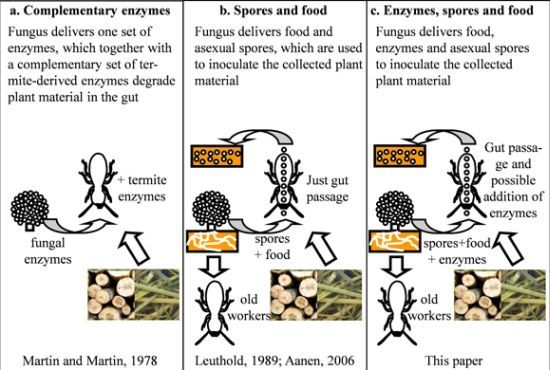
Fungiculture or Termite Husbandry? The Ruminant Hypothesis
Abstract
:1. Introduction
2. The Mutualism between Macrotermitine Termites and Termitomyces Fungi
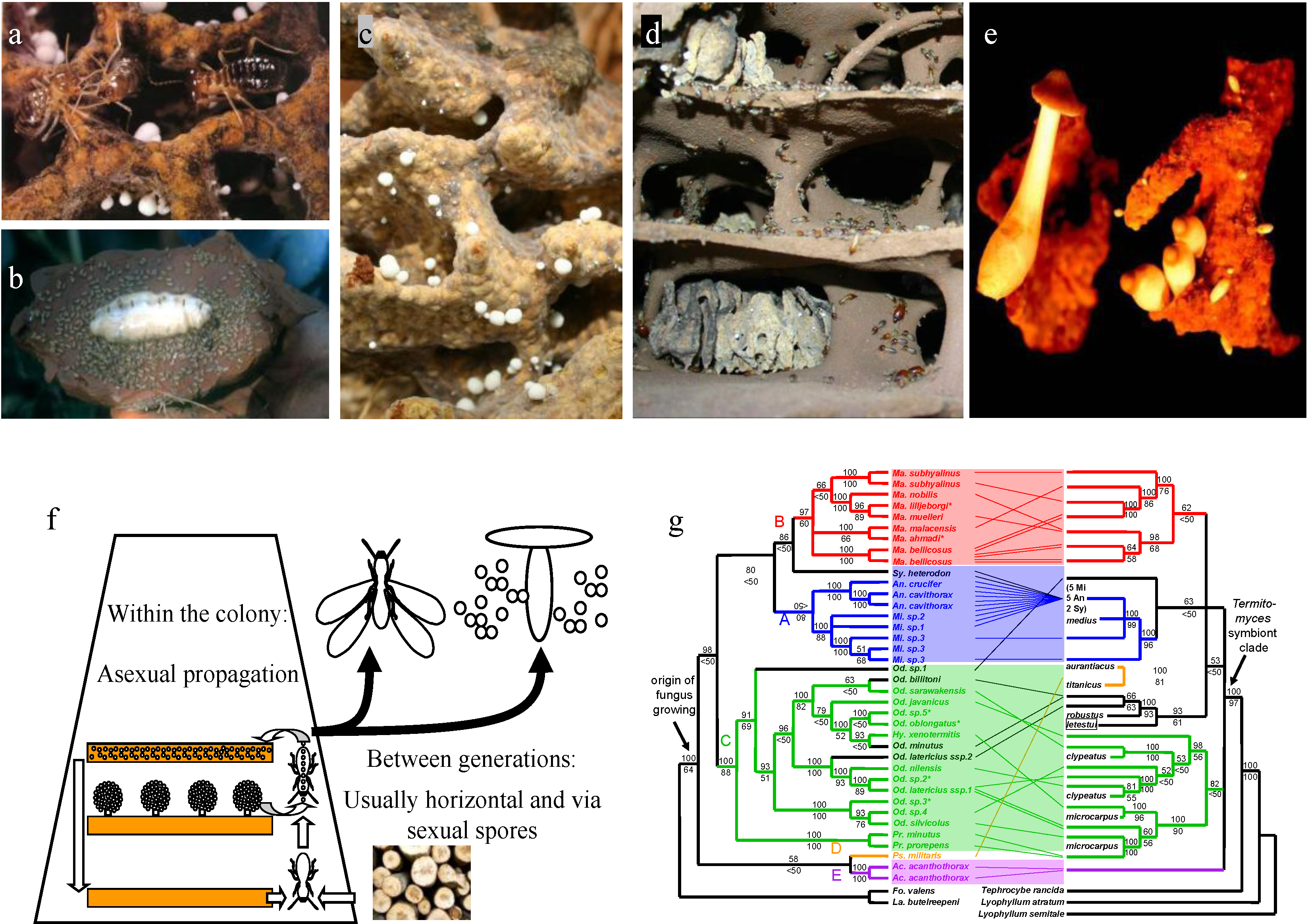
3. Large Differences in Host-Fungus Specificity
4. Hypotheses on the Role of the Symbiotic Fungus
- (1)
