Biogeography and Phylogeny of Wood-feeding Cockroaches in the Genus Cryptocercus
Abstract
: Subsocial, xylophagous cockroaches of the genus Cryptocercus exhibit a disjunct distribution, with representatives in mature montane forests of North America, China, Korea and the Russian Far East. All described species are wingless and dependent on rotting wood for food and shelter at all stages of their life cycle; consequently, their distribution is tied to that of forests and strongly influenced by palaeogeographical events. Asian and American lineages form distinct monophyletic groups, comprised of populations with complex geographic substructuring. We review the phylogeny and distribution of Cryptocercus, and discuss splitting events inferred from molecular data.1. Introduction
Woodroaches of the genus Cryptocercus (Dictyoptera: Cryptocercidae) are subsocial, xylophagous cockroaches that occur in mountain forests of temperate regions, living in galleries that they chew within rotten logs [1–5]. Numerous recent phylogenetic studies of both the insects and their symbionts strongly suggest that the genus is a sister group to termites (reviewed in [6]). The genus has a disjunct distribution, occurring in eastern and western North America, and in China, Korea and the Russian Far East. All Cryptocercus spp. are wingless cockroaches (see Figure 1), and depend on dead wood for food and shelter at all stages of their life cycle. It is therefore likely that their distributional pattern has been strongly affected by palaeogeographic events that influenced their source tree hosts, such as the appearance of land bridges or the uplift of mountains. Both North American [7–11] and Asian taxa [10,12] have been the subjects of independent phylogeographic analyses, and the phylogenetic relationships and divergence times within and between Palaearctic and Nearctic taxa have been analyzed based on molecular data [13]. Here we review the distribution and phylogeny of Cryptocercus (1) worldwide, (2) in the Palaearctic, and (3) in the Nearctic.
2. Worldwide Distribution and Phylogeny of Cryptocercus
2.1. When did the Splitting Events Occur?
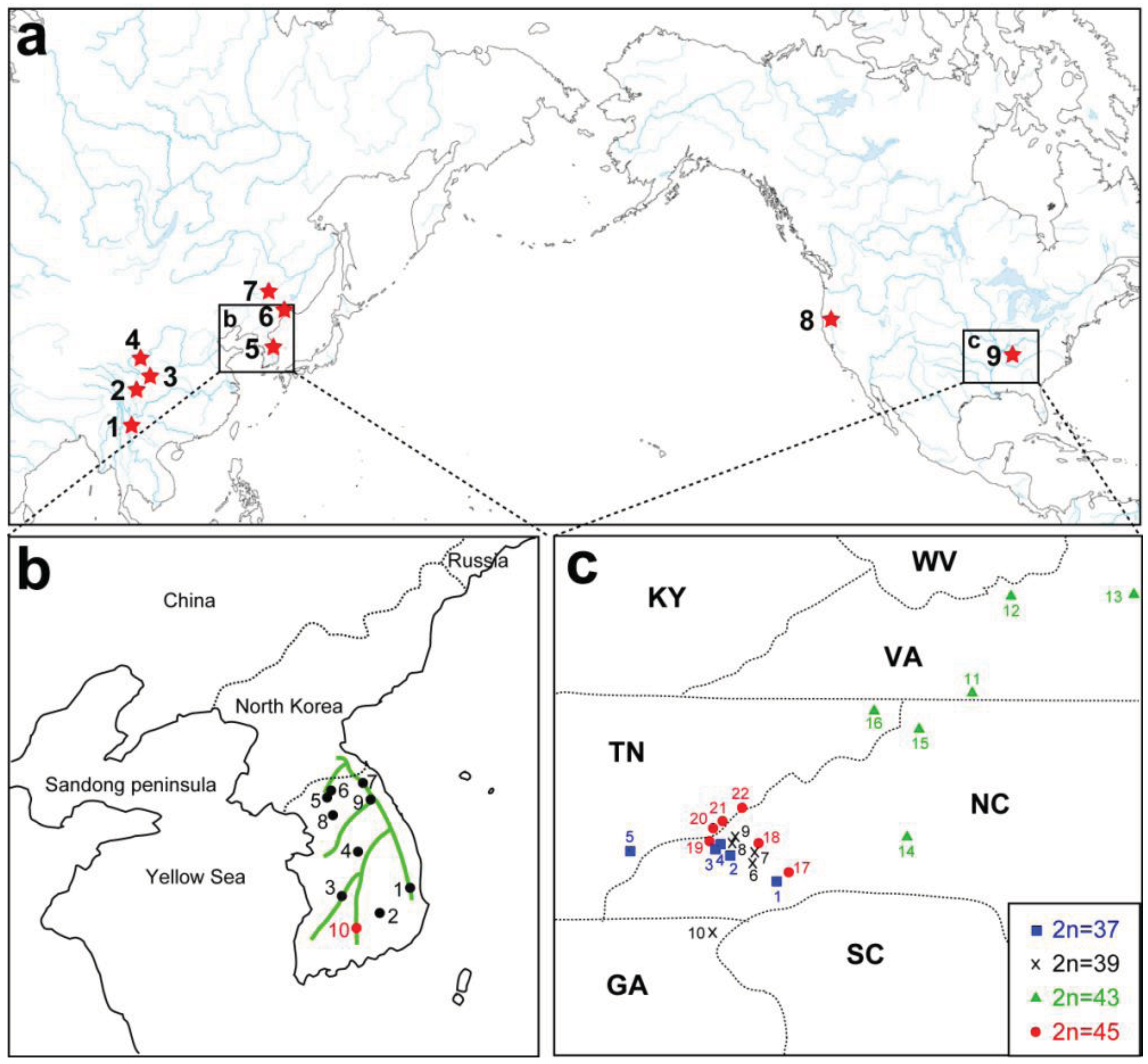
A remarkable feature of the present geographic distribution of Cryptocercus is the disjunct distribution found between the Nearctic and Palearctic regions, and between eastern and western North America (Figure 2A). Grandcolas [14] hypothesized that the American lineage evolved from Asian taxa. If this hypothesis is supported, then Asian taxa would be ancestral to the American lineage, with the latter as apical. However, independent analyses using multiple representatives of both North American and Asian taxa show that the two groups form respective monophyletic groups (e.g., [12]).
