.69 (7.72–17.66) | W × Indoxacarb (1X) | C | 32.61 (25.59–39.64) | |||
| Indoxacarb (4X) | D | 11.34 (6.37–16.31) | W × Indoxacarb (2X) | C | 26.41 (19.39–33.44) | |||
| W × Lambda-cyhalothrin | C | 24.81 (17.79–31.84) | ||||||
| W × Indoxacarb (4X) | C | 21.61 (14.58–28.64) | ||||||
| T × Indoxacarb (1X) | C D | 17.84 (10.82–24.87) | ||||||
| T × Indoxacarb (2X) | DE | 4.26 (−2.77–11.28) | ||||||
| T × Indoxacarb (4X) | E | 1.07 (−5.96–8.1) | ||||||
| T × Lambda-cyhalothrin | E | 0.57 (−6.46–7.6) | ||||||

3.2. Bait Study
| Model parameters | Parameter estimates (± SE) | p > t | |
|---|---|---|---|
| Intercept | 79.44 ± 5.82 | <0.0001* | |
| Time after initial application | −0.75 ± 0.38 | 0.0489* | |
| Treatment | |||
| Indoxacarb | 1.07 ± 6.63 | 0.8736 | |
| Sodium tetraborate | 7.78 ± 6.63 | 0.2543 | |
| Imidacloprid | −55.2 ± 6.63 | <0.0001* | |
| Time after initial application × Treatment | |||
| Time after initial application × Indoxacarb | −0.25 ± 0.53 | 0.6439 | |
| Time after initial application × Sodium tetraborate | −1.42 ± 0.53 | 0.0087* | |
| Time after initial application × Imidacloprid | −1.38 ± 0.53 | 0.0107* | |


4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Martínez, J.J.; Quirán, E.M.; Bachmann, A.O. The neotropical genus Brachymyrmex Mayr, 1868 (Hymenoptera: Formicidae) in Argentina. Redescription of the type species, B. patagonicus mayr, 1868, B. bruchi Forel, 1912 and B. oculatus Santschi, 1919. Acta Zool. Mex. 2004, 20, 273–285. [Google Scholar]
- Wheeler, G.C.; Wheeler, J. Brachymyrmex musculus, a new ant in the United States. Entomol. News 1978, 89, 189–190. [Google Scholar]
- MacGown, J.A.; Forster, J.A. A preliminary list of the ants (Hymenoptera: Formicidae) of Alabama, USA. Entomol. News 2005, 116, 61–74. [Google Scholar]
- MacGown, J.A.; Hill, J.G.; Deyrup, M.A. Brachymyrmex patagonicus (Hymenoptera: Formicidae), an emerging pest species in the southeastern United States. Fla. Entomol. 2007, 90, 457–464. [Google Scholar] [CrossRef]
- Miguelena, J.; Baker, P.B. Ruining your picnic: Prevalence of ants in urban parks in Tucson, AZ. In the Proceedings of the National Conference on Urban Entomology, Atlanta, USA, 20–23 May 2012; pp. 47–51.
- Robbins, M.; Miller, T.E. Patterns of ant activity on Opuntia stricta (cactaceae), a native host-plant of the invasive cactus moth, Cactoblastis cactorum (Lepidoptera: Pyralidae). Fla. Entomol. 2009, 92, 391–393. [Google Scholar] [CrossRef]
- JMP, 8.0; SAS Institute, Inc: Cary, NC, USA, 2008.
- Soeprono, A.M.; Rust, M.K. Effect of delayed toxicity of chemical barriers to control Argentine ants (Hymenoptera: Formicidae). J. Econ. Entomol. 2004, 97, 2021–2028. [Google Scholar] [CrossRef] [PubMed]
- Buczkowski, G.; Scharf, M.E.; Ratliff, C.R.; Bennett, G.W. Efficacy of simulated barrier treatments against laboratory colonies of pharaoh ant. J. Econ. Entomol. 2005, 98, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Wiltz, B.; Suiter, D.; Gardner, W. Activity of bifenthrin, chlorfenapyr, fipronil, and thiamethoxam against red imported fire ants (Hymenoptera: Formicidae). J. Econ. Entomol. 2010, 103, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Hannum, C.D.; Miller, D.M. Intercolony variation in the black carpenter ant (Camponotus pennsylvanicus) (Hymenoptera: Formicidae) response to fipronil (0.06%) residues. Sociobiology 2008, 52, 729–750. [Google Scholar]
- Warner, J.; Scheffrahn, R.H. Laboratory evaluation of baits, residual insecticides, and an ultrasonic device for control of white-footed ants, Technomyrmex albipes (Hymenoptera: Formicidae). Sociobiology 2005, 45, 317–330. [Google Scholar]
- Warner, J.; Scheffrahn, R.H.; Yang, R.-L. Arboreal bioassay for toxicity of residual and liquid bait insecticides against white-footed ants, Technomyrmex difficilis (Hymenoptera: Formicidae). Sociobiology 2010, 55, 847–860. [Google Scholar]
- Knight, R.L.; Rust, M.K. Repellency and efficacy of insecticides against foraging workers in laboratory colonies of Argentine ants (Hymenoptera: Formicidae). J. Econ. Entomol. 1990, 83, 1402–1408. [Google Scholar]
- Osbrink, W.L.; Lax, A.R. Effect of tolerance to insecticides on substrate penetration by formosan subterranean termites (Isoptera: Rhinotermitidae). J. Econ. Entomol. 2002, 95, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Wege, P.; Hoppe, M.; Bywater, A.; Weeks, S.; Gallo, T. A microencapsulated formulation of lambda-cyhalothrin. In the Proceedings of the Third International Conference on Urban Pests, Prague, Czech Republic, 19–22 July 1999; pp. 301–310.