- Termitomyces has a role in lignin degradation, which enhances the digestibility of the cellulose (proposed by Grassé and Noirot [43]);
- (2)
- Termitomyces is an additional protein-rich food source (mainly the fungal nodules);
- (3)
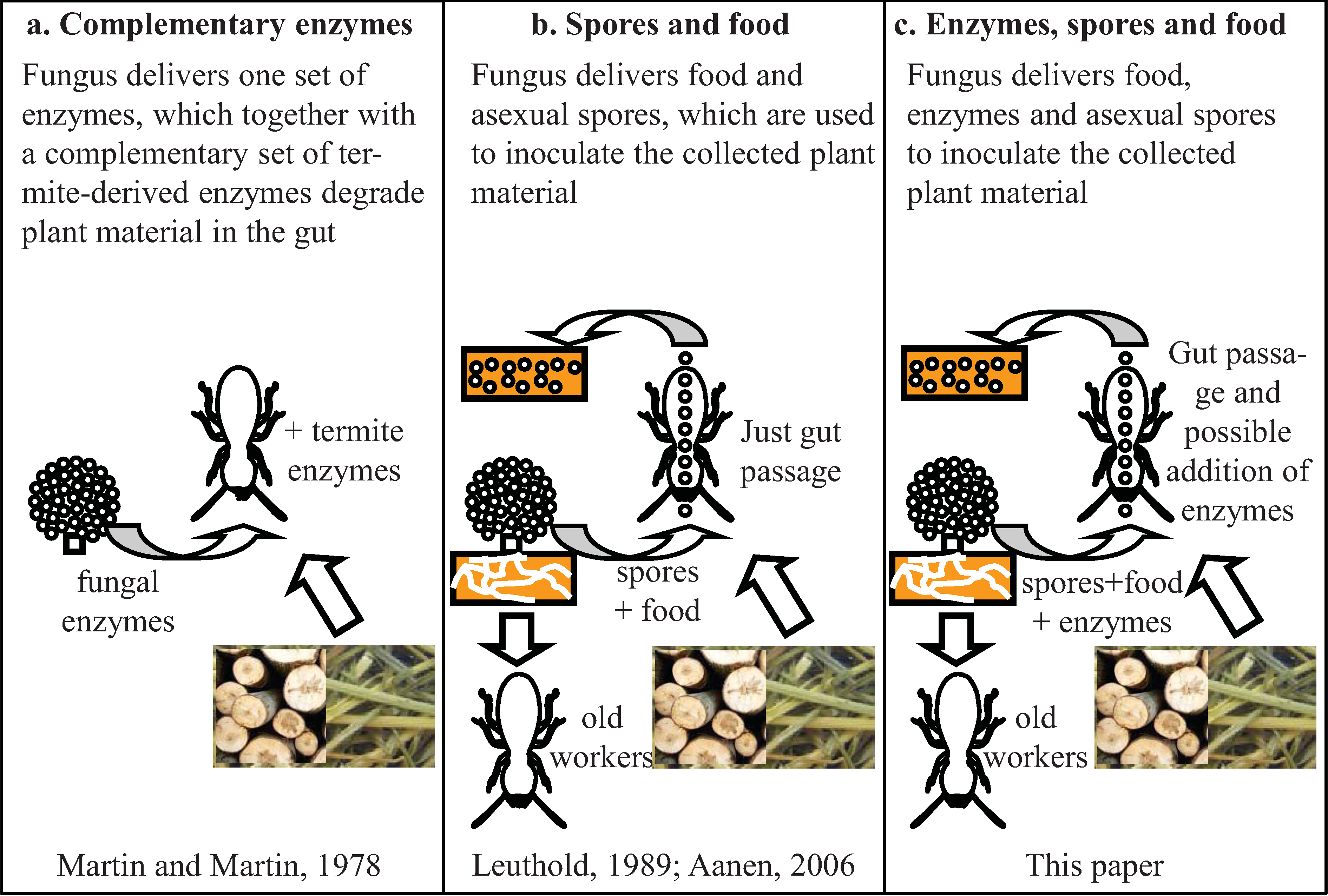
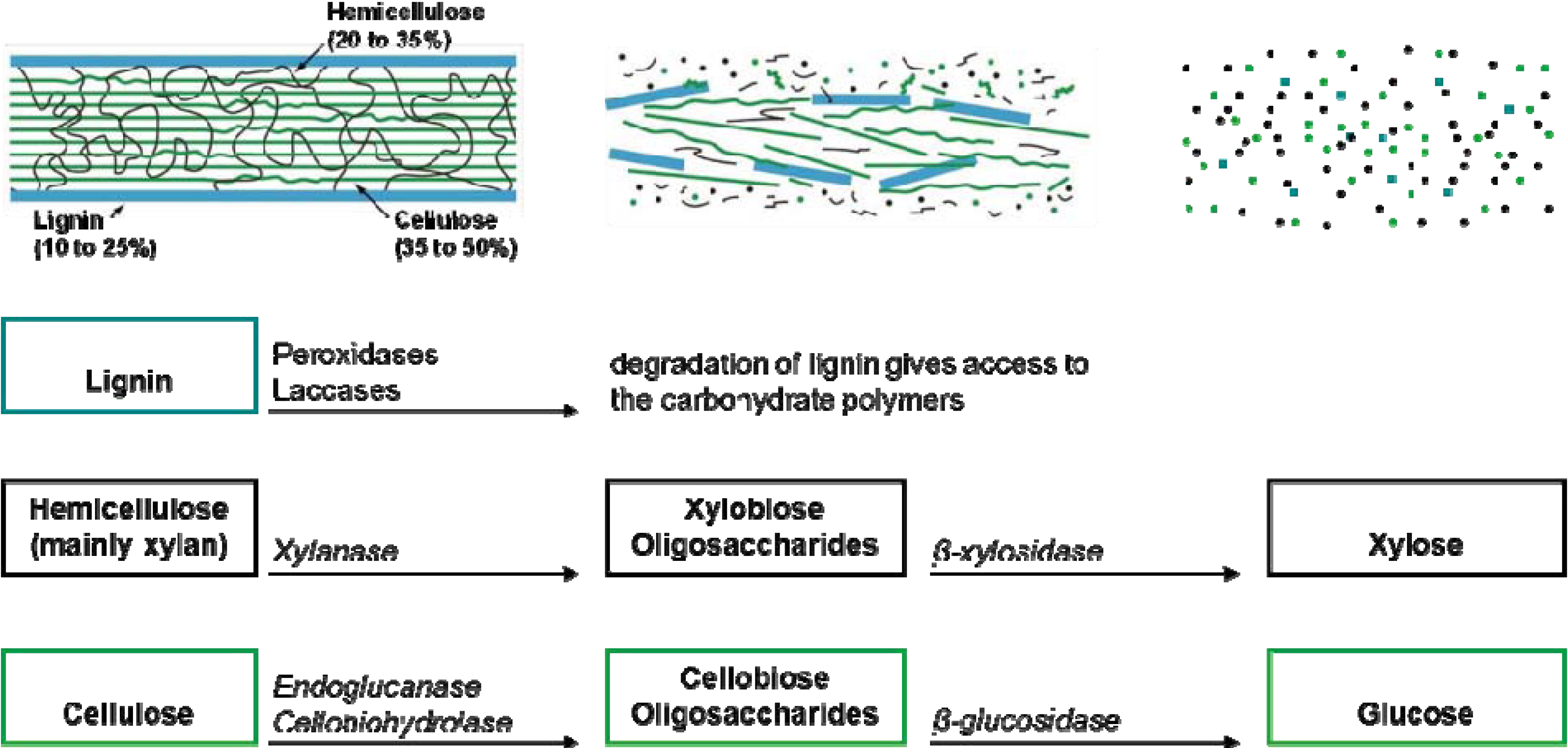
5. The Ruminant Hypothesis: Termites Helping the Fungus to Degrade Lignocellulose
6. Other Putative Nutritional Symbionts
7. Selection of Specific Fungal Strains When Starting a Colony
8. Conclusions
Acknowledgments
References
- Watson, R.A.; Pollack, J.B. How Symbiosis Can Guide Evolutio. In Proceedings of the 5th European Conference on Advances in Artificial Life (ECAL ’99), Lausanne, Switzerland, 15 September 1999; pp. 29–38.