There are several hypotheses regarding the divergence times between Asian and American taxa. Grandcolas [14] proposed that “the ancestor of Cryptocercus was distributed in Asia, extending posteriorly its distribution to North America”. He hypothesized that these movements occurred via the connection of the two landmasses during the late Tertiary. Later, Grandcolas et al. [4] proposed that the splitting events between Asian and American species occurred between ∼18 and 2 million years ago (MYA), based on the genetic divergence of partial mitochondrial rDNA sequences.
Clark et al. [9] proposed a markedly different scenario: “During the Jurassic (213-144 MYA), the ancestor of extant Cryptocercus inhabited the temperate deciduous forests of the Arcto-Tertiary complex in the extreme northern regions of the Northern Hemisphere…A general cooling trend began during the Cretaceous (144–65 MYA), which forced the Arcto-Tertiary community (and the ancestor of Cryptocercus) to move south into Asia and North America”. In formulating their hypothesis, Clark et al. [9] utilized data from not only mitochondrial and nuclear genes of the cockroaches, but also from 16S rRNA genes of their fat body endosymbionts (Blattabacterium spp.). These endosymbionts have been found in all cockroaches examined to date (with one exception-Nocticolidae), and just one termite species, the basal taxon Mastotermes darwiniensis (reviewed in [6]). Clark et al. [9] demonstrated topological congruence between the endosymbionts and their hosts, and using a 0.6–1.0% per 50 million years sequence divergence rate, estimated the divergence times between Asian and American species at about 115–70 MYA. This sequence divergence rate was originally proposed by Bandi et al. [21], based on the assumption that cockroaches and termites are each monophyletic and that they diverged from each other sometime between 250 and 135 MYA. However, Maekawa et al. [13] pointed out that the former hypothesis was not supported by more recent phylogenetic studies among cockroaches, termites and mantids (which together form the Dictyoptera). Both molecular and morphological evidence show a sister-group relationship between termites and Cryptocercus, and the paraphyly of cockroaches in relation to termites (reviewed in [6]). Moreover, Maekawa et al. [13] pointed out that the fossil record was not consistent with the idea that termites and cockroaches diverged from each other 250 MYA. Cockroaches are one of the most ancient insect groups, and their fossils are observed in Carboniferous strata. However, cockroach fossils from the Carboniferous to the Jurassic possess a distinct, external ovipositor, while the ovipositors of more recent fossils and of extant Blattaria either do not exceed the tip of the abdomen (cockroaches) or are vestigial (termites except for Mastotermes). Consequently, ancient fossil cockroaches with external ovipositors are likely to be paraphyletic with respect to the Dictyoptera [22]. Indeed, termite, mantid and modern cockroach fossils first appear in early Cretaceous strata (∼130 MYA). Consequently, Maekawa et al. [13] suggested that a more realistic estimate for the split of Dictyoptera was sometime between 135 and 180 MYA. The older value was based on fossil records of the Mesoblattinidae, which has been proposed as the stem group of Dictyoptera [23,24].
The monophyly of Asian taxa could not be verified because in the studies of both Grandcolas et al. [4] and Clark et al. [9] only one Asian representative was used. Park et al. [12] estimated divergence times between Asian and American lineages based on the mitochondrial COII gene sequences of multiple Asian and two American taxa. They suggested that Asian and American lineages diverged around 61–26 MYA. These values are intermediate between those of Clark et al. [9] and Grandcolas et al. [4].
2.2. Endosymbiont 16S Phylogeny Reveals a Late Cretaceous-Early Tertiary Split of Asian and American Groups
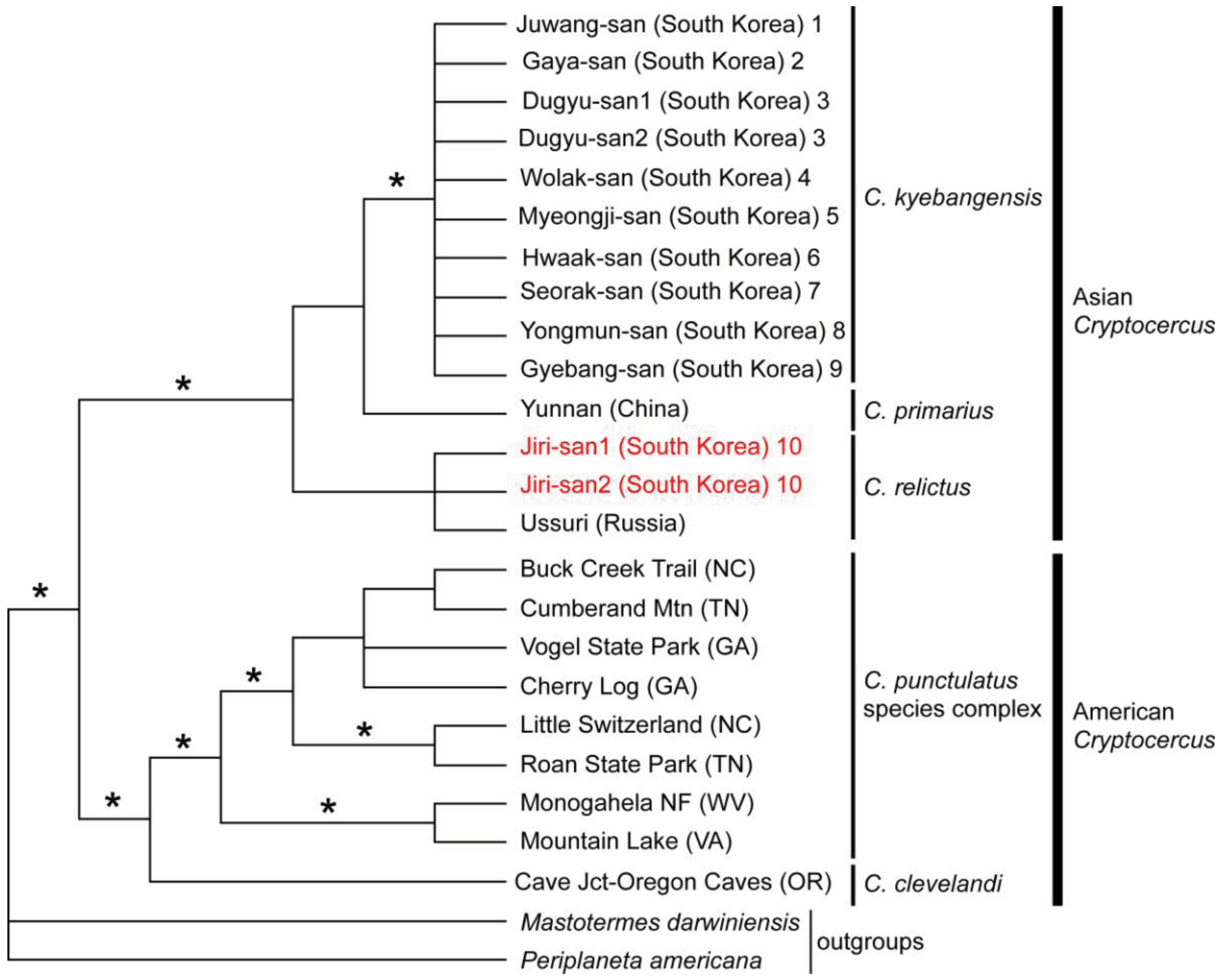
No reports of molecular phylogenetic analyses using Blattabacterium endosymbionts from multiple individuals of Asian and American Cryptocercus existed until Maekawa et al. [13] estimated their phylogenetic relationships and divergence times using endosymbiont 16S sequences of Korean hosts combined with published data. The monophyly of Asian taxa, as well as that of American taxa, was supported by more than 90% Bayesian posterior probabilities and most parsimonious (MP) bootstrap probabilities (Figure 3). The data suggested, then, that there had been only one divergence event between Asian and American Cryptocercus.