- Choe, D.-H.; Rust, M.K. Horizontal transfer of insecticides in laboratory colonies of the Argentine ant (Hymenoptera: Formicidae). J. Econ. Entomol. 2008, 101, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Brooks, M.; Nentwig, G.; Gutsmann, V. Elimination of a Tapinoma melanocephalum (hymenoptera: Formicidae) infestation using imidacloprid bait. Int. Pest Control 2009, 51, 240–243. [Google Scholar]
- Rust, M.K.; Reierson, D.A.; Klotz, J.H. Delayed toxicity as a critical factor in the efficacy of aqueous baits for controlling Argentine ants (hymenoptera: Formicidae). J. Econ. Entomol. 2004, 97, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Furman, B.D.; Gold, R.E. Trophallactic transmission and metabolism of the active ingredient indoxacarb in Advion™ (Hymenoptera: Formicidae). Sociobiology 2006, 48, 335–353. [Google Scholar]
- Hansen, L.D. Indoxacarb as a management tool for carpenter ants. In the Proceedings of The National Conference on Urban Entomology, Tulsa, OK, USA, May 18–21 2008; pp. 62–64.
- Mo, Y. Temporal food preference and effectiveness of selected bait products against Pachycondyla chinensis (Emery) (Hymenoptera: Formicidae). Master Thesis, Clemson University, Clemson, SC, USA, 2013. [Google Scholar]
- Mathieson, M.; Toft, R.; Lester, P.J. Influence of toxic bait type and starvation on worker and queen mortality in laboratory colonies of Argentine ant (Hymenoptera: Formicidae). J. Econ. Entomol. 2012, 105, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Keefer, T.C.; Gold, R.E. Biology and management of the dark rover ant (Hymenoptera: Formicidae). In the Proceedings of the National Conference on Urban Entomology, Atlanta, GA, USA, 20–23 May 2012; pp. 36–37.
- Klotz, J.H.; Vail, K.M.; Williams, D.F. Toxicity of a boric acid-sucrose water bait to Solenopsis invicta (Hymenoptera: Formicidae). J. Econ. Entomol. 1997, 90, 488–491. [Google Scholar]
- Klotz, J.H.; Greenberg, L.; Amrhein, C.; Rust, M.K. Toxicity and repellency of borate-sucrose water baits to Argentine ants (Hymenoptera: Formicidae). J. Econ. Entomol. 2000, 93, 1256–1258. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.D. Inconsistencies in the use of baits in field trials and comparison to laboratory trials with carpenter ants (Hymenoptera: Formicidae). In the Proceedings of the Sixth International Conference on Urban Pests, Budapest, Hungary, 13–16 July 2008; pp. 65–69.
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miguelena, J.G.; Baker, P.B. Evaluation of Liquid and Bait Insecticides against the Dark Rover Ant (Brachymyrmex patagonicus). Insects 2014, 5, 832-848. https://doi.org/10.3390/insects5040832
Miguelena JG, Baker PB. Evaluation of Liquid and Bait Insecticides against the Dark Rover Ant (Brachymyrmex patagonicus). Insects. 2014; 5(4):832-848. https://doi.org/10.3390/insects5040832
Chicago/Turabian StyleMiguelena, Javier G., and Paul B. Baker. 2014. "Evaluation of Liquid and Bait Insecticides against the Dark Rover Ant (Brachymyrmex patagonicus)" Insects 5, no. 4: 832-848. https://doi.org/10.3390/insects5040832