- Margulis, L. Biodiversity: Molecular biological domains, symbiosis and kingdom origins. Biosystems 1992, 27, 39–51. [Google Scholar]
- Humphreys, C.P.; Franks, P.J.; Rees, M.; Bidartondo, M.I.; Leake, J.R.; Beerling, D.J. Mutualistic mycorrhiza-like symbiosis in the most ancient group of land plants. Nat. Commun. 2010, 1, 103. [Google Scholar]
- Groombridge, B. Global Biodiversity: Status of the Earth’s Living resources; Springer: Berlin, Heidelberg, Germany, 1992; Volume 1992. [Google Scholar]
- Erwin, T. The Biodiversity Question: How Many Species of Terrestrial Arthropods Are There? In Forest Canopies; Lowman, M.D., Rinker, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 259–269. [Google Scholar]
- Gullan, P.; Cranston, P. The Insects: An Outline of Entomology, 4th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2010. [Google Scholar]
- Baumann, P. Biology of Bacteriocyte-Associated Endosymbionts of Plant Sap-Sucking Insects. In Annual Review of Microbiology; Annual Reviews: Palo Alto, CA, USA, 2005; Volume 59, pp. 155–189. [Google Scholar]
- Feldhaar, H.; Gross, R. Insects as hosts for mutualistic bacteria. Int. J. Med. Microbiol. 2009, 299, 1–8. [Google Scholar]
- Douglas, A.E. Nutritional interactions in insect-microbial symbioses: Aphids and their symbiotic bacteria Buchnera. Annu. Rev. Entomol. 1998, 43, 17–37. [Google Scholar]
- Shigenobu, S.; Wilson, A. Genomic revelations of a mutualism: The pea aphid and its obligate bacterial symbiont. Cell. Mol. Life Sci. 2011, 68, 1297–1309. [Google Scholar]
- Gibson, C.M.; Hunter, M.S. Extraordinarily widespread and fantastically complex: Comparative biology of endosymbiotic bacterial and fungal mutualists of insects. Ecol. Lett. 2010, 13, 223–234. [Google Scholar]
- Scott, J.J.; Oh, D.-C.; Yuceer, M.C.; Klepzig, K.D.; Clardy, J.; Currie, C.R. Bacterial protection of beetle-fungus mutualism. Science 2008, 322. [Google Scholar] [CrossRef]
- Aanen, D.K.; Eggleton, P.; Rouland-Lefevre, C.; Guldberg-Froslev, T.; Rosendahl, S.; Boomsma, J.J. the evolution of fungus-growing termites and their mutualistic fungal symbionts. Proc. Natl. Acad. Sci. USA 2002, 99, 14887–14892. [Google Scholar]
- Mueller, U.G.; Schultz, T.R.; Cameron, R.C.; Adams, R.M.M.; Malloch, D. The origin of the attine ant-fungus mutualism. Q. Rev. Biol. 2001, 76, 169–197. [Google Scholar]
- Farrell, B.D.; Sequeira, A.S.; O’Meara, B.C.; Normark, B.B.; Chung, J.H.; Jordal, B.H. The evolution of agriculture in beetles (Curculionidae: Scolytinae and Platypodinae). Evolution 2009, 55, 2011–2027. [Google Scholar]
- Aanen, D.K.; Eggleton, P. Fungus-growing termites originated in African rain forest. Curr. Biol. 2005, 15, 851–855. [Google Scholar]
- Nobre, T.; Koné, N.A.; Konaté, S.; Linsenmair, K.E.; Aanen, D.K. Dating the fungus-growing termites’ mutualism shows a mixture between ancient codiversification and recent symbiont dispersal across divergent hosts. Mol. Ecol. 2011, 20, 2619–2627. [Google Scholar]
- Jones, D.T.; Eggleton, P.; Bignell, D.E.; Roisin, Y.; Lo, N. Global Biogeography of Termites: A Compilation of Sources. In Biology of Termites: A Modern Synthesis; Springer: Amsterdam, The Netherlands, 2011; pp. 477–498. [Google Scholar]
- Wood, T.G.; Sands, W.A. The Role of Termites in Ecosystems. In Production Ecology of Ants and Termites; Brian, M.V., Ed.; Cambridge Univ. Press: Cambridge, UK, 1978; pp. 245–292. [Google Scholar]
- Buxton, R.D. Changes in the composition and activities of termite communities in relation to changing rainfall. Oecologia 1981, 51, 371–378. [Google Scholar]
- Wilson, E. The Insect Societies; Belknap Press of Harvard University Press: Cambridge, MA, USA, 1971. [Google Scholar]