Maekawa et al. [13] calculated the average maximum likelihood distance between endosymbiont 16S from M. darwiniensis and the Cryptocercus spp. (6.05 ± 0.57%, average ± SD, n = 15), and suggested that 0.0302 substitutions per site occurred in each of these lineages. Using an estimate for the split of the Dictyoptera (135 and 180 MYA; Section 2.1), they calculated that the evolutionary rate was 0.0084–0.0111 per site per 50 MY. Moran et al. [25] reported that the evolutionary rates of aphid endosymbiont 16S were 0.0076–0.0232 per site per 50 MY, and the above estimates of Blattabacterium 16S were within this range. Using this evolutionary rate, Maekawa et al. [13] suggested that the divergence times between Asian and American Cryptocercus (which have a mean ML distance of 2.61 ± 0.25% (n = 54)) were between 77.8–58.7 MYA.
These authors then addressed the correspondence between the estimated divergence times and hypotheses regarding the paleogeography of the region. During the late Cretaceous/early Tertiary (∼65 MYA), Laurasia had been subdivided into the Aquillapollenites Province (eastern Asia and Western North America) and the Normapolles Province (eastern North America and Europe) [26–28], and the temperate climates of the early Tertiary allowed a “boreotropical flora” to dominate the Northern Hemisphere [29]. The boreotropical flora spread between Eurasia and America during the early Eocene (∼55 MYA), aided by the existence of the North Atlantic land bridge and the Bering Land Bridge [28]. Although the source origin of the genus is still unclear, the results of both Maekawa et al. [13] and Park et al. [12] suggest that the ancestor of Cryptocercus had evolved by this point and existed in both Asia and America. Disruptions of both the Bering Land Bridge and the North Atlantic Bridge during the middle Eocene (∼50–45 MYA) (see [28]) probably contributed to the divergence between Asian and American Cryptocercus. The Bering Land Bridge may have reformed in the late Eocene [28], with Asia and America continuously connected with each other until the formation of the Bering Strait in the late Miocene [29–30]. The results suggest that gene flow between Asian and American Cryptocercus had been disrupted by the late Eocene, despite the continuous connection and warm climates of the two continents until the late Tertiary. Widespread grassland in these regions [29] might have contributed to this cessation of gene flow between Asian and American Cryptocercus.
3. Distribution and Phylogeny of Palaearctic Cryptocercus
3.1. Taxonomy and Phylogenetic Relationships
Currently, seven Asian species of Cryptocercus are recognized. Cryptocercus primarius and C. matilei are found in Sichuan Province, West China [16,17] and C. primarius was rediscovered in two forests within the Hengduan Mountains of the northwestern Yunnan Province, West China [18]. The other Asian species, C. relictus, is found in the Ussuri region and Siberia (Russia), eastern Manchuria, and South Korea [19,31]. A South Korean species, C. kyebangensis, was described by Grandcolas et al. [4]. Later, Grandocolas et al. [15] described three additional species, C. hirtus, C. meridianus and C. parvus, from China (see Figure 2A and 2B for the map).
The phylogeography of Northeast Asian Cryptocercus spp. was inferred from mitochondrial COII and 16S genes and nuclear 18S gene sequences [12]. The data show two distantly related groups in the Korean taxa: the southwestern population (Cryptocercus from Jiri-san) and the population containing the remaining Korean individuals (C. kyebangensis). The former was shown to be most closely related to the populations in Northeast China and eastern Russia (C. relictus). This is an unexpected finding, because Jiri-san is located in the extreme southwest region of the Korean Peninsula.
Lo et al. [10] subsequently sequenced mitochondrial COII and 16S genes in C. primarius to determine its phylogenetic position. Despite being geographically proximate to one another, C. primarius samples from the Yunnan region in China were genetically distant, and precise phylogenetic relationships among the Asian species (C. primarius, C. relictus and C. kyebangensis used in this study) were not clearly delineated.
3.2. Biogeography of Cryptocercus in South Korea
Park et al. [12] utilized COII transversion rates of 0.13–0.30%/MY [32] to infer the biogeography of Korean Cryptocercus. They suggested that the divergence of the three groups (Cryptocercus in Northeast China and eastern Russia, those in Jiri-san, and those in the remainder South Korea) occurred during the Miocene (7.5–17.4 MYA), and that the first and second populations diverged from each other sometime between 0.8–1.9 MYA. Based on present distributions, Park et al. [12] proposed that the common ancestor of extant C. relictus and C. kyebangensis may have been distributed in East China or Northeast China, and then migrated into the Korean Peninsula during the Miocene (7.5–17.4 MYA).
