Ecological and Evolutionary Determinants of Bark Beetle —Fungus Symbioses
Abstract
:1. Scolytinae-Fungus Symbioses

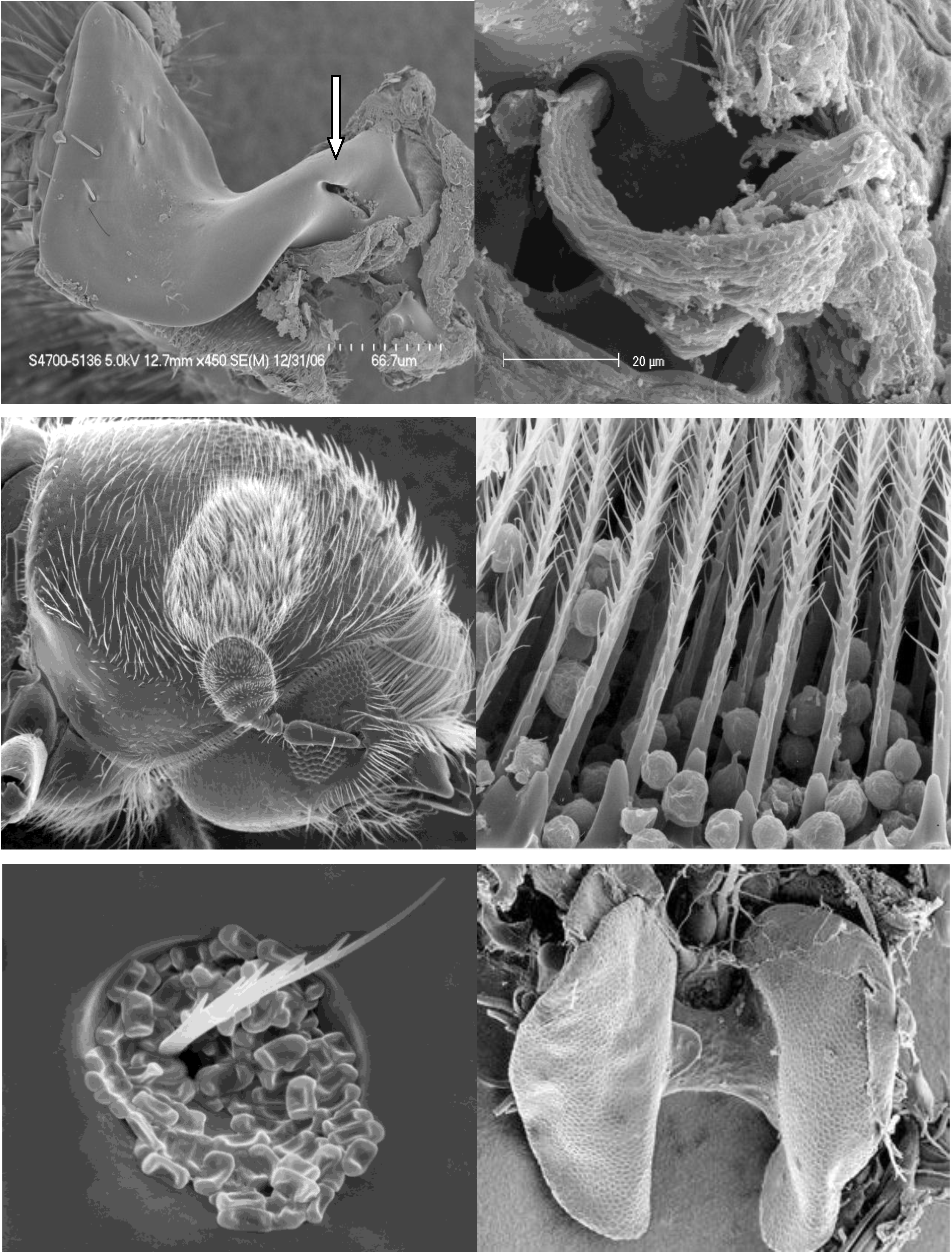

2. Evolution of Scolytinae-Fungus Symbioses
3. The Role of Biotic and Abiotic Factors in Shaping Scolytinae-Fungus Symbioses
3.1. The Host Plant
3.2. Microbes
3.3. Arthropods
3.4. Temperature
4. Stability and Redundancy in Multipartite Systems
5. Conclusions and Future Directions
Acknowledgements
References
- Sapp, J. Evolution by Association: A History of Symbiosis; Oxford University Press: New York, NY, USA, 1994. [Google Scholar]
- Margulis, L.; Fester, R. Symbiosis as a Source of Evolutionary Innovation; MIT Press: Cambrage, MA, USA, 1991. [Google Scholar]
- Douglas, A.E. Symbiotic Interactions; Oxford Science Publications: Oxford, UK, 1994. [Google Scholar]
- Maynard Smith, J.; Szathmary, E. The Major Transitions in Evolution; Oxford University Press: Cary, NC, USA, 1995. [Google Scholar]
- Bronstein, J. Mutualisms. In Evolutionary Ecology, Perspectives and Synthesis; FOX, C., Fairbairn, D., Roff, D., Eds.; Oxford University Press: Oxford, UK, 2001; pp. 315–330. [Google Scholar]
- Paine, T.D.; Raffa, K.F.; Harrington, T.C. Interactions among scolytid bark beetles, their associated fungi, and live host conifers. Annu. Rev. Entomol. 1997, 42, 179–206. [Google Scholar] [CrossRef]
- Six, D.L. Bark Beetle-Fungus Symbioses. In Insect Symbiosis; Bourtzis, K., Miller, T., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 97–114. [Google Scholar]
- Six, D.L.; Klepzig, K.D. Dendroctonus bark beetles as model systems for the study of symbiosis. Symbiosis 2004, 37, 207–232. [Google Scholar]
- Kirisits, T. Fungal Associates of European Bark Beetles with Special Emphasis on the Ophiostomatoid Fungi. In Bark and Wood Boring Insects in Living Trees in Europe, a Synthesis; Lieutier, F., Day, K.R., Battisti, A., Gregoire, J.-C., Evans, H.F., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 181–236. [Google Scholar]
- Harrington, T.C. Ecology and Evolution of Mycophagous Bark Beetles and Their Fungal Partners. In Insect-Fungal Associations: Ecology and Evolution; Vega, F.E., Blackwell, M., Eds.; Oxford University Press: Oxford, UK, 2005; pp. 257–291. [Google Scholar]
- Mueller, U.; Gerardo, N.; Aanen, D.; Six, D.; Schultz, T. The evolution of agriculture in insects. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 563–595. [Google Scholar]
- Six, D.L.; Wingfield, M.J. The role of phytopathogenicity in bark beetle-fungus symbioses: A challenge to the classic paradigm. Annu. Rev. Entomol. 2011, 56, 255–272. [Google Scholar]
- Wood, S.L. The bark and ambrosia beetles of North and Central America (Coleoptera: Scolytidae), a taxonomic monograph. Great Basin Nat. Mem. 1982, 6, 1–1359. [Google Scholar]
- Bright, D.E. Systematics of Bark Beetles. In Beetle-Pathogen Interactions in Conifer Forests; Schowalter, T.D., Philip, G.M., Academic Press: New York, NY, USA, 1993; pp. 23–33. [Google Scholar]
- Marvaldi, A.E.; Sequiera, A.S.; O’brien, C.W.; Farrell, B.W. Molecular and morphological phylogenetics of weevils (Coleoptera, Curculionoidea): Do niche shifts accompany diversification? Syst. Biol. 2002, 51, 761–785. [Google Scholar] [CrossRef]
- Beaver, R.A. Insect-Fungus Relationships in the Bark and Ambrosia Beetles. In Insect-Fungus Interactions; Wilding, N., Collins, N.M., Hammond, P.M., Webber, J.F., Eds.; Academic Press: London, UK, 1989; pp. 121–143. [Google Scholar]
- Morales-Ramos, J.A.; Rojas, M.G.; Sittertz-Bhatkar, H.; Saldana, G. Symbiotic relationship between Hypothenemus hampei (Coleoptera: Scolytidae) and Fusarium solani (Moniliales: Tuberculariaceae). Ann. Entomol. Soc. Am. 2000, 93, 541–547. [Google Scholar] [CrossRef]
- Zipfel, R.D.; Debeer, Z.W.; Jacobs, K.; Wingfield, B.D.; Wingfield, M.J. Multigene phylogenies define Ceratocystiopsis and Grosmannia distinct from Ophiostoma. Stud. Mycol. 2006, 55, 75–97. [Google Scholar] [CrossRef]
