Differences in Immune Defense Evasion of Selected Inbred Lines of Heterorhabditis Bacteriophora in Two White Grub Species
Abstract
:1. Introduction
2. Experimental Section
2.1. Sources of White Grubs
2.2. Source of H. bacteriophora Inbred Lines
2.3. Virulence of Inbred Lines against P. japonica and C. borealis
2.4. Penetration, Encapsulation and Survival of Inbred Lines in P. japonica and C. borealis
2.5. Temporal Interactions of Inbred Lines with Immune Response of P. japonica
3. Results
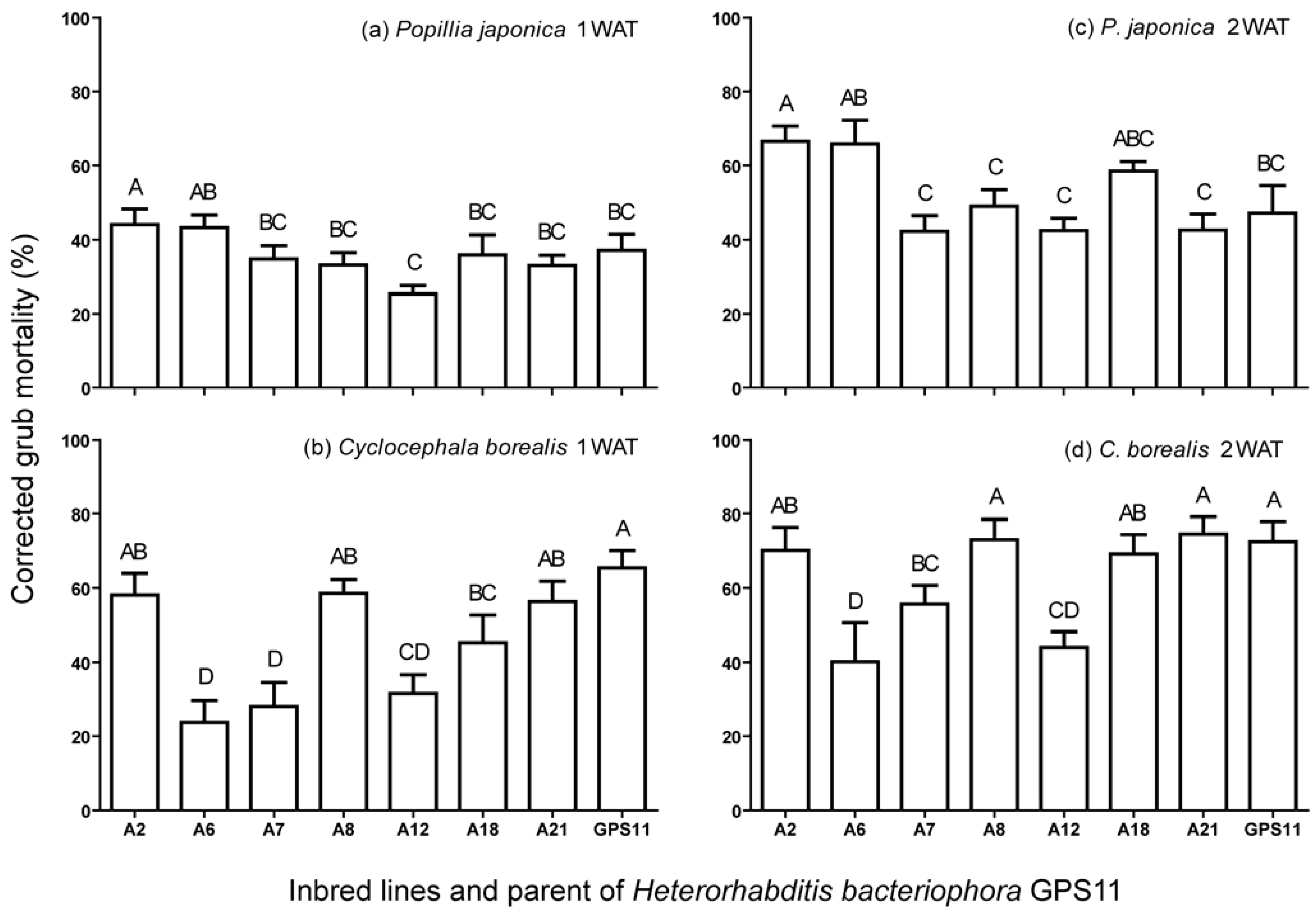
3.1. Virulence of Inbred Lines against P. japonica and C. borealis
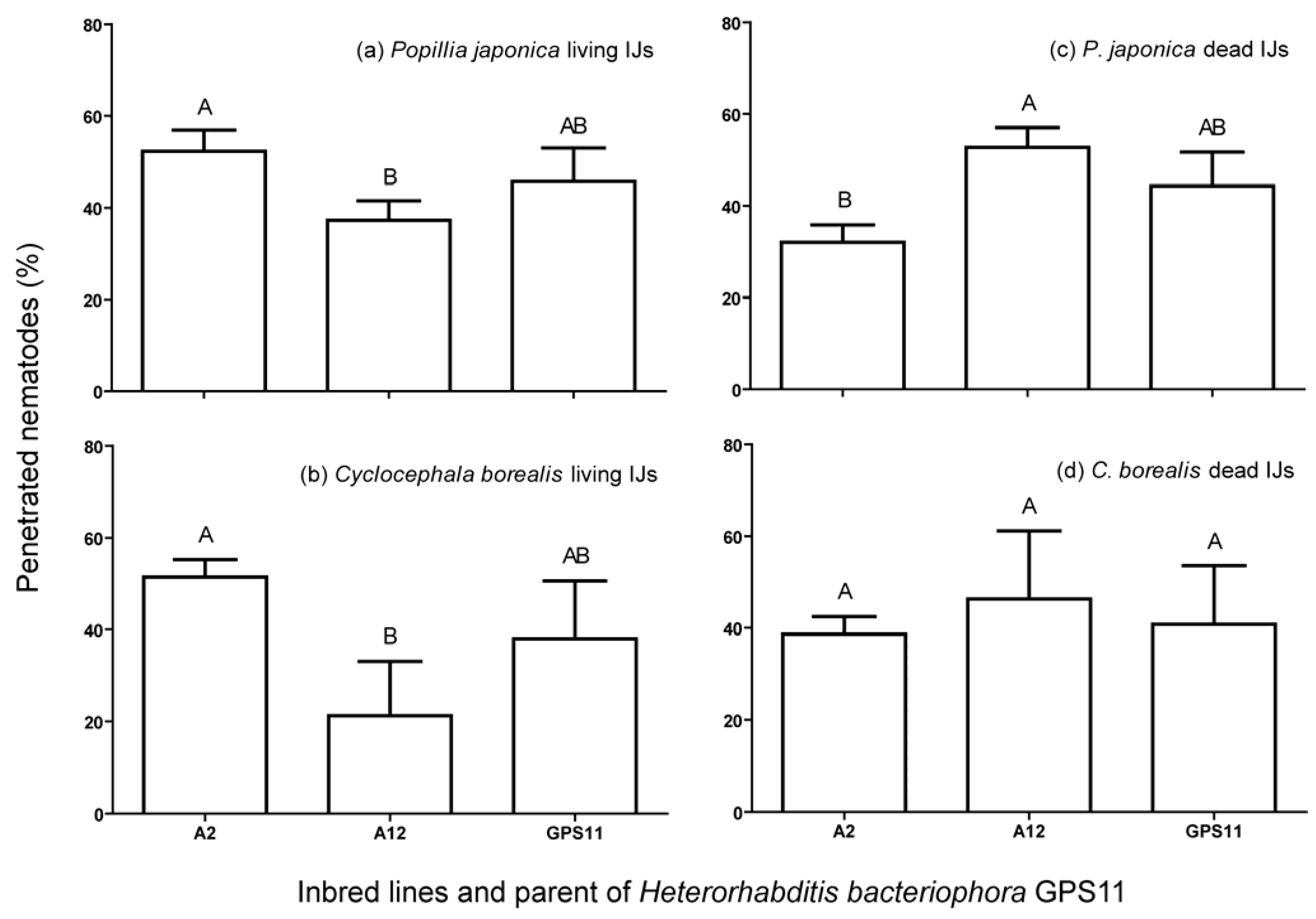
3.2. Penetration, Encapsulation and Survival of Inbred Lines in P. japonica and C. borealis
3.3. Temporal Interactions of Inbred Lines with Immune Response of P. japonica
4. Discussion and Conclusions

Acknowledgments
References
- Dowds, B.C.A.; Peters, A. Virulence Mechanisms. In Entomopathogenic Nematology; Gaugler, R., Ed.; CABI Publishing: New York, NY, USA, 2002; pp. 79–98. [Google Scholar]