These authors pointed out two possible routes for the migration into the Korean Peninsula: via mountain chains that connect Manchuria to South Korea, or via the Yellow Sea basin. Regarding the first possible route, potential connections include the Taebaek Mountains in South Korea, and the Jangbai Mountains and Nangnim Mountains in North Korea. As to the second route, Park et al. [12] noted that Cryptocercus is found in some mountainous areas (e.g., Yongmun-san) in the western part of the Korean Peninsula, and that the western coastline of the Korean Peninsula is located only about 250 km from that of the Sandong Peninsula of East China. Furthermore, the Yellow Sea basin was above sea-level during the early-mid Miocene (especially, 11.5–16.5 MYA) [33]. If ancestral populations of C. kyebnagensis had migrated into Korea via the Yellow Sea basin, they could have later spread along the Taebaek Mountains during the Pleistocene (0.4–0.8 MYA).
Park et al. [12] offered two hypotheses for the unexpected distribution of C. relictus in Jiri-san. The first is that ancestral populations in Manchuria might have moved south into the Korean Peninsula. The divergence time (about 13.5 MYA) between populations in Manchuria and Jiri-san corresponds to the early Pleistocene, when advances and retreats of glaciers might have affected divergence between the populations. An alternate hypothesis is that, during the estimated time period (0.8–1.9 MYA), a portion of the ancestral population of extant C. relictus moved to Manchuria, whereas another population moved into the southwestern parts of South Korea via the Yellow Sea basin. The Yellow Sea basin was completely or partially exposed during the Pleistocene [34–36], and the bones of extinct animals (Elephas namadicus and Mammuthus primigenius) have been collected there (reviewed in [34]). It is therefore possible that ancestral populations of C. relictus (Jiri-san) in East China could have migrated into the southwestern part of the Korean Peninsula via a forested land route on the Yellow Sea basin.
4. Distribution and Phylogeny of Nearctic Cryptocercus
4.1. Taxonomy of Nearctic Species
Until 1997, C. punctulatus was the sole species reported in the United States. Its distribution, however, was strongly disjunct; an eastern population lived in the Appalachian Mountains from Pennsylvania to Alabama, and a western population inhabited the Pacific Northwest (Washington, Oregon, and northern California) [1,37] (see Figure 2A for the map). The distance separating the two populations (>3400 km), as well as the winglessness and consequent low vagility of the insect hinted at taxonomic division of the two populations. Cleveland et al. [1] noted that the western population of C. punctulatus had a longer developmental time, a larger body size, and 5 species of cellulolytic protozoa in the hindgut not found in the gut fauna of the Appalachian group. Later investigations documented that chromosome numbers (see the following section), mitochondrial gene sequences encoding 12S and 16S [38], and the chemistry of tergal gland secretions [39] also differed. Preliminary field studies suggested that the two populations may be reproductively isolated [38], and Nalepa et al. [7] reported not only differences in sequences of endosymbiont 16S, but also several biological differences between the two groups (egg number/ootheca, density of sternal punctation, male abdomen). These authors concluded that C. punctulatus as then represented in the literature included at least two species. The name C. punctulatus applied to populations in the eastern United States, and those in the northwestern United States were described as a new species, C. clevelandi.
Burnside et al. [8] later named three new species in the eastern population (C. wrighti, C. darwini, and C. garciai) based on differences in the sequences of mitochondrial genes (12S and 16S) and chromosome number. The validity of the species-level status proposed by these authors was questioned [20], however, because: (1) chromosome numbers were known for only part of the sample; (2) the evolutionary relationships among members of the species complex were unclear, (3) reproductive compatibility had not been investigated; and (4) although morphological variation was apparently present, it had not been demonstrated that this variation consistently distinguished the proposed species. Consequently, we do not use the species-level status proposed by Burnside et al. [8] for the populations in the eastern United States, but do recognize that C. punctulatus is a cryptic species complex.
4.2. Chromosome Evolution in the C. punctulatus Species Complex
Although karyotype studies consistently show that C. clevelandi has a diploid chromosome number of 48 in females (2n = 47 in males), four karyotypes have been detected in the C. punctulatus species complex. A diploid chromosome number of 40 for females (2n = 39 in males) was reported by Cohen and Roth [40] from the area of Highlands, North Carolina. Luykx [41] found males with 2n = 37 chromosomes, also from the Highlands area, and with 2n = 43 chromosomes at Mountain Lake, Virginia. A fourth chromosome number, 2n = 45, was reported from the Asheville, North Carolina, area [38], but the mid-point of the karyotype series (2n = 41) has not yet been detected. Palearctic species of Cryptocercus display a similar pattern of chromosomal variation in geographically proximate regions, with male chromosome numbers of both 2n = 19 and 2n = 21 reported from C. primarius samples collected in Yunnan Province, China [10]. Robertsonian changes that involve the fission or fusion of nonhomologous autosomes is responsible for the reported variations in chromosome number [41], and although these changes shift the number of chromosomes, they do not alter the fundamental genic content of the arms of the chromosomes involved. Either a chromosome with a median centromere is split into two chromosomes with terminal centromeres, or two chromosomes with terminal centromeres are fused into one chromosome with a centromere near the middle [42]. Because chromosome numbers of Palearctic Cryptocercus were approximately half those reported in Nearctic species, Lo et al. [10] suggested the possibility of either genome dupulication in the ancestor of the Nearctic lineage, or a reduction in the ancestor of the Palearctic lineage. There are reports of highly variable chromosome numbers among species of the other cockroach genera (i.e., male 2n = 23–49 in Blattella spp., 34–50 in Ischnoptera spp., 38–74 in Blaberus spp., and 36–50 in Epilampra spp.) [40].