- Hausner, G.; Reid, J.; Klassen, G.R. Ceratocystiopsis—A reappraisal based on molecular criteria. Mycol. Res. 1993, 97, 625–633. [Google Scholar] [CrossRef]
- Spatafora, J.W.; Blackwell, M. The polyphyletic origins of ophiostomatoid fungi. Mycol. Res. 1994, 98, 1–9. [Google Scholar]
- Harrington, T.C. Diseases of Conifers Caused by Species of Ophiostoma and Leptographium. In Ceratocystis and Ophiostoma: Taxonomy, Ecology and Pathogenicity; Wingfield, M.J., Seifert, K.A., Webber, J.F., Eds.; American Phytopathological Society: St. Paul, MN, USA, 1993; pp. 161–172. [Google Scholar]
- Paulin-Mahady, A.E.; Harrington, T.C.; Mcnew, D. Phylogenetic and taxonomic evaluation of Chalara, Chalaropsis, and Thielaviopsis anamorphs associated with Ceratocystis. Mycologia 2002, 94, 62–72. [Google Scholar] [CrossRef]
- Whitney, H.S., Bandoni; Oberwinkler, F. Entomocorticum dendroctoni gen. et sp. nov. (Basidomycotina), a possible nutritional symbiote of the mountain pine beetle in British Columbia. Can. J. Bot. 1987, 65,, 95–102. [Google Scholar] [CrossRef]
- Hsiau, P.-T.W.; Harrington, T.C. Phylogenetics and adaptations of basidiomycetous fungi fed upon by bark beetles (Coleoptera: Scolytidae). Symbiosis 2003, 34, 111–131. [Google Scholar]
- Kok, L.T. Lipids of Ambrosia Fungi and the Life of Mutualistic Beetles. In Insect-Fungus Symbiosis: Nutrition, Mutualism, and Commensalism; Batra, L., Ed.; John Wiley and Sons: Hoboken, NJ, USA, 1979; pp. 33–52. [Google Scholar]
- Norris, D.M. The Mutualistic Fungi of the Xyleborini Beetles. In Insect-Fungus Symbiosis: Nutrition, Mutualism, and Commensalism; Batra, L., Ed.; John Wiley and Sons: Hoboken, NJ, USA, 1979; pp. 53–64. [Google Scholar]
- Cassar, S.; Blackwell, M. Convergent origins of ambrosia fungi. Mycologia 1996, 88, 596–601. [Google Scholar]
- Gebhardt, H.; Bergerow, D.; Oberwinkler, F. Identification of the ambrosia fungus of Xyleborus monographus and X. dryographus (Coleoptera: Curculionidae, Scolytinae). Mycol. Prog. 2004, 3, 95–102. [Google Scholar] [CrossRef]
- Jones, K.G.; Blackwell, M. Phylogenetic analysis of ambrosial species in the genus Raffaelea based on 18S rDNA sequences. Mycol. Res. 1998, 102, 661–665. [Google Scholar] [CrossRef]
- Rollins, F.; Jones, K.G.; Krokene, P.; Solheim, H.; Blackwell, M. Phylogeny of asexual fungi associated with bark and ambrosia beetles. Mycologia 2001, 93, 991–996. [Google Scholar]
- Gebhardt, H.; Weiss, M.; Oberwinkler, F. Dryadomyces amasae: A nutritional fungus associated with ambrosia beetles of the genus Amasa (Coleoptera: Curculionidae, Scolytinae). Mycol. Res. 2005, 109, 687–696. [Google Scholar] [CrossRef]
- Harrington, T.C.; Aghayeva, D.N.; Fraedrich, S.W. New combinations in Raffaelea, Ambrosiella, and Hyalorhinocladiella, and four new species from the red bay ambrosia beetle, Xyleborus glabratus. Mycotaxon 2010, 111, 337–361. [Google Scholar] [CrossRef]
- Kok, L.T.; Norris, D.M.; Chu, H.M. Sterol metabolism as a basis for a mutualistic symbiosis. Nature 1970, 225, 661–662. [Google Scholar]
- Biedermann, P.H.W.; Taborsky, M. Larval helpers and age polyethism in ambrosia beetles. Proc. Natl. Acad. Sci.USA 2011, 108, 17064–17069. [Google Scholar]
- Francke-Grosmann, H. Ectosymbiosis in Wood-Inhabiting Insects. In Symbiosis; Henrym, S.M., Ed.; Academic Press: New York, NY, USA, 1967; volume II, pp. 17064–17069. [Google Scholar]
- Scriber, J.M.; Slansky, F., Jr. The nutritional ecology of immature insects. Annu. Rev.Entomol. 1981, 26, 183–211. [Google Scholar]
- Slansky, F.; Scriber, J.M. Food Consumption and Utilization. In Comprehensive Insect Physiology, Biochemistry, and Pharmacology; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon Pr: London, UK, 1985; pp. 87–163. [Google Scholar]
- Ayres, M.P.; Wilkens, R.T.; Ruel, J.J. Nitrogen budgets of phloem-feeding bark beetles with and without symbiotic fungi. Ecology 2000, 81, 2198–2210. [Google Scholar]
- Mattson, W.J. Herbivory in relation to plant nitrogen content. Annu. Rev. Ecol.Syst. 1980, 11, 119–161. [Google Scholar]