- Darlington, J. Nutrition and Evolution in Fungus-Growing Termites. In Nourishment and Evolution in Insect Societies; Hunt, J.H., Nalepa, C.A., Eds.; Westview Press: Boulder, CO, USA, 1994; pp. 105–130. [Google Scholar]
- Leuthold, R.H.; Badertscher, S.; Imboden, H. The inoculation of newly formed fungus comb with Termitomyces in Macrotermes colonies (Isoptera, Macrotermitinae). Insectes Sociaux 1989, 36, 328–338. [Google Scholar] [CrossRef]
- Aanen, D.K. As you reap, so shall you sow: Coupling of inoculating and harvesting stabilizes the mutualism between termites and fungi. Biol. Lett. 2006, 2, 209–212. [Google Scholar]
- Korb, J.; Aanen, D.K. The evolution of uniparental transmission of fungal symbionts in fungus-growing termites (Macrotermitinae). Behav. Ecol. Sociobiol. 2003, 53, 65–71. [Google Scholar]
- Grassé, P.-P.; Noirot, C. La fondation de nouvelles sociétés par Bellicositermes natalensis Hav. Insectes Sociaux 1955, 2, 213–220. [Google Scholar] [CrossRef]
- Johnson, R. Colony development and establishment of the fungus comb in Microtermes sp. nr. usambaricus (Sjöstedt) (Isoptera: Macrotermitinae) from Nigeria. Insectes Sociaux 1981, 28, 3–12. [Google Scholar] [CrossRef]
- Johnson, R.A.; Thomas, R.J.; Wood, T.G.; Swift, M.J. The inoculation of the fungus comb in newly founded colonies of some species of the Macrotermitinae (Isoptera) from Nigeria. J. Nat. Hist. 1981, 15, 751–756. [Google Scholar]
- Nobre, T.; Fernandes, C.; Boomsma, J.J.; Korb, J.; Aanen, D.K. Farming termites determine the genetic population structure of Termitomyces fungal symbionts. Mol. Ecol. 2011, 20, 2023–2033. [Google Scholar] [CrossRef]
- Aanen, D.K.; de Fine Licht, H.H.; Debets, A.J.M.; Kerstes, N.A.G.; Hoekstra, R.F.; Boomsma, J.J. High symbiont relatedness stabilizes mutualistic cooperation in fungus-growing termites. Science 2009, 326, 1103–1106. [Google Scholar]
- Rouland-Lefevre, C. Symbiosis with Fungi. In Termites: Evolution, Sociality, Symbioses, Ecology; Abe, T., Bignell, D.E., Higashi, M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 289–306. [Google Scholar]
- Rouland-Lefevre, C.; Diouf, M.N.; Brauman, A.; Neyra, M. Phylogenetic relationships in Termitomyces (family Agaricaceae) based on the nucleoticle sequence of ITS: A first approach to elucidate the evolutionary history of the symbiosis between fungus-growing termites and their fungi. Mol. Phylogenet. Evol. 2002, 22, 423–429. [Google Scholar]
- Aanen, D.K.; Ros, V.; de Fine Licht, H.; Mitchell, J.; de Beer, Z.W.; Slippers, B.; Rouland-LeFevre, C.; Boomsma, J. Patterns of interaction specificity of fungus-growing termites and Termitomyces symbionts in South Africa. BMC Evol. Biol. 2007, 7. [Google Scholar] [CrossRef]
- Katoh, H.; Miura, T.; Maekawa, K.; Shinzato, N.; Matsumoto, T. Genetic variation of symbiotic fungi cultivated by the macrotermitine termite Odontotermes formosanus (Isoptera: Termitidae) in the Ryukyu Archipelago. Mol. Ecol. 2002, 11, 1565–1572. [Google Scholar] [CrossRef]
- Nobre, T.; Eggleton, P.; Aanen, D.K. Vertical transmission as the key to the colonization of Madagascar by fungus-growing termites? Proc. R. Soc. B Biol. Sci. 2010, 277, 359–365. [Google Scholar] [CrossRef]
- De Fine Licht, H.H.; Boomsma, J.J.; Aanen, D.K. Presumptive horizontal symbiont transmission in the fungus-growing termite Macrotermes natalensis. Mol. Ecol. 2006, 15, 3131–3138. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar]
- Breznak, J.A.; Brune, A. Role of microorganisms in the digestion of lignocellulose by termites. Annu. Rev. Entomol. 1994, 39, 453–487. [Google Scholar]
- Bignell, D.E. Introduction to Symbiosi. In Termites: Evolution, Sociality, Symbioses, Ecology; Abe, T., Bignell, D.E., Higashi, M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 189–208. [Google Scholar]