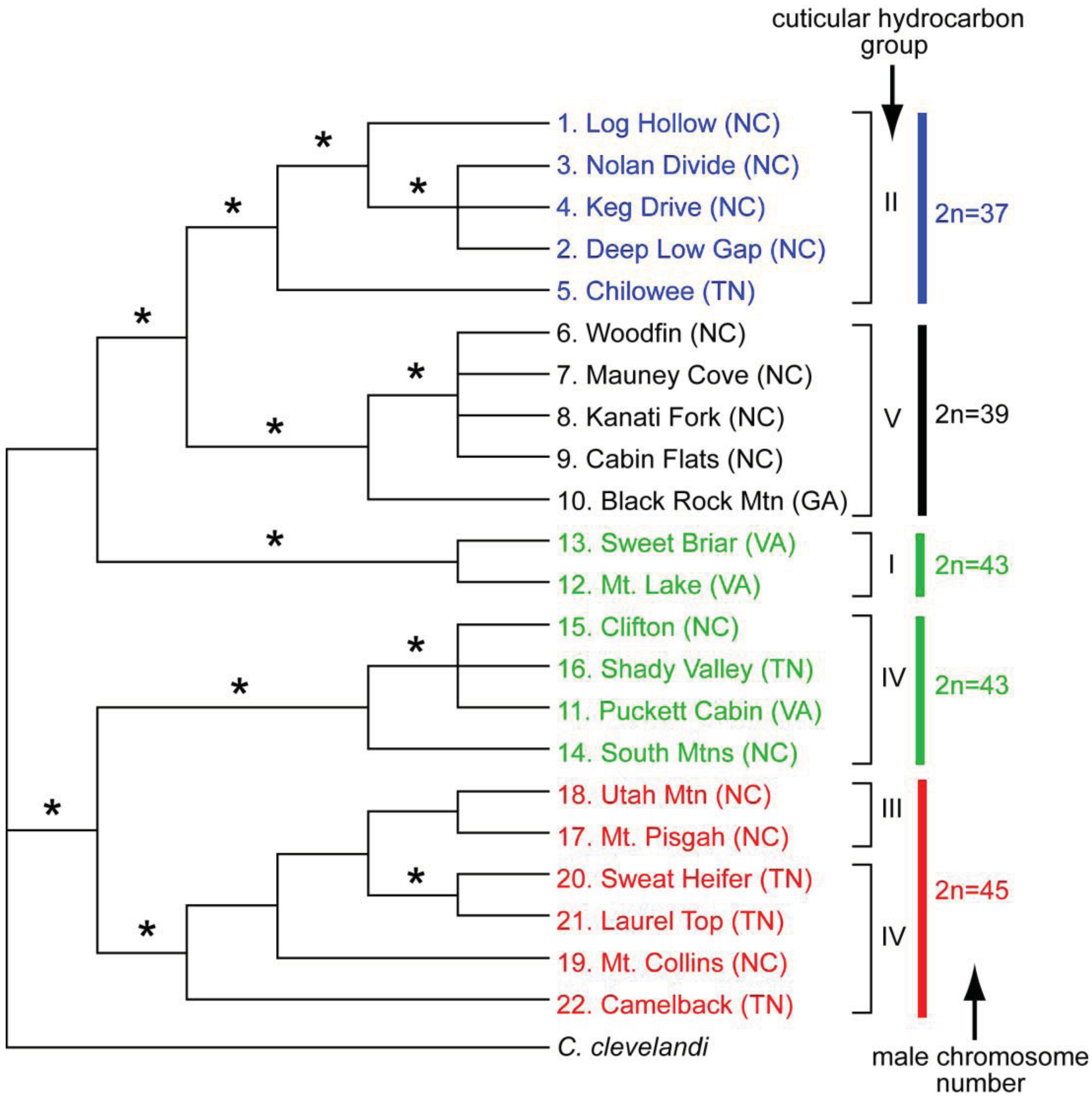
The geographic distributional pattern of the four known karyotypes (male 2n = 37, 39, 43, 45) of the C. punctulatus species complex was reported by Nalepa et al. [20], based on 71 sites in the Southern Appalachian Mountains with a concentration on western North Carolina. Cockroach populations with different karyotypes were geographically structured in a mosaic marked by abrupt geographic transitions between them, and with at least one karyotype (2n = 39) occurring in two disjunct regions. These authors offered two evolutionary scenarios that could account for the four known karyotype groups in the C. punctulatus species complex. In the extreme version of the parallel scenario, all the karyotype variants arose independently from an ancestral population via centric fusions, and possibly more than once. In the alternate, sequential scenario, an ancestral karyotype (2n = 47) was the source of a chromosomal fusion (2n = 45), which in turn gave rise to an additional chromosomal fusion (2n = 43), and so on. Lo et al. [10] analysed the COII gene from members of the C. punctulatus species complex from 15 locations. Although their results upheld the serial reduction hypothesis, support for the relationships among the chromosomal lineages was not high. Taxa with the same chromosome number formed monophyletic groups, the exception being the two disjunct populations of the 2n = 39 karyotype.
Although molecular analyses of the karyotype groups in the eastern United States have resulted in three competing phylogenetic trees (43(39(45 + 37))) [37], (45(43(37 + 39))) [8,10], and (43(45(37 + 39))) [9], the emerging consensus is that the 2n = 37 and 39 groups are closely related and relatively apical. The 2n = 39 group appears to be divided into two geographically disjunct populations [39], and is likely not monophyletic [10]. Placement of the 2n = 43 and 45 groups within the tree varies among currently available analyses. Everaerts et al. [11] examined species limits by analyzing cuticular hydrocarbons, as well as mitochondrial and nuclear genes, in the four karyotype groups of C. punctulatus species complex. They found five distinct hydrocarbon phenotypes, but these were only partially congruent with chromosome number and therefore with purported species descriptions (Figure 4).
Molecular as well as cuticular hydrocarbon data indicate that Cryptocercus with a male 2n = 43 karyotype belong to at least two discrete, distantly related lineages. One is sister group to the 2n = 37 and 2n = 39 clade, and has a unique hydrocarbon profile. The other 2n = 43 lineage is sister group to the 2n = 45 samples, with cuticular hydrocarbons that group with four samples of the 2n = 45 lineage. Cuticular hydrocarbons of two other 2n = 45 samples diverge from this assemblage. Overall, the data suggest that cuticular hydrocarbons and chromosome number have some measure of evolutionary independence, so that neither is entirely reliable in delineating historical lineages. The Everaerts et al. [11] study is supportive of the parallel model of chromosomal evolution in the C. punctulatus species complex.
4.3. Biogeography of Nearctic Species
Based on the rate of evolution proposed above (see Section 1.2.), the endosymbionts of C. clevelandi and the eastern C. punctulatus species complex diverged around 45–58 MYA (Palaeocene-Eocene), and the divergence among endosymbionts of the eastern groups occurred at the beginning of the Miocene (18–24 MYA). These data are roughly congruent with the biogeographical discussion proposed by Nalepa et al. [7,20]. Here we revisit the biogeography of the Nearctic species, particularly the C. punctulatus species complex.