- Hodges, J.E.; Lorio, P.L., Jr. Carbohydrate and nitrogen fractions of the inner bark of loblolly pine s under moisture stress. Can. J. Bot. 1969, 47, 1651–1657. [Google Scholar]
- Fajer, E.D. The effects of enriched CO2 atmospheres on plant insect herbivore interactions: Growth responses of larvae of the specialist butterfly, Junonia coenia (Lepidoptera: Nymphalidae). Oecologia 1989, 81, 514–520. [Google Scholar] [CrossRef]
- Slansky, F.; Feeny, P. Stabilization of the rate of nitrogen accumulation by larvae of the cabbage butterfly on wild and cultivated plants. Ecol. Monogr. 1997, 47, 209–228. [Google Scholar]
- Hodges, J.E.; Barras, S.J.; Maudlin, J. Amino acids in the inner bark of loblolly pine as affected by the southern pine beetle and associated organisms. Can. J. Bot. 1968, 46, 1467–1472. [Google Scholar]
- Safranyik, L. Size- and sex-related emergence, and survival in cold storage, of mountain pine beetle adults. Can. Entomol. 1976, 108, 209–212. [Google Scholar] [CrossRef]
- Botterweg, P.F. Dispersal and flight behavior of the spruce beetle Ips typographus in relation to sex, size and fat content. Z. Angew. Entomol. 1982, 94, 466–489. [Google Scholar] [CrossRef]
- Botterweg, P.F. The effect of attack density on size, fat content and emergence of the spruce beetle, Ips typographus. Z. Angew. Entomol. 1983, 96, 7–55. [Google Scholar]
- Anderbrandt, O. Survival of parent and brood bark beetles, Ips typographus, in relation to size, lipid content and reemergence or emergence day. Physiol. Entomol. 1988, 13, 121–129. [Google Scholar] [CrossRef]
- Atkins, M.D. Lipid loss with flight in the Douglas-fir beetle. Can. Entomol. 1969, 101, 164–165. [Google Scholar]
- Thompson, S.N.; Bennett, R.B. Oxidation of fat during flight of male Douglas-fir beetles, Dendroctonus pseudotsugae. J. Insect Physiol. 1971, 17, 1555–1563. [Google Scholar] [CrossRef]
- Reid, R.W. Biology of the mountain pine beetle, Dendroctonus monticolae Hopkins, in the east Kootenay region of British Columbia. II. Behavior in the host, fecundity, and the internal changes in the female. Can. Entomol. 1962, 94, 605–613. [Google Scholar] [CrossRef]
- Amman, G.D. Some factors affecting oviposition behavior of the mountain pine beetle. Environ. Entomol. 1972, 1, 691–695. [Google Scholar]
- Clarke, A.L.; Webb, J.W.; Franklin, F.T. Fecundity of the southern pine beetle in laboratory pine bolt. Ann. Entomol. Soc. Am. 1979, 72, 229–231. [Google Scholar]
- Coppedge, B.R.; Stephen, F.M.; Felton, G.W. Variation in female southern pine beetle size and lipid content in relation to fungal associates. Can. Entomol. 1995, 127, 145–154. [Google Scholar]
- Bridges, R. Mycangial fungi of Dendroctonus frontalis (Coleoptera: Scolytidae) and their relationship to beetle population trends. Environ. Entomol. 1983, 12, 858–861. [Google Scholar]
- Goldhammer, D.S.; Stephen, F.M.; Paine, T.D. The effect of the fungi Ceratocystis minor (Hedgecock) Hunt var. barrasii Taylor, and SJB 122 on the reproduction of the southern pine beetle, Dendroctonus frontalis Zimmermann (Coleoptera: Scolytidae). Can. Entomol. 1991, 122, 407–418. [Google Scholar]
- Rumbold, C.T. Two blue-staining fungi associated with bark beetle infestation of pines. J. Agric. Res. 1931, 43, 847–873. [Google Scholar]
- Stone, C.; Simpson, J.A. Species associations in Ips grandicollis galleries in Pinustaeda. N. Z. J. For. Sci. 1990, 20, 75–96. [Google Scholar]
- Clayton, R.B. The utilization of sterols by insects. J. Lipid Res. 1964, 5, 3–19. [Google Scholar]
- Richmond, J.A.; Thomas, H.A. Hylobius pales: Effect of dietary sterols on development and on sterol content of somatic tissue. Ann. Entomol. Soc. Am. 1975, 68, 329–332. [Google Scholar]
- Svoboda, J.A.; Thompson, M.J.; Robbins, W.R.; Kaplanis, J.N. Insect sterol metabolism. Lipids 1978, 13, 742–753. [Google Scholar]
- Kramer, P.J.; Kozlowski, T.T. Physiology of Trees; McGraw Hill: New York, NY, USA, 1960. [Google Scholar]