- An, R.; Sreevatsan, S.; Grewal, P.S. Comparative in vivo gene expression of the closely related bacteria Photorhabdus temperata and Xenorhabdus koppenhoeferi upon infection of the same insect host, Rhizotrogus majalis. BMC Genomics 2009, 10, 433. [Google Scholar] [CrossRef]
- Grewal, P.S.; Ehlers, R.-U.; Shapiro-Ilan, D.I. Nematodes as Biocontrol Agents, 1st ed.; CABI Publishing: Oxon, UK, 2005; p. 505. [Google Scholar]
- Klein, M.G.; Grewal, P.S.; Jackson, T.A. Lawn, Turf, and Grassland Pests. In Field Manual of Techniques in Invertebrate Pathology; Lacey, L.A., Kaya, H.K., Eds.; Kluwer Academic Publishers: AH Dordrecht, Netherlands, 2000; pp. 681–706. [Google Scholar]
- Grewal, P.S.; Grewal, S.K.; Malik, V.S.; Klein, M.G. Differences in the susceptibility of introduced and native white grub species to entomopathogenic nematodes from various geographic localities. Biol. Contr. 2002, 24, 230–237. [Google Scholar] [CrossRef]
- Grewal, P.S.; Wang, X.; Taylor, R.A.J. Dauer juvenile longevity and stress tolerance in natural populations of an entomopathogenic nematode: Is there a relationship? Int. J. Parasitol. 2002, 32, 717–725. [Google Scholar] [CrossRef]
- Shapiro-Ilan, D.I.; Glazer, I.; Segal, D. Trait stability and fitness of the heat tolerant entomopathogenic nematode Heterorhabditis bacteriophora IS5 strain. Biol. Contr. 1996, 6, 238–244. [Google Scholar] [CrossRef]
- Wang, X.; Grewal, P.S. Rapid genetic deterioration of environmental tolerance and reproductive potential of an entomopathogenic nematode during laboratory maintenance. Biol. Contr. 2002, 23, 71–78. [Google Scholar] [CrossRef]
- Bai, C.; Shapiro-Ilan, D.I.; Gaugler, R.; Hopper, K.R. Stabilization of beneficial traits in Heterorhabditis bacteriophora through creation of inbred lines. Biol. Contr. 2005, 32, 220–227. [Google Scholar] [CrossRef]
- Roush, R.T. Genetic Considerations in the Propagation of Entomophagous Species. In Critical Issues in Biological Control; Baker, R.R., Dunn, P.E., Eds.; Liss: New York, NY, USA, 1990; pp. 373–387. [Google Scholar]
- Sandhu, S.K. Genetic and Molecular Analysis of Infective Juvenile Longevity and Stress Tolerance in the Entomopathogenic Nematode Heterorhabditis bacteriophora.