Nalepa et al. [20] pointed out that, although glaciers did not extend to the southeastern United States during the Quaternary, the area was nonetheless affected by the shifts in global atmospheric circulation patterns that caused glacial advances and retreats. Both landform and pollen records indicate that periglacial conditions prevailed in the Central and Southern Appalachian Mountains during the last full glacial interval (the Wisconsin Glacial Interval in North America), and were harsh in some locales [43,44]. The composition and location of vegetation changed, and boreal forests covered much of the region between 34° and 40° N latitude [44,45]. Like many detritivores, however, Cryptocercus likely would have had little problem weathering plant species shifts in Southern Appalachian forests; host choice is more dependent on decompositional status than on source tree species. Cryptocercus is also protected against climatic extremes, because an insulating buffer zone encapsulates the interior of large logs. These properties, among others, may explain the historical persistence of the genus [29,46].
Two factors may have been primary in influencing historic distributional shifts in Southern Appalachians Cryptocercus [20]. The first is absence of mature forest and thus coarse woody debris, and the second is adequate moisture. The former may have forced Cryptocercus from higher elevations during cold, wet, full glacial periods, and the latter may have restricted the cockroaches to mountaintops during the warmer, drier interglacials.
All mountains of the southeastern U.S. are below timber line today, but during glacial advances the tree-line in the Southern Appalachians was depressed to elevations ranging from 914 to 1500 m [47,48]. Mountain summits were covered by extensive areas of alpine tundra, with discontinuous regions of spruce and fir krumholtz [44,49]. Nalepa et al. [20] suggested that the lack of habitable logs at high elevations during this time would have forced Cryptocercus off mountains and into local refugia; the prevailing cool, moist climate concurrently made lower elevation forested sites more habitable for the cockroach. When glaciers began their undulating retreat, the warming climate allowed for the migration of species out of refugia, and forests could re-invade high altitude areas [44,49,50]. At low elevations, warmth, drought, and anthropogenic changes increasingly restricted mesic forest and Cryptocercus to favorable islands of habitat [44,51–54].
All four known karyotypes of Cryptocercus in the eastern United States are present in North Carolina between 35° 27′ and 35° 36′ N, where the Southern Appalachian Mountains are geographically widest and topographically the most complex [55]. Disconnected, differently oriented mountain ranges are separated by narrow, often riparian valleys frequently more than 900 m lower in elevation. The consequent variation in habitat types and potential for local habitat shifts during climatic changes assured many organisms of suitable but discontinuous refugia during glacial advance. Cryptocercus likely became spatially subdivided along with their habitat. If gene flow between refugial populations of the cockroach was suspended for a sufficient period of time, the insects would have differentiated in situ; consequently diversification was allopatric, because refuge formation is an ecologically vicariant process [56]. Nalepa et al. [20] noted that the winglessness of Cryptocercus would have accentuated population subdivision, because in organisms with restricted dispersal capability, ranges are known to be easily fragmented [57].
It is possible that the current parapatric boundaries between karyotypes of Cryptocercus, then, are relatively recent [20]. The most recent spread of Appalachian endemics out of refugia occurred during the past 20,000 years, the time interval since the last major glaciation [44]. However, the question of when the karyotypes actually differentiated is more problematic. Diversification of many species complexes has been linked to cycles of habitat expansion and contraction related to glacial advance and retreat. The first ice advance into the Appalachians equivalent to that of the late Wisconsin occurred in the Pliocene about 2.4 MYA; glaciation intensified in the middle and late Pleistocene [43]. Nonetheless, sequence divergence rates of the 16S rDNA of bacterial endosymbionts of the fat body suggest that divergence among the Southern Appalachian populations of Cryptocercus began at the onset of the Miocene (18–24 MYA) [20]. If so karyotype differentiations may have long predated the start of the ice ages [20].
5. Conclusions
Complex mountain topography in both Asia and North America strongly contributes to the known distribution of taxonomic groups of Cryptocercus. Glacial advances and retreats of the Tertiary and Quaternary influenced forests and consequently, the presence of the rotting log hosts required by genus. Anthropogenic land use changes have an impact on current distributions. The geographic origin of the genus is still unknown, and phylogenetic relationships among all described species need clarification. Asian species appear to be more morphologically distinct than those in Eastern North America, but the full range of species diversity on both continents requires further study. One approach to improve understanding of Cryptocercus spp. phylogeography may be to estimate divergence times among lineages using recently developed methods (e.g., Bayesian methods [58]).

Acknowledgments
We thank Ryan Garrick for giving us the opportunity to write this review.
