Screening Commercially Available Entomopathogenic Biocontrol Agents for the Control of Aethina tumida (Coleoptera: Nitidulidae) in the UK
Abstract
:1. Introduction
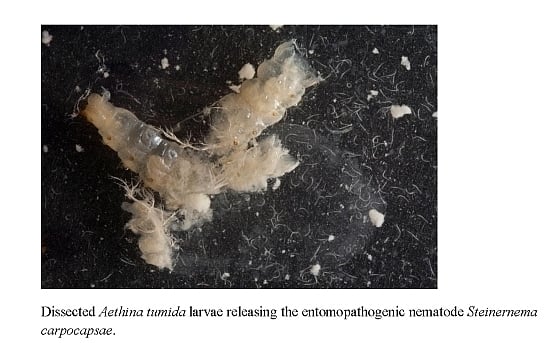
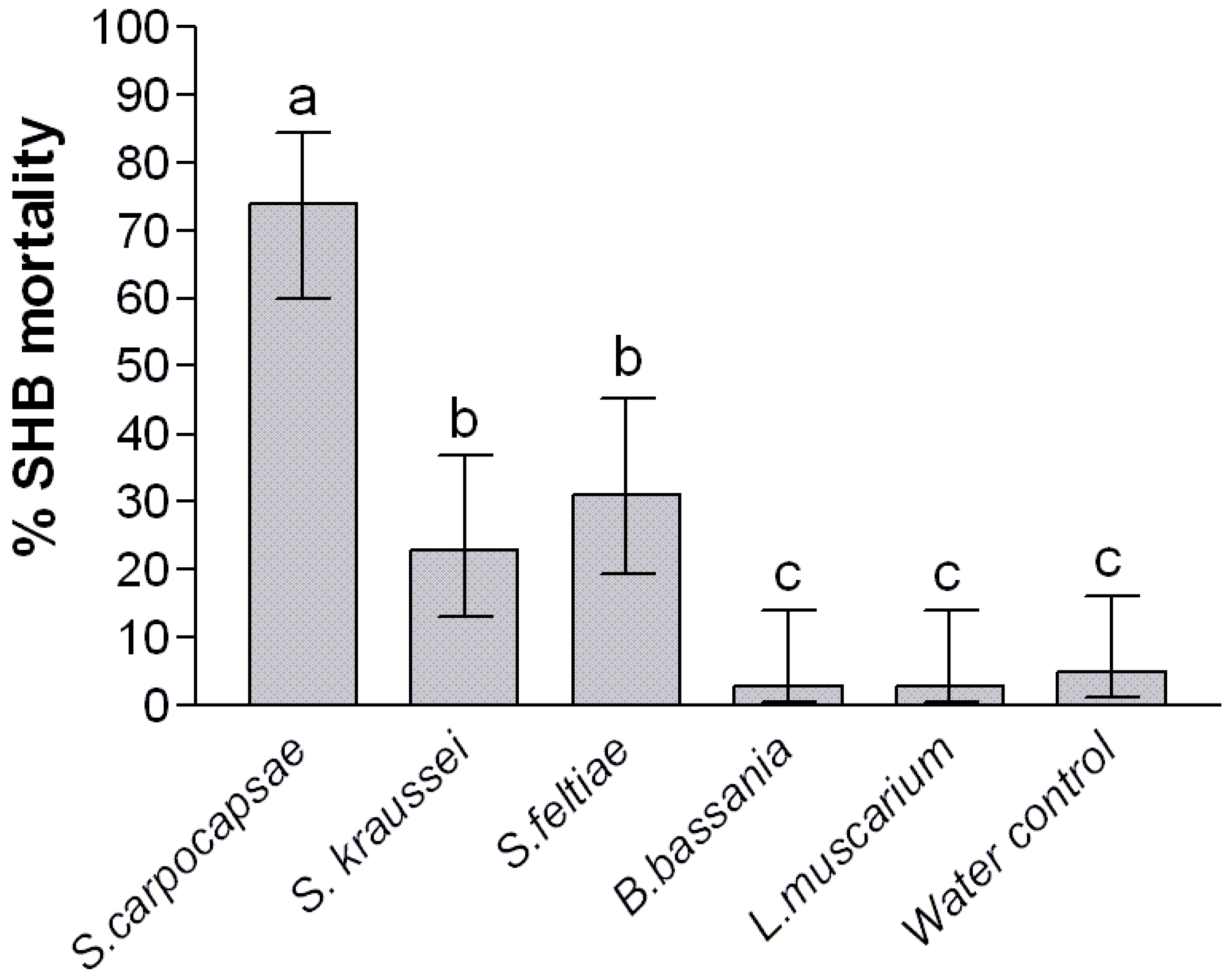
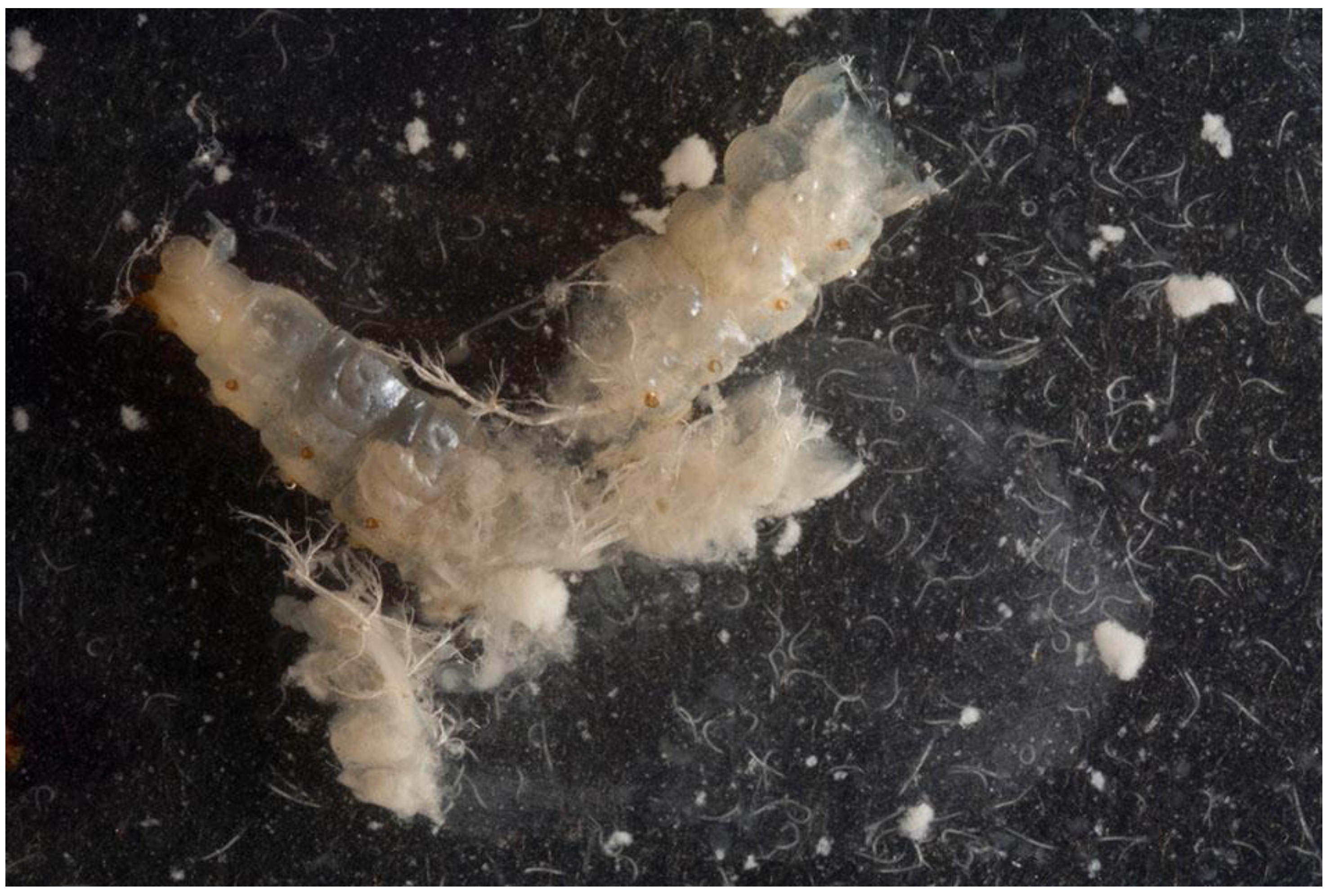
2. Results and Discussion


3. Experimental Section
3.1. Insect Rearing and Control Agents
3.2. Direct Exposure of Larvae to Control Agents
3.3. Indirect Exposure of Larvae to Control Agents
3.4. Sequential Application of Nematodes against Beetle Larvae
3.5. Analysis of Data
4. Conclusions
Acknowledgements
References
- Lundie, A.E. The Small Hive Beetle, Aethina tumida; Science Bulletin; Department of Agriculture and Forestry: Stellenbosch, Western Cape Province, South Africa, 1940.