- Maurer, P.; Debieu, D.; Malosse, C.; Leroux, P.; Riba, G. Sterols and symbiosis in the leaf-cutter and Acromyrmex octospinosus (Reich) (Hymenoptera, Formicidea: Attini). Arch. Insect Biochem. Physiol. 1992, 20, 13–21. [Google Scholar] [CrossRef]
- Nasir, H.; Noda, H. Yeast-like symbiotes as a sterol source in anobiid beetles (Coleoptera: Anobiidae): Possible metabolic pathways from fungal sterols of 7-dehydrocholesterol. Arch. Insect Biochem. Physiol. 2003, 52, 175–182. [Google Scholar]
- Norris, D.M.; Baker, J.K.; Chu, H.M. Symbiotic interrelationships between microbes and ambrosia beetles III. Ergosterol as the source of sterol to the insect. Ann. Entomol. Soc. Am. 1969, 62, 413–414. [Google Scholar]
- Norris, D.M. Dependence of Fertility and Progeny Development of Xyleborus ferrugineus upon Chemicals from Its Symbiotes. In Insect and Mite Nutrition; Rodriguez, J.C., Ed.; North-Holland Pub. Co.: North-Holland, The Netherlands, 1972; pp. 299–310. [Google Scholar]
- Perez, J.; Infante, F.; Vega, F.E. Does the coffee berry borer (Coleoptera: Scolytidae) have mutualistic fungi? Ann. Entomol. Soc. Am. 2005, 98, 483–490. [Google Scholar] [CrossRef]
- Bentz, B.J.; Six, D.L. Ergosterol content of four fungal symbionts associated with Dendroctonus ponderosae and D. rufipennis (Coleoptera: Curculionidae, Scolytinae). Ann. Entomol. Soc. Am. 2006, 99, 189–194. [Google Scholar] [CrossRef]
- Kok, L.T.; Norris, D.M. Comparative sterol compositions of adult female Xyleborus ferrugineus and its mutualistic fungal ectosysmbionts. Compr. Biochem. Physiol. 1973, 44, 499–505. [Google Scholar]
- Whitney, H.S. Association of Dendroctonus ponderosae (Coleoptera: Scolytidae) with blue-stain fungi and yeasts during brood development in lodgepole pine. Can. Entomol. 1971, 103, 1495–1503. [Google Scholar] [CrossRef]
- Adams, A.S.; Six, D.L. Temporal variation in mycophagy and prevalence of fungi associated with developmental stages of the mountain pine beetle, Dendroctonus ponderosae (Coleoptera: Scolytinae, Curculionidae). Environ. Entomol. 2006, 36, 64–72. [Google Scholar] [CrossRef]
- Fox, J.W.; Wood, D.L.; Akers, P.P.; Parmeter, J.R., Jr. Survival and development of Ips paraconfusus Lanier (Coleoptera: Scolytidae) reared axenically and with tree pathogeneic fungi vectored by cohabiting Dendroctonus species. Can. Entomol. 1992, 124, 1157–1167. [Google Scholar] [CrossRef]
- Nevill, R.J.; Safranyik, L. Interaction between the bluestain fungal associates of mountain pine and pine engraver beetles, (Dendroctonus ponderosae and Ips pini, Coleoptera: Scolytidae) and their effects on the beetles. J. Entomol. Soc. Br. Columbia 1996, 93, 39–48. [Google Scholar]
- Bleiker, K.; Six, D.L. Dietary benefits of fungal associates to an eruptive herbivore: Potential implications of multiple associates on host population dynamics. Environ. Entomol. 2007, 36, 1384–1396. [Google Scholar]
- Barras, S.J. Antagonism between Dendroctonus frontalis and the fungus Ceratocystis minor. Ann. Entomol. Soc. Am. 1970, 63, 1187–1190. [Google Scholar]
- Chapman, R.F. The Insects: Structure and Function; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Six, D.L.; Paine, T.D. Effects of mycangial fungi and host tree species on progeny survival and emergence of Dendroctonus ponderosae (Coleoptera: Scolytidae). Environ. Entomol. 1998, 27, 1393–1401. [Google Scholar]
- Mousseau, T.A.; Dingle, H. Maternal effects in insect life histories. Annu. Rev.Entomol. 1991, 36, 511–534. [Google Scholar]
- Strongman, D.B. A method for rearing Dendroctonus ponderosae Hopk. (Coleoptera: Scolytidae) from eggs to pupae on host tissue with or without a fungal complement. Can. Entomol. 1987, 119, 207–208. [Google Scholar] [CrossRef]
- Yearian, W.C.; Gouger, R.J.; Wilkinson, R.C. Effects of the bluestain fungus, Ceratocystis ips, on the development of Ips bark beetles in pine bolts. Ann. Entomol. Soc. Am. 1972, 65, 481–487. [Google Scholar]