References
- Cleveland, L.R.; Hall, S.R.; Sanders, E.P.; Collier, J. The wood-feeding roach Cryptocercus, its protozoa, and the symbiosis between protozoa and roach. Mem. Am. Acad. Arts Sci. 1934, 17, 185–342. [Google Scholar]
- Seelinger, G.; Seelinger, U. On the social organization, alarm and fighting in the primitive cockroach Cryptocercus punctulatus Scudder. Zeitschrift für Tierpsychologie 1983, 61, 315–333. [Google Scholar]
- Nalepa, C.A. Colony composition, protozoan transfer and some life history characteristics of the woodroach Cryptocercus punctulatus Scudder (Dictyoptera: Cryptocercidae). Behav. Ecol. Sociobiol. 1984, 14, 273–279. [Google Scholar]
- Grandcolas, P.; Park, Y.C.; Choe, J.C.; Piulachs, M.D.; Belles, X.; D'Haese, C.; Farine, J.P.; Brossut, R. What does Cryptocercus kyebangensis, n. sp. from South Korea about Cryptocercus evolution? A study in morphology, molecular phylogeny and chemistry of tergal glands (Dictyoptera, Blattaria, Polyphagidae). Proc. Acad. Nat. Sci. Phila. 2001, 151, 61–79. [Google Scholar]
- Park, Y.C.; Grandcolas, P.; Choe, J.C. Colony composition, social behavior and some ecological characteristics of the Korean wood-feeding cockroach (Cryptocercus kyebangensis). Zool. Sci. 2002, 19, 1133–1139. [Google Scholar]
- Lo, N.; Eggleton, P. Termite phylogenetics and co-cladogenesis with symbionts. In Biology of Termites: A Modern Synthesis; Bignell, D.E., Roisin, Y., Lo, N., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 27–50. [Google Scholar]
- Nalepa, C.A.; Byers, G.W.; Bandi, C.; Sironi, M. Description of Cryptocercus clevelandi (Dictyoptera: Cryptocercidae) from the northwestern United States, molecular analysis of bacterial symbionts in its fat body, and notes on biology, distribution, and biogeography. Ann. Entomol. Soc. Am. 1997, 90, 416–424. [Google Scholar]
- Burnside, C.A.; Smith, P.T.; Kambhampati, S. Three new species of the wood roach, Cryptocercus (Blattodea: Cryptocercidae) from the eastern United States. J. Kans. Entomol. Soc. 2000, 72, 361–378. [Google Scholar]
- Clark, J.W.; Hossain, S.; Burnside, C.; Kambhampati, S. Coevolution between a cockroach and its bacterial endosymbiont: A biogeographical perspective. Proc. R. Soc. Lond. B. 2001, 268, 393–398. [Google Scholar]
- Lo, N.; Luykx, P.; Santoni, R.; Beninati, T.; Bandi, C.; Casiraghi, M.; Lu, W.H.; Zakharov, E.V.; Nalepa, C.A. Molecular phylogeny of Cryptocercus wood-roaches based on mitochondrial COII and 16S sequences, and chromosome numbers in Palearctic representatives. Zool. Sci. 2006, 23, 393–398. [Google Scholar]
- Everaerts, C.; Maekawa, K.; Farine, J.P.; Shimada, K.; Luykx, P.; Brossut, R.; Nalepa, C.A. The Cryptocercus punctulatus species complex (Dictyoptera: Cryptocercidae) in the eastern United States: Comparsion of cuticular hydrocarbons, chromosome number, and DNA sequences. Mol. Phylogenet. Evol. 2008, 47, 950–959. [Google Scholar]
- Park, Y.C.; Maekawa, K.; Matsumoto, T.; Santoni, R.; Choe, J.C. Molecular phylogeny and biogeography of the Korean woodroaches Cryptocercus spp. Mol. Phylogenet. Evol. 2004, 30, 450–464. [Google Scholar]
- Maekawa, K.; Park, Y.C.; Lo, N. Phylogeny of endosymbiont bacteria harbored by the woodroach Cryptocercus spp. (Cryptocercidae: Blattaria): Molecular clock evidence for a late Cretaceous-early Tertiary split of Asian and American lineages. Mol. Phylogenet. Evol. 2005, 36, 728–733. [Google Scholar]
- Grandcolas, P. Systematics, endosymbiosis and biogeography of Cryptocercus clevelandi and C. punctulatus (Blattaria: Polyphagidae) from North America: A phylogenetic perspective. Ann. Entomol. Soc. Am. 1999, 92, 285–291. [Google Scholar]
- Grandcolas, P.; Legendre, F.; Park, Y.C.; Belles, X.; Murienne, J.; Pellens, R. The genus Cryptocercus in East Asia: Distribution and new species (Insecta, Dictyoptedra, Blattaria, Polyphagidae). Zoosystema 2005, 27, 725–732. [Google Scholar]
- Cryptocercus matilei n. sp., du Sichuan de Chine (Dictyoptera, Blattaria, Polyphaginae). Rev. Française d'Entomol. (N.S.) 2000, 22, 223–226.
- Bey-Bienko, G. On some new or interesting Asiatic Blattodea. Ann. Mag. Nat. Hist. 1 1938, 1, 230–238. [Google Scholar]
- Nalepa, C.A.; Li, L.; Wen-Hua, L.; Lazell, J. Rediscovery of the wood-eating cockroach Cryptocercus primarius (Dictyoptera: Cryptocercidae) in China, with notes on ecology and distribution. Acta Zootaxonomica Sin. 2001, 26, 184–190. [Google Scholar]
- Bey-Bienko, G. Description of six new species of Palearctic Blattodea. Konowia 1935, 14, 117–134. [Google Scholar]
- Nalepa, C.A.; Luykx, P.; Klass, K.-D.; Deitz, L.L. Distribution of karyotypes of the Cryptocercus punctulatus species complex (Dictyoptera: Cryptocercidae) in the Southern Appalachians: Relation to habitat and history. Ann. Entomol. Soc. Am. 2002, 95, 276–287. [Google Scholar]
- Bandi, C.; Sironi, M.; Damiani, G.; Magrassi, L.; Nalepa, C.A.; Laudani, U.; Sacchi, L. The establishment of intracellular symbiosis in an ancestor of cockroaches and termites. Proc. R. Soc. Lond. B 1995, 259, 293–299. [Google Scholar]
- Grimaldi, D. A fossil mantis (Insecta: Mantodea) in Cretaceous amber of New Jersey, with comments on the early history of the Dictyoptera. Am. Mus. Novit. 1997, 3204, 1–11. [Google Scholar]
- Labandeira, C.C. A compendium of fossil insect families. Milwaukee Public Mus. Contrib. Biol. Geol. 1994, 88, 1–71. [Google Scholar]
- Vrsansky, P.; Vishniakova, V.N.; Rasnitsyn, A.P. Order Blattida Latreille, 1810. In History of insects; Rasnitsyn, A.P., Quicke, D.L.J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 263–270. [Google Scholar]
- Moran, N.A.; Munson, M.A.; Baumann, P.; Ishikawa, H. A molecular clock in endosymbiotic bacteria is calibrated using the insect hosts. Proc. R. Soc. Lond. B 1993, 253, 167–171. [Google Scholar]