- Rouland-Lefèvre, C.; Bignell, D. Cultivation of Symbiotic Fungi by Termites of the Subfamily Macrotermitinae. In Symbiosis; volume 4, Seckbach, J., Ed.; Springer: Amsterdam, The Netherlands, 2004; pp. 731–756. [Google Scholar]
- Veivers, P.C.; Mühlemann, R.; Slaytor, M.; Leuthold, R.H.; Bignell, D.E. Digestion, diet and polyethism in two fungus-growing termites: Macrotermes subhyalinus Rambur and M. michaelseni Sjøstedt. J. Insect Physiol. 1991, 37, 675–682. [Google Scholar] [CrossRef]
- Wood, T.G.; Thomas, R.J. The Mutualistic Association between Macrotermitinae and Termitomyces. In Insect—Fungus Interactions; Wilding, N., Collins, N.M., Hammond, P.M., Webber, J.F., Eds.; Academic Press: London, UK, 1989; pp. 113–128. [Google Scholar]
- Grassé, P.-P.; Noirot, C. Le meule des termites champignonnistes et sa signification symbiotique. Ann. Sci. Nat. Zool. Biol. Anim. 1958, 11, 113–128. [Google Scholar]
- Martin, M.M.; Martin, J.S. Cellulose digestion in the midgut of the fungus-growing termite Macrotermes natalensis: The role of acquired digestive enzymes. Science 1978, 199, 1453–1455. [Google Scholar]
- Rouland-Lefevre, C.; Lenoir, F.; Lepage, M. The role of the symbiotic fungus in the digestive metabolism of several species of fungus-growing termites. Comp. Biochem. Physiol. 1991, 99A, 657–663. [Google Scholar]
- Bignell, D.E.; Eggleton, P. Termites in Ecosystems. In Termites: Evolution, Sociality, Symbioses, Ecology; Abe, T., Bignell, D.E., Higashi, M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 363–387. [Google Scholar]
- Hyodo, F.; Tayasu, I.; Inoue, T.; Azuma, J.I.; Kudo, T.; Abe, T. Differential role of symbiotic fungi in lignin degradation and food provision for fungus-growing termites (Macrotermitinae: Isoptera). Funct. Ecol. 2003, 17, 186–193. [Google Scholar]
- Hyodo, F.; Inoue, T.; Azuma, J.I.; Tayasu, I.; Abe, T. Role of the mutualistic fungus in lignin degradation in the fungus-growing termite Macrotermes gilvus (Isoptera; Macrotermitinae). Soil Biol. Biochem. 2000, 32, 653–658. [Google Scholar] [CrossRef]
- Rohrmann, G. The origin, structure, and nutritional importance of the comb in two species of Macrotermitinae. Pedobiologia 1978, 18, 89–98. [Google Scholar]
- Abo-Khatwa, N. Cellulase of fungus-growing termites: A new hypothesis on its origin. Cell. Mol. Life Sci. 1978, 34, 559–560. [Google Scholar]
- Rouland, C.; Civas, A.; Renoux, J.; Petek, F. Purification and properties of cellulases from the termite Macrotermes mulleri (Termitidae, Macrotermitinae) and its symbiotic fungus Termitomyces sp. Comp. Biochem. Physiol. Part B Biochem. 1988, 91, 449–458. [Google Scholar] [CrossRef]
- Ikhouane, A.; Rouland-Lefevre, C. In Etude de la biodégradation, in vivo et in vitro, de polymères glucidiques par trois champignons du genre Termitomyces. Actes Colloques Insectes Sociaux 1996, 10, 101–109. [Google Scholar]
- Matoub, M.; Rouland, C. Purification and properties of the xylanases from the termite Macrotermes bellicosus and its symbiotic fungus Termitomyces sp. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1995, 112, 629–635. [Google Scholar] [CrossRef]
- Faulet, B.M.; Niamke, S.; Gonnety, J.T.; Kouame, L.P. Purification and biochemical properties of a new thermostable xylanase from symbiotic fungus, Termitomyces sp. Afr. J. Biotechnol. 2006, 5, 273–282. [Google Scholar]
- Deng, T.F.; Chen, C.R.; Cheng, M.L.; Pan, C.Y.; Zhou, Y.; Mo, J.C. Differences in cellulase activity among different castes of Odontotermes formosanus (Isoptera : Termitidae) and the symbiotic fungus Termitomyces albuminosus. Sociobiology 2008, 51, 697–704. [Google Scholar]