- Whitney, H.S.; Spanier, O.J. An improved method for rearing axenic mountain pine beetles, Dendroctonus ponderosae (Coleoptera: Scolytidae). Can. Entomol. 1982, 114, 1095–1101. [Google Scholar] [CrossRef]
- Conn, J.E.; Borden, J.H.; Hunt, D.W.A.; holman, J.; Whitney, H.S.; Spanier, O.J.; Pierce, H.D., Jr.; Oehlschlager, A.C. Pheromone production by axenically reared Dendroctonus ponderosae and Ips paraconfusus (Coleoptera: Scolytidae). J. Chem. Ecol. 1984, 10, 281–289. [Google Scholar] [CrossRef]
- Fleming, T.H.; Sahley, C.T.; Holland, J.N.; Nason, J.D.; Hamrick, J.L. Sonoran desert columnar cacti and the evolution of generalized pollination systems. Ecol. Monogr. 2001, 71, 511–530. [Google Scholar]
- Lombardero, M.J.; Ayres, M.P.; Hofstetter, R.W.; Moser, J.C.; Klepzig, K.D. Strong indirect interactions of Tarsonemus mites (Acarina: Tarsonemidae) and Dendroctonus frontalis (Coleoptera: Scolytidae). Oikos 2003, 102, 243–252. [Google Scholar] [CrossRef]
- Hofstetter, R.W.; Cronin, J.; Klepzig, K.D.; Moser, J.C.; Ayres, M.P. Antagonisms, mutualisms and commensalisms affect outbreak dynamics of the southern pine beetle. Oecologia 2006, 147, 679–691. [Google Scholar]
- Hemingway, R.W.; Mcgraw, G.W.; Barras, S.J. Polyphenols in Ceratocystisminor-infected Pinus taeda: Fungal metabolites, phloem and xylem phenols. Agric. Food Chem. 1977, 25, 717–722. [Google Scholar] [CrossRef]
- Kirejtshuk, A.G.; Azar, D.; Beaver, R.A..; Mandelshtam, M.Y.; Nel, A. The most ancient bark beetle known; A new tribe, genus and species from Lebanese amber (Coleoptera, Curculionidae, Scolytinae). Syst. Entomol. 2009, 34, 101–112. [Google Scholar] [CrossRef]
- Cognato, A.I.; Grimaldi, D. 100 Million years of morphological conservation in bark beetles (Coleoptera: Curculionidae: Scolytinae). Syst. Entomol. 2009, 34, 93–100. [Google Scholar]
- Jordal, B.H.; Sequeira, A.; Cognato, A. The age and phylogeny of wood boring weevils and the origin of subsociality. Mol. Phylogenetics Evol. 2011, 59, 708–724. [Google Scholar]
- Sequiera, A.S.; Normark, B.B.; Farrell, B.D. Evolutionary assemblage of the conifer fauna: Distinguishing ancient from recent associations in bark beetles. Proc. R. Soc. Lond. B 2000, 267, 2359–2366. [Google Scholar]
- Farrell, B.D.; Sequiera, A.S.; O’meara, B.C.; Normark, B.B.; Chung, J.H.; Jordahl, B.H. The evolution of agriculture in beetles (Curculionidae: Scolytinae and Platypodinae). Evolution 2001, 55, 2011–2027. [Google Scholar]
- Berbee, M.L.; Taylor, J.W. Fungal Molecular Evolution: Gene Trees and Geologic Time. In The Mycota. Vol. VIIB; Mclaughlin, D.J., Mclaughlin, E., Lemke, P.A., Eds.; Springer Verlag: Heidelberg, Germany, 2001; pp. 229–246. [Google Scholar]
- Hulcr, J.; Kolarick, M.; Kirkendall, L. A new record of a fungus-beetle symbiosis in Scolytodes bark beetles (Scolytine, Curculionidae, Coleoptera). Symbiosis 2007, 43, 151–159. [Google Scholar]
- Fagan, W.F.; Siemann, E.; Mitter, C.; Denno, R.F.; Huberty, A.F.; Woods, H.A.; Elser, J.J. Nitrogen in insects: Implications for trophic complexity and species diversity. Am. Nat. 2002, 160, 784–802. [Google Scholar]
- Farrell, B.D. “Inordinate fondness” explained: Why are there so many beetles? Science 1998, 281, 553–557. [Google Scholar]
- Jordal, B.H.; Normark, B.B.; Farrell, B.D. Evolutionary radiation of an inbreeding haplodiploid beetle lineage. Biol. J. Linnaean Soc. 2000, 71, 483–499. [Google Scholar]
- Atkinson, T.H.; Equihua-Martinez, A. Biology of bark and ambrosia beetles (Coleoptera: Scolytidae and Platypodidae) of a tropical rainforest in southeastern Mexico with an annotated checklist of species. Ann. Entomol. Soc. Am. 1986, 79, 414–423. [Google Scholar]
- Lackner, A.L.; Alexander, S.A. Occurrence and pathogeneicity of Verticicladiella procera in Christmas tree plantations in Virginia. Plant Dis. 1982, 66, 211–212. [Google Scholar] [CrossRef]
- Alexander, S.A.; Horner, W.E.; Lewis, K.J. Leptographium procerum as a Pathogen of Pines. In Leptographium Root Diseases in Conifers; Harrington, T.C., Cobb, F.W., Jr., Eds.; American Phytopathological Society: St. Paul, MN, USA, 1988; pp. 97–112. [Google Scholar]