- Grewal, P.S.; Converse, V.; Georgis, R. Influence of production and bioassay methods on virulence of two ambush foragers (Nematoda: Steinernematidae). J. Invertebr. Pathol. 1999, 73, 40–44. [Google Scholar] [CrossRef]
- Poinar, G.O., Jr. Taxonomy and Biology Steinernematidae and Heterorhabditidae. In Entomopathogenic Nematodes in Biological Control; Gaugler, R., Kaya, H.K., Eds.; CRC Press: Boca Raton, FL, USA, 1990; pp. 23–61. [Google Scholar]
- White, G.F. A method for obtaining infective nematode larvae from cultures. Science 1927, 66, 302–303. [Google Scholar]
- Kaya, H.K.; Stock, S.P. Techniques in Insect Nematology. In Manual of Techniques in Insect Pathology; Lacey, L.A., Ed.; Academic Press: London, UK, 1997; pp. 281–324. [Google Scholar]
- Abbott, W.S. A method for computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar]
- Glazer, I.; Gaugler, R.; Segal, D. Genetics of the nematode Heterorhabditis bacteriophora HP88 strain: The diversity of beneficial traits. J. Nematol. 1991, 23, 324–333. [Google Scholar]
- Wang, Y.; Gaugler, R.; Cui, L. Variations in immune response of Popillia japonica and Acheta domesticus to Heterorhabditis bacteriophora and Steinernema species. J. Nematol. 1994, 26, 11–18. [Google Scholar]
- Wang, Y.; Campbell, J.F.; Gaugler, R. Infection of entomopathogenic nematodes Steinernema glaseri and Heterorhabditis bacteriophora against Popillia japonica (Coleoptera, Scarabaeidae) larvae. J. Invertebr. Pathol. 1995, 66, 178–184. [Google Scholar] [CrossRef]
- Brivio, M.F.; Pagani, M.; Restelli, S. Immune suppression of Galleria mellonella (Insecta, Lepidoptera) humoral defenses induced by Steinernema feltiae (Nematoda, Rhabditida): Involvement of the parasite cuticle. Exp. Parasitol. 2002, 101, 149–156. [Google Scholar] [CrossRef]
- Wang, Y.; Gaugler, R. Steinernema glaseri surface coat protein suppresses the immune response of Popillia japonica (Coleoptera : Scarabaeidae). Biol. Contr. 1999, 14, 45–50. [Google Scholar] [CrossRef]
- Dunphy, G.B.; Webster, J.M. Partially characterized components of the epicuticle of dauer juvenile Steinernema feltiae and their influence on hemocyte activity in Galleria mellonella. J. Parasitol. 1987, 73, 584–588. [Google Scholar] [CrossRef]
- Han, R.; Ehlers, R.-U. Pathogenicity, development, and reproduction of Heterorhabditis bacteriophora and Steinernema carpocapsae under axenic in vivo conditions. J. Invertebr. Pathol. 2000, 75, 55–58. [Google Scholar] [CrossRef]
- Hallem, E.A.; Rengarajan, E.A.; Rengarajan, M.; Ciche, T.A.; Sternberg, P.W. Nematodes, bacteria, and flies: A tripartite model for nematode parasitism. Curr. Biol. 2007, 17, 898–904. [Google Scholar] [CrossRef]
- Eleftherianos, I.; Joyce, S.; ffrench-Constant, R.H.; Clarke, D.J.; Reynolds, S.E. Probing the tri-trophic interaction between insects, nematodes and Photorhabdus. Parasitology 2010, 137, 1695–1706. [Google Scholar]
- Bowen, D.; Rocheleau, T.A.; Blackburn, M.; Andreev, O.; Golubeva, E.; Bhartia, R.; ffrench-Constant, R.H. Insecticidal toxins from the bacterium Photorhabdus luminescens. Science 1998, 280, 2129–2132. [Google Scholar]
- An, R.; Grewal, P.S. Differences in the virulence of Heterorhabditis bacteriophora and Steinernema scarabaei to three white grub species: The relative contribution of the nematodes and their symbiotic bacteria. Biol. Contr. 2007, 43, 310–316. [Google Scholar] [CrossRef]
- Strauch, O.; Oestergaard, J.; Hollmer, S.; Ehlers, R.-U. Genetic improvement of the desiccation tolerance of the entomopathogenic nematode Heterorhabditis bacteriophora through selective breeding. Biol. Contr. 2004, 31, 218–226. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
An, R.; Voss, M.; Jagdale, G.B.; Grewal, P.S. Differences in Immune Defense Evasion of Selected Inbred Lines of Heterorhabditis Bacteriophora in Two White Grub Species. Insects 2012, 3, 378-389. https://doi.org/10.3390/insects3020378
An R, Voss M, Jagdale GB, Grewal PS. Differences in Immune Defense Evasion of Selected Inbred Lines of Heterorhabditis Bacteriophora in Two White Grub Species. Insects. 2012; 3(2):378-389. https://doi.org/10.3390/insects3020378
Chicago/Turabian StyleAn, Ruisheng, Marcio Voss, Ganpati B. Jagdale, and Parwinder S. Grewal. 2012. "Differences in Immune Defense Evasion of Selected Inbred Lines of Heterorhabditis Bacteriophora in Two White Grub Species" Insects 3, no. 2: 378-389. https://doi.org/10.3390/insects3020378