- Yang, T.; Mo, J.C.; Cheng, J. Purification and some properties of cellulase from Odontotermes formosanus (Isoptera: Termitidae). Entomol. Sin. 2004, 11, 1–10. [Google Scholar]
- Sengupta, S.; Ghosh, A.K.; Sengupta, S. Purification and characterisation of a β-glucosidase (cellobiase) from a mushroom Termitomyces clypeatus. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 1991, 1076, 215–220. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Banerjee, P.C.; Sengupta, S. Purification and properties of xylan hydrolase from mushroom Termitomyces clypeatus. Biochim. Biophys. Acta (BBA) Enzymol. 1980, 612, 143–152. [Google Scholar]
- Sinha, N.; Sengupta, S. Simultaneous production of α-arabinofuranosidase and xylanase by Termitomyces clypeatus. World J. Microbiol. Biotechnol. 1995, 11, 359–360. [Google Scholar] [CrossRef]
- Bose, S.; Mazumder, S.; Mukherjee, M. Laccase production by the white-rot fungus Termitomyces clypeatus. J. Basic Microbiol. 2007, 47, 127–131. [Google Scholar] [CrossRef]
- Bignell, D.E.; Slaytor, M.; Veivers, P.C.; Muhlemann, R.; Leuthold, R.H. Functions of symbiotic fungus gardens in higher termites of the genus Macrotermes: Evidence against the acquired enzyme hypothesis. Acta Microbiol. Immunol. Hung. 1994, 41, 391–401. [Google Scholar]
- Slaytor, M. Cellulose digestion in termites and cockroaches: What role do symbionts play? Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1992, 103, 775–784. [Google Scholar]
- Badertscher, S.; Gerber, C.; Leuthold, R.H. Polyethism in food supply and processing in termite colonies of Macrotermes subhyalinus (Isoptera). Behav. Ecol. Sociobiol. 1983, 12, 115–119. [Google Scholar] [CrossRef]
- Traniello, J.F.A.; Leuthold, R.H. The Behavior and Ecology of Foraging in Termites. In Termites: Evolution, Sociality, Symbioses, Ecology; Abe, T., Bignell, D.E., Higashi, M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 141–168. [Google Scholar]
- Swift, M.; Heal, O.; Anderson, M. Decomposition in Terrestrial Ecosystems; Blackwell Scientific: Oxford, UK, 1979; p. 372. [Google Scholar]
- Collins, N. The Utilization of Nitrogen Resources by Termites (Isoptera). In Nitrogen as an Ecological Factor; Lee, J., McNeill, S., Rorison, I., Eds.; Blackwell Scientific Publications: Oxford, UK, 1983; pp. 381–412. [Google Scholar]
- Martin, M.M. Acquired Enzymes in the Fungus-Growing Termite Macrotermes natalensis. In Invertebrate Microbial Interactions. Ingested Fungal Enzymes in Arthropod Biology; Comstock Publishing Associates: Ithaca, NY, USA, 1987; pp. 17–36. [Google Scholar]
- Martin, M.M.; Martin, J.S. The distribution and origins of the cellulolytic enzymes of the higher termite, Macrotermes natalensis. Physiol. Zool. 1979, 52, 11–21. [Google Scholar]
- Bignell, D.E. Morphology, Physiology, Biochemistry and Functional Design of the Termite Gut: An Evolutionary Wonderland. In Biology of Termites: A Modern Synthesis; Bignell, D.E., Roisin, Y., Lo, N., Eds.; Springer: Amsterdam, The Netherlands, 2011; pp. 375–412. [Google Scholar]
- Misra, J.; Ranganathan, V. Digestion of cellulose by the mound building termite, Termes (Cyclotermes) obesus (Rambur). Proc. Plant Sci. 1954, 39, 100–113. [Google Scholar]
- Pasti, M.B.; Belli, M.L. Cellulolytic activity of actinomycetes isolated from termites (Termitidae) gut. FEMS Microbiol. Lett. 1985, 26, 107–112. [Google Scholar]
- Paul, J.; Saxena, S.; Varma, A. Ultrastructural studies of the termite (Odontotermes obesus) gut microflora and its cellulolytic properties. World J. Microbiol. Biotechnol. 1993, 9, 108–112. [Google Scholar] [CrossRef]
- Varm, A.; Bala Krishna, K.; Jaishree, P.; Shailendra, S.; Helmut, K. Lignocellulose degradation by microorganisms from termite hills and termite guts: A survey on the present state of art. FEMS Microbiol. Rev. 1994, 15, 9–28. [Google Scholar]