- Hoover, K.; Wood, D.L.; Storer, A.J.; Fox, J.W.; Bros, W.E. Transmission of the pitch canker fungus, Fusarium subglutinans f.sp. pini, to Monterey pine, Pinus radiata, by cone- and twig-infesting beetles. Can. Entomol. 1996, 128, 981–994. [Google Scholar]
- Bruno, J.F.; Satchowicz, J.J.; Bertness, M.D. Inclusion of facilitation into ecological theory. Trends Ecol. Evol. 2003, 18, 119–125. [Google Scholar]
- Six, D.L.; Paine, T.D. A phylogenetic comparison of ascomycete mycangial fungi and Dendroctonus bark beetles (Coleoptera: Scolytidae). Ann. Entomol. Soc. Am. 1999, 92, 159–166. [Google Scholar]
- Mueller, U.G.; Schultz, T.R.; Currie, C.R.; Adams, R.M.M.; Mallock, D. The origin of attine ant-fungus mutualism. Q. Rev. Biol. 2001, 76, 169–197. [Google Scholar]
- Malloch, D.; Blackwell, M. Dispersal Biology of the Ophiostomatoid Fungi. In Ceratocystis and Ophiostoma: Taxonomy, Ecology and Pathogenicity; Wingfield, M.J., Seifert, K.A., Webber, J.F., Eds.; American Phytopathological Society: St. Paul, MN, USA, 1993; pp. 195–206. [Google Scholar]
- Hulcr, J.; Cognato, A.I. Repeated evolution of crop theft in fungus-farming ambrosia beetles. Evolution 2010, 64, 3205–3212. [Google Scholar]
- Reed, D.L.; Hafner, M.S. Host specificity of chewing lice on pocket gophers: A potential mechanism for cospeciation. J. Mammol. 1997, 78, 655–660. [Google Scholar]
- Page, R.D.M.; Paterson, A.M.; Clayton, D.M. Lice and cospeciation: A response to Barker. Int. J. Parasitol. 1996, 26, 213–218. [Google Scholar]
- Kirkendall, L.R. The evolution of mating systems in bark and ambrosia beetles (Coleoptera: Scolytidae and Platypodidae). Zool. J. Linnaean Soc. 1983, 77, 293–352. [Google Scholar]
- Biedermann, P.H.W. Observations on sex ratio and behavior of males in Xyleborinus saxesnii Ratzeburg (Scolytinae, Coleoptera). Zookeys 2010, 56, 253–267. [Google Scholar]
- Keller, L.; Peer, K.; Bernasconi, C.; Taborsky, M.; Shuker, D.M. Inbreeding and selection on sex ratio in the bark beetle, Xylosandrus gemanus. BMC Evol. Biol. 2011, 11, 359. [Google Scholar]
- Six, D.L. A comparison of mycangial and phoretic fungi of individual mountain pine beetles. Can. J. For. Res. 2003, 33, 1331–1334. [Google Scholar]
- Lewinsohn, D.; Lewinsohn, E.; Bertagnolli, C.T.; Partridge, A.D. Blue-stain fungi and their transport structures on the Douglas-fir beetle. Can. J. For. Res. 1994, 24, 2275–2283. [Google Scholar]
- Storer, A.J.; Wood, D.L.; Gordon, T.R. Modification of coevolved insecplant interactions by an exotic plant pathogen. Ecol. Entomol. 1999, 24, 238–243. [Google Scholar]
- Jacobs, K.; Bergdahl, D.R.; Wingfield, M.J.; Halik, S.; Seifert, K.A.; Bright, D.E.; Wingfield, B.D. Leptographium wingfieldii introduced into North America and found associated with exotic Tomicus piniperda and native bark beetles. Mycol. Res. 2004, 108, 411–418. [Google Scholar]
- Six, D.L.; Bentz, B.J. Temperature determines the relative abundance of symbionts in a multipartite bark beetle-fungus symbiosis. Microb. Ecol. 2007, 54, 112–118. [Google Scholar]
- Anderson, R.S. Weevils and plants: Phylogenetic versus ecological mediation of evolution of host plant associations in Curculioninae (Coleoptera: Curculionidae). Mem. Entomol. Soc. Can. 1993, 165, 197–232. [Google Scholar]
- Debeer, Z.W.; Harrington, T.C.; Vissmer, H.F.; Wingfield, B.D. Phylogeny of the Ophiostoma stenocerus—Sporothrix schenckii complex. Mycologia 2003, 95, 434–441. [Google Scholar]
- Roets, F.; Debeer, Z.W.; Dreyer, L.L.; Zipfel, R.; Crous, P.W.; Wingfield, M.J. Multi-gene phylogeny for Ophiostoma reveals two new species from Protea infructescences. Stud. Mycol. 2006, 55, 199–212. [Google Scholar] [CrossRef] [Green Version]
- Lieutier, F. Host Resistance to Bark Beetles and Its Variations. In Bark and Wood Boring Insects in Living Trees in Europe, a Synthesis; Lieutier, F., Day, K.R., Battisti, A., Gregoire, J.-C., Evans, H.F., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 135–180. [Google Scholar]