- Neumann, P.; Elzen, P.J. The biology of the small hive beetle (Aethina tumida, Coleoptera: Nitidulidae): Gaps in our knowledge of an invasive species. Apidologie 2004, 35, 229–247. [Google Scholar] [CrossRef]
- Spiewok, S.; Pettis, J.S.; Duncan, M.; Spooner-Hart, R.; Westervelt, D.; Neumann, P. Small hive beetle, Aethina tumida, populations I: Infestation levels of honey bee colonies, apiaries and regions. Apidologie 2007, 38, 595–605. [Google Scholar] [CrossRef]
- Cuthbertson, A.G.S.; Brown, M.A. Issues affecting British honey bee biodiversity and the need for conservation of this important ecological component. Int. J. Environ. Sci. Tech. 2009, 6, 695–699. [Google Scholar]
- Cuthbertson, A.G.S.; Mathers, J.J.; Blackburn, L.F.; Wakefield, M.E.; Collins, L.E.; Luo, W.; Brown, M.A. Maintaining Aethina tumida (Coleoptera:Nitidulidae) under quarantine laboratory conditions in the UK and preliminary observations on its behaviour. J. Apicult. Res. 2008, 47, 192–193. [Google Scholar]
- De Guzman, L.I.; Frake, A.M. Temperature affects Aethina tumida (Coleoptera: Nitidulidae) development. J. Apicult. Res. 2007, 46, 88–93. [Google Scholar] [CrossRef]
- Hood, W.M. The small hive beetle, Aethina tumida: A review. Bee World 2004, 85, 51–59. [Google Scholar]
- Hemingway, J.; Ranson, H. Insecticide resistance in insect vectors of human diseases. Annu. Rev. Entomol. 2000, 45, 371–391. [Google Scholar] [CrossRef]
- Hassan, S.A.; Bigler, F.; Bogenschütz, H. Results of the second joint pesticide testing programme by the IOBC/WPRS-Working Group “Pesticides and Beneficial Arthropods”. Z. Ang. Entomol. 1983, 95, 151–158. [Google Scholar]
- World Health Organization (WHO). Permethrin. (Environmental Health Criteria 94); World Health Organization, United Nations Environment Program and International Labour Organization: Geneva, Switzerland, 1990; pp. 76–78.