- Yara, K.; Jahan, K.; Hayashi, H. In situ morphology of the gut microbiota of the fungus-growing termite Odontotermes formosanus (Termitidae: Macrotermitidae). Sociobiology 1989, 15, 2247–2260. [Google Scholar]
- Anklinmuhlemann, R.; Bignell, D.E.; Veivers, P.C.; Leuthold, R.H.; Slaytor, M. Morphological, microbiological and biochemical studies of the gut flora in the fungus-growing termite Macrotermes subhyalinus. J. Insect Physiol. 1995, 41, 929–940. [Google Scholar] [CrossRef]
- Shah, H.N.; Gharbia, S.E. Ecophysiology and taxonomy of bacteroides and related taxa. Clin. Infect. Dis. 1993, 16, S160–S167. [Google Scholar]
- Ohkuma, M.; Noda, S.; Hongoh, Y.; Kudo, T. Diverse bacteria related to the bacteroides subgroup of the CFB phylum within the gut symbiotic communities of various termites. Biosci. Biotechnol. Biochem. 2002, 66, 78–84. [Google Scholar]
- Hongoh, Y.; Ekpornprasit, L.; Inoue, T.; Moriya, S.; Trakulnaleamsai, S.; Ohkuma, M.; Noparatnaraporn, N.; Kudo, T. Intracolony variation of bacterial gut microbiota among castes and ages in the fungus-growing termite Macrotermes gilvus. Mol. Ecol. 2006, 15, 505–516. [Google Scholar]
- Mackenzie, L.M.; Muigai, A.T.; Osir, E.O.; Lwande, W.; Keller, M.; Toledo, G.; Boga, H.I. Bacterial diversity in the intestinal tract of the fungus-cultivating termite Macrotermes michaelseni (Sjöstedt). Afr. J. Biotechnol. 2007, 6. [Google Scholar]
- Liu, N.; Yan, X.; Zhang, M.; Xie, L.; Wang, Q.; Huang, Y.; Zhou, X.; Wang, S.; Zhou, Z. Microbiome of fungus-growing termites: A new reservoir for lignocellulase genes. Appl. Environ. Microbiol. 2011, 77, 48–56. [Google Scholar]
- Long, Y.-H.; Xie, L.; Liu, N.; Yan, X.; Li, M.-H.; Fan, M.-Z.; Wang, Q. Comparison of gut-associated and nest-associated microbial communities of a fungus-growing termite Odontotermes yunnanensis. Insect Sci. 2010, 17, 265–276. [Google Scholar] [CrossRef]
- Brauman, A.; Dore, J.; Eggleton, P.; Bignell, D.; Breznak, J.A.; Kane, M.D. Molecular phylogenetic profiling of prokaryotic communities in guts of termites with different feeding habits. FEMS Microbiol. Ecol. 2001, 35, 27–36. [Google Scholar]
- Ohkuma, M. Termite symbiotic systems: Efficient bio-recycling of lignocellulose. Appl. Microbiol. Biotechnol. 2003, 61, 1–9. [Google Scholar]
- Scharf, M.E.; Boucias, D.G. Potential of termite-based biomass pre-treatment strategies for use in bioethanol production. Insect Sci. 2010, 17, 166–174. [Google Scholar]
- Bathellier, J. Contribution à l’ etude systématique et biologique de termites de l’Indo-Chine. Faune Colonies Franc. 1927, 1, 125–365. [Google Scholar]
- De Fine Licht, H.H.; Andersen, A.; Aanen, D.K. Termitomyces sp associated with the termite Macrotermes natalensis has a heterothallic mating system and multinucleate cells. Mycol. Res. 2005, 109, 314–318. [Google Scholar] [CrossRef]
- Heim, R. Termites et Champignons; Société nouvelle de Éditions Boubée: Paris, France, 1977. [Google Scholar]
- Thomas, R.J. Distribution of Termitomyces Heim and other fungi in the nests and major workers of several nigerian Macrotermitinae. Soil Biol. Biochem. 1987, 19, 335–341. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nobre, T.; Aanen, D.K. Fungiculture or Termite Husbandry? The Ruminant Hypothesis. Insects 2012, 3, 307-323. https://doi.org/10.3390/insects3010307
Nobre T, Aanen DK. Fungiculture or Termite Husbandry? The Ruminant Hypothesis. Insects. 2012; 3(1):307-323. https://doi.org/10.3390/insects3010307
Chicago/Turabian StyleNobre, Tânia, and Duur K. Aanen. 2012. "Fungiculture or Termite Husbandry? The Ruminant Hypothesis" Insects 3, no. 1: 307-323. https://doi.org/10.3390/insects3010307