- Coulson, R.N. Population dynamics of bark beetles. Annu. Rev. Entomol. 1979, 24, 417–447. [Google Scholar]
- Harrington, T.C.; Cobb, F.W., Jr. Pathogenicity of Leptographium and Verticicladiella species isolated from roots of North American conifers. Phytopathology 1983, 73, 596–599. [Google Scholar] [CrossRef]
- Klepzig, K.D.; Raffa, K.F.; Smalley, E.B. Association of an insect-fungal complex with red pine decline in Wisconsin. For. Sci. 1991, 37, 1119–1139. [Google Scholar]
- Eckhardt, L.G.; Goyer, R.A.; Klepzig, K.D.; Jones, J.P. Interactions of Hylastes species (Coleoptera: Scolytidae) with Leptographium species associated with Loblolly pine decline. J. Econ. Entomol. 2004, 97, 468–474. [Google Scholar] [CrossRef]
- Amman, G.D.; Cole, W.D. Mountain Pine Beetle Dynamics in Lodgepole Pine Forests Part II: Population Dynamics. USDA FS Intermountain Forest Range and Experiment Station General Technical Report INT-145. Ogden, UT, USA, 1983. [Google Scholar]
- Bleiker, K.; Six, D.L. Competition and coexistence in a multi-partner mutualism: Interactions between two fungal symbionts of the mountain pine beetle in beetle-attacked trees. Microb. Ecol. 2008, 57, 191–202. [Google Scholar]
- Solheim, H.; Krokene, P. Growth and virulence of mountain pine beetle associated blue-stain fungi, Ophiostoma clavigerum and Ophiostoma montium. Can. J. Bot. 1998, 76, 561–566. [Google Scholar]
- Klepzig, K.D.; Flores-Otero, J.; Hofstetter, R.W.; Aryes, M.P. Effects of available water on growth and competition of southern pine beetle associated fungi. Mycol. Res. 2004, 108, 183–188. [Google Scholar]
- Bridges, R.; Marler, J.E.; Mcsparrin, B.H. A quantitative study of the yeasts and bacteria associated with laboratory-reared Dendroctonus frontalis Zimm. (Coleoptera: Scolytidae). Z. Angew. Entomol. 1984, 97, 261–267. [Google Scholar]
- Bridges, R. Nitrogen-fixing bacteria associated with bark beetles. Microb. Ecol. 1981, 7, 131–137. [Google Scholar]
- Hunt, D.W.A.; Borden, J.H. Conversion of verbenols to verbenone by yeasts isolated from Dendroctonus ponderosae (Coleoptera: Scolytidae). J. Chem. Ecol. 1990, 16, 1385–1397. [Google Scholar] [CrossRef]
- French, J.R.J.; Robinson, P.J.; Minko, G.; Pahl, P.J. Response of the European elm bark beetle, Scolytus multistriatus, to host bacterial isolates. J. Chem. Ecol. 1984, 10, 1133–1149. [Google Scholar] [CrossRef]
- Cardoza, Y.J.; Klepzig, K.D.; Raffa, K.F. Bacteria in oral secretions of an endophytic insect inhibit antagonistic fungi. Ecol. Entomol. 2006, 31, 636–645. [Google Scholar]
- Davis, S.T.; Hofstetter, R.W. Interactions between the yeast Orgataea pini and filamentous fungi associated with the western pine beetle. Microb. Ecol. 2011, 61, 626–634. [Google Scholar]
- Nair, J.R.; Singh, G.; Sekar, V. Isolation and characterization of a novel bacillus strain from coffee phyllosphere showing anti-fungal activity. J. Appl. Microbiol. 2002, 93, 772–780. [Google Scholar]
- Adams, A.S.; Six, D.L.; Adams, S; Holben, W. In vitro interactions among yeasts, bacteria and the fungal symbionts of the mountain pine beetle, Dendroctonus ponderosae. Microb. Ecol. 2008, 56, 460–466. [Google Scholar] [CrossRef]
- Adams, A.S.; Currie, C.R..; Cardoza, Y.; Klepzig, K.; Raffa, K.F. Effects of symbiotic bacteria and tree chemistry on the growth and reproduction of bark beetle fungal symbionts. Can. J. For. Res. 2009, 39, 1133–1147. [Google Scholar] [CrossRef]
- Scott, J.J.; Dong-Chan, O.; Yuceer, M.C.; Klepzig, K.D.; Clardy, J.; Currie, C.R. Bacterial protection of beetle-fungus mutualism. Science 2008, 322. [Google Scholar] [CrossRef]
- Vasanthkumar, A.; Delalibera, I.; Handelsman, J.; Klepzig, K.; Schloss, P.; Raffa, K. Characterization of gut-associated bacteria in larvae and adults of the southern pine beetle, Dendroctonus frontalis Zimmermann. Environ. Entomol. 2006, 35, 1710–1717. [Google Scholar] [CrossRef]