- Glazer, I.; Salame, L.; Goldenberg, S.; Blumberg, D. Susceptibility of sap beetles (Coleoptera: Nitidulidae) to entomopathogenic nematodes. Biocontrol Sci. Technol. 1999, 9, 259–266. [Google Scholar] [CrossRef]
- Cabanillas, H.F.; Elzen, P.J. Infectivity of entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) against the small hive beetle Aethina tumida (Coleoptera: Nitidulidae). J. Apic. Res. 2006, 45, 49–50. [Google Scholar]
- Cuthbertson, A.G.S.; Blackburn, L.F.; Eyre, D.P.; Cannon, R.J.C.; Millar, J.; Northing, P. Bemisia tabaci: The current situation in the UK and the prospect of developing strategies for eradication using entomopathogens. Insect Sci. 2011, 18, 1–10. [Google Scholar]
- Petersen, J.J.; Willis, O.R. Some factors affecting parasitism by mermithid nematodes in southern house mosquito larvae. J. Econ. Entomol. 1970, 63, 175–178. [Google Scholar]
- Gaugler, R.; Molloy, D. Instar susceptibility of Simulium vittatum (Diptera: Simuliidae) to the entomogenous nematode Neoaplectana carpocapsae. J. Nematol. 1981, 13, 1–5. [Google Scholar]
- Cuthbertson, A.G.S.; Mathers, J.J. Personal Communication. The Food and Environment Research Agency: Sand Hutton, York, UK, 2011. [Google Scholar]
- Ellis, J.D.; Spiewok, S.; Delaplane, K.S.; Buchholz, S.; neumann, p.; Tedders, W.L. Susceptibility of Aethina tumida (Coleoptera: Nitidulidae) larvae and pupae to entomopathogenic nematodes. J. Econ. Entomol. 2010, 103, 1–9. [Google Scholar]
- Shapiro-Ilan, D.I.; Gaugler, R.; Tedders, W.L.; Brown, I.; Lewis, E.E. Optimization of inoculation for in vivo production of entomopathogenic nematodes. J. Nematol. 2002, 34, 343–350. [Google Scholar]
- Shapiro-Ilan, D.I. Personal Communication. United States Department of Agriculture, Southeastern Fruit and Tree Nut Research Laboratory: Byron, GA, USA, 2011. [Google Scholar]
- Lacey, L.A.; Frutos, H.K.; Vail, P. Insect pathogens as biological control agents: Do they have a future? Biol. Control 2001, 21, 230–248. [Google Scholar] [CrossRef]
- Boucias, D.G.; Pendland, J.C. Principles of Insect Pathology; Kluwer: Boston, MA, USA, 1998; pp. 1–147. [Google Scholar]
- Muerrle, T.M.; Neumann, P.; Dames, J.F.; Hepburn, H.R.; Hill, M.P. Susceptibility of adult Aethina tumida (Coleoptera: Nitidulidae) to entomopathgenic fungi. J. Econ. Entomol. 2006, 99, 1–6. [Google Scholar] [CrossRef]
- Ellis, J.D.; Rong, I.H.; Hill, M.P.; Hepburn, H.R.; Elzen, P.J. The susceptibility of small hive beetle (Aethina tumida Murray) pupae to fungal pathogens. Am. Bee J. 2004, 144, 486–488. [Google Scholar]
- Morse, R.A.; Flottum, K. Honey Bee Pests, Predators and Diseases; Root Company: Medina, OH, USA, 1997; pp. 1–137. [Google Scholar]
- Cuthbertson, A.G.S.; Mathers, J.J.; Blackburn, L.F.; Brown, M.A.; Marris, G. Small hive beetle: The next threat to British honey bees? Biologist 2010, 57, 35–39. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cuthbertson, A.G.S.; Mathers, J.J.; Blackburn, L.F.; Powell, M.E.; Marris, G.; Pietravalle, S.; Brown, M.A.; Budge, G.E. Screening Commercially Available Entomopathogenic Biocontrol Agents for the Control of Aethina tumida (Coleoptera: Nitidulidae) in the UK. Insects 2012, 3, 719-726. https://doi.org/10.3390/insects3030719
Cuthbertson AGS, Mathers JJ, Blackburn LF, Powell ME, Marris G, Pietravalle S, Brown MA, Budge GE. Screening Commercially Available Entomopathogenic Biocontrol Agents for the Control of Aethina tumida (Coleoptera: Nitidulidae) in the UK. Insects. 2012; 3(3):719-726. https://doi.org/10.3390/insects3030719
Chicago/Turabian StyleCuthbertson, Andrew G. S., James J. Mathers, Lisa F. Blackburn, Michelle E. Powell, Gay Marris, Stephane Pietravalle, Mike A. Brown, and Giles E. Budge. 2012. "Screening Commercially Available Entomopathogenic Biocontrol Agents for the Control of Aethina tumida (Coleoptera: Nitidulidae) in the UK" Insects 3, no. 3: 719-726. https://doi.org/10.3390/insects3030719