- Adams, A.S.; Adams, S.M.; Currie, C.R.; Gillette, N.E.; Raffa, K.F. Geographic variation in bacteria commonly associated with the red turpentine beetle (Coleoptera: Curculionidae). Environ. Entomol. 2010, 39, 406–414. [Google Scholar]
- Lombardero, M.J.; Klepzig, K.D.; Moser, J.C.; Ayres, M.P. Biology, demography and community interactions of Tarsonmeus (Acarina: Tarsonemidae) mites phoretic on Dendroctonus frontalis (Coleoptera: Scolytidae). Agric. For. Entomol. 2000, 2, 193–202. [Google Scholar] [CrossRef]
- Hoffstetter, R.W.; Klepzig, K.D.; Moser, J.C.; Ayres, M.P. Seasonal dynamics of mites and fungi and their effects on the southern pine beetle. Environ. Entomol. 2006, 147, 679–691. [Google Scholar]
- Boone, C.K.; Six, D.L.; Zheng, Y.; Raffa, K.F. Parasitoids and dipteran predators exploit volatiles from microbial symbionts to locate bark beetles. Environ. Entomol. 2008, 37, 150–161. [Google Scholar]
- Adams, A.S.; Six, D.L. Detection of host habitat by parasitoids using cues associated with mycangial fungi of the mountain pine beetle, Dendroctonus ponderosae. Can. Entomol. 2008, 140, 124–127. [Google Scholar] [CrossRef]
- Sullivan, B.T.; Berisford, C.W. Semiochemicals from fungal associates if bark beetles may mediate host location behavior of parasitoids. J. Chem. Ecol. 2004, 30, 703–717. [Google Scholar]
- Bleiker, K.; Six, D.L. Effects of water potential and solute on the growth and interactions of the two fungal symbionts of the mountain pine beetle. Mycol. Res. 2008, 113, 3–15. [Google Scholar]
- Robinson, R.C. Blue stain fungi in lodgepole pine (Pinus contorta Dougl. var. latifolia Engelm.) infested by the mountain pine beetle (Dendroctonus monticolae Hopk.). Can. J. Bot. 1962, 40, 609–614. [Google Scholar] [CrossRef]
- Whitney, H.S.; Farris, S.H. Maxillary mycangium in the mountain pine beetle. Science 1970, 167, 54–55. [Google Scholar]
- Doebeli, M.; Knowlton, N. The evolution of interspecific mutualisms. Proc. Natl. Acad. Sci. USA 1988, 95, 8676–8680. [Google Scholar]
- Keddy, P.A. Working with Heterogeneity: An Operator’s Guide to Environmental Gradients. In Ecological Heterogeneity; Kolassa, J., Pickett, S.T.A., Eds.; Springer Verlag: New York, NY, USA, 1991; pp. 191–201. [Google Scholar]
- Wellnitz, T.; Poff, N.L. Functional redundancy in heterogeneous environments: Implications for conservation. Ecol. Lett. 2001, 4, 177–179. [Google Scholar]
- Power, M.E.; Tilman, D.; Estes, J.A.; Menge, B.A.; Bond, W.J.; Mills, L.S.; Daily, G.; Castilla, J.C.; Lubchenco, J.; Paine, R.T. Challenges in the quest for keystones. BioScience 1996, 46, 609–620. [Google Scholar]
- Dunson, W.A.; Travis, J. The role of abiotic factors in community organization. Am. Nat. 1991, 138, 1067–1091. [Google Scholar]
- Six, D.L.; Paine, T.D. Ophiostoma clavigerum is the mycangial fungus of the Jeffrey pine beetle, Dendroctonus jeffreyi. Mycologia 1997, 89, 858–866. [Google Scholar] [CrossRef]
- Roe, A.D.; James, P.M.A.; Rice, A.V.; Cooke, J.E.K.; Sperling, F.A.H. Spatial community structure of mountain pine beetle fungal symbionts across a latitudinal gradient. Microb. Ecol. 2011, 62, 347–360. [Google Scholar]
- Walker, B. Conserving biological diversity through ecosystem resilience. Conserv. Biol. 1995, 9, 747–752. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Six, D.L. Ecological and Evolutionary Determinants of Bark Beetle —Fungus Symbioses. Insects 2012, 3, 339-366. https://doi.org/10.3390/insects3010339
Six DL. Ecological and Evolutionary Determinants of Bark Beetle —Fungus Symbioses. Insects. 2012; 3(1):339-366. https://doi.org/10.3390/insects3010339
Chicago/Turabian StyleSix, Diana L. 2012. "Ecological and Evolutionary Determinants of Bark Beetle —Fungus Symbioses" Insects 3, no. 1: 339-366. https://doi.org/10.3390/insects3010339
APA StyleSix, D. L. (2012). Ecological and Evolutionary Determinants of Bark Beetle —Fungus Symbioses. Insects, 3(1), 339-366. https://doi.org/10.3390/insects3010339