- Raven, P.H.; Axelrod, D.I. Angiosperm biogeography and past continental movements. Ann. Mo. Bot. Gard. 1974, 61, 539–673. [Google Scholar]
- Wolfe, J.A. Some aspects of plant geography of the Northern Hemisphere during the late Cretaceous and Tertiary. Ann. Mo. Bot. Gard. 1975, 62, 264–279. [Google Scholar]
- Tiffney, B.H. The Eocene North Atlantic Bridge: Its importance in Tertiary and modern phytogeography of the Northern Hemisphere. J. Arnold Arbor. 1985, 66, 243–273. [Google Scholar]
- Nalepa, C.A.; Bandi, C. Phylogenetic status, distribution, and biogeography of Cryptocercus. Ann. Entomol. Soc. Am. 1999, 92, 292–302. [Google Scholar]
- Sher, A. Traffic lights at the Beringian Crossroads. Nature 1999, 397, 103–104. [Google Scholar]
- Asahina, S. Notes on two small collections of the Blattaria from China and Korea. Akitu 1991, 121, 1–5. [Google Scholar]
- Beckenbach, A.T.; Wei, Y.W.; Liu, H. Relationships in the Drosophila obscura species group, inferred from mitochondrial cytochrome oxidase II sequences. Mol. Biol. Evol. 1993, 10, 619–634. [Google Scholar]
- Yi, S.H.; Yi, S.S.; Batten, D.J.; Yun, H.S.; Park, S.J. Cretaceous and Cenozoic non-marine deposits of the Northern South Yellow Sea Basin, offshore western Korea: Palynostratigraphy and palaeoenvironments. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2003, 191, 15–44. [Google Scholar]
- Park, Y.A.; Yi, H.I. Late quaternary climatic changes and sea-level history along the Korean coasts. J. Coastal Res. Spec. Issue 1989, 17, 163–168. [Google Scholar]
- Park, Y.A. The changes of sea level and climate during the late Pleistocene and Holocene in the Yellow Sea egion. Korean J. Quat. Res. 1992, 6, 13–19. [Google Scholar]
- Park, Y.A.; Kim, B.K.; Zhao, S. Sea level fluctuation in the Yellow Sea Basin. J. Korean Soc. Oceanogr. 1994, 29, 42–49. [Google Scholar]
- Atkinson, T.H.; Koehler, P.G.; Patterson, R.S. Catalogue and Atlas of the Cockroaches (Dictyoptera) of North America North of Mexico. Misc. Publ. Entomol. Soc. Am. 1991, 78, 1–86. [Google Scholar]
- Kambhampati, S.; Luykx, P.; Nalepa, C.A. Evidence for sibling species in Cryptocercus punctulatus, the wood roach, from variation in mitochondrial DNA and karyotype. Heredity 1996, 76, 485–496. [Google Scholar]
- Brossut, R.; Nalepa, C.A.; Bonnard, O.; Le Quere, J.L.; Farine, J.P. Tergal glands of male and female Cryptocercus punctulatus scudder (Dictyoptera: Cryptocercidae): Composition, sexual dimorphism, and geographic variation of secretion. J. Chem. Ecol. 1991, 17, 823–831. [Google Scholar]
- Cohen, S.H.; Roth, L.M. Chromosome numbers of the Blattaria. Ann. Entomol. Soc. Am. 1970, 63, 1520–1547. [Google Scholar]
- Luykx, P. XO-XX sex chromosomes and Robertsonian variation in the autosomes of the wood-roach Cryptocercus punctulatus (Dictyoptera: Blattaria: Cryptocercidae). Ann. Entomol. Soc. Am. 1983, 76, 518–522. [Google Scholar]
- White, M.J.D. Animal Cytology and Evolution, 3rd Ed. ed; Cambridge University Press: Cambridge, MA, USA, 1973. [Google Scholar]
- Braun, D.D. Glacial and periglacial erosion of the Appalachians. Geomorphology 1989, 2, 233–256. [Google Scholar]
- Delcourt, P.A.; Delcourt, H.R.; Morse, D.F.; Morse, P.A. History, evolution, and organization of vegetation and human culture. In Biodiversity of the Southeastern United States: Lowland Terrestrial Communities; Martin, W.H., Boyce, S.G., Echternacht, A.C., Eds.; John Wiley & Sons: New York, NY, USA, 1993; pp. 47–79. [Google Scholar]
- Watts, W.A. The full-glacial vegetation of northwestern Georgia. Ecology 1970, 51, 17–33. [Google Scholar]
- Nalepa, C.A.; Bignell, D.E.; Bandi, C. Detritivory, coprophagy, and the evolution of digestive mutualisms in Dictyoptera. Insectes Soc. 2001, 48, 194–201. [Google Scholar]
- King, P.B.; Stupka, A. The Great Smoky Mountains-their geology and natural history. Sci. Mon. 1950, 71, 31–42. [Google Scholar]
- Michalek, D.D. Fanlike Features and Related Periglacial Phenomena of the Southern Blue Ridge. Ph.D. dissertation, Department of Geology, University of North Carolina, Chapel Hill, NC, USA, 1969. [Google Scholar]
- Mills, H.H.; Delcourt, P.A. Quarternary geology of the Appalachian highlands and interior low plateaus. In Quarternary Nonglacial Geology; Morrison, R.B., Ed.; Conterminous U.S. Geological Society of America: Boulder, CO, USA, 1991; pp. 611–628. [Google Scholar]
- Webb, T.I.; Bartlein, P.J. Global changes during the last 3 million years: Climatic controls and biotic responses. Annu. Rev. Ecol. Syst. 1992, 23, 141–173. [Google Scholar]
- Folkerts, G.W. The range of the eastern woodeating cockroach Cryptocercus punctulatus Scudder (Blattaria: Cryptocercidae) in the United States. Entomol. News 2006, 117, 101–108. [Google Scholar]
- Hardin, J.W.; Cooper, A.W. Mountain disjuncts in the eastern Piedmont of North Carolina. J. Elisha Mitchell Sci. Soc. 1967, 83, 139–150. [Google Scholar]
- Martin, P.S. Pleistocene ecology and biogeography of North America. In Zoogeography; Hubbs, C.L., Ed.; AAAS Publ.: Cambridge, MA, USA, 1958; pp. 375–420. [Google Scholar]
- Nalepa, C.A. Cryptocercus punctulatus (Dictyoptera: Cryptocercidae) from monadnocks in the Piedmont of North Carolina. J. Entomol. Sci. 2001, 36, 329–334. [Google Scholar]
- White, P.S.; Buckner, E.R.; Pittillo, J.D.; Cogbill, C.V. High-elevation forests: Spruce-fir forests, northern hardwoods forests, and associated communities. In Biodiversity of the Southeastern United States; Martin, W.H., Boyce, S.G., Echternacht, A.C., Eds.; John Wiley & Sons: New York, NY, USA, 1993; pp. 305–337. [Google Scholar]
- Patton, J.L.; da Silva, M.N.F. Rivers, refuges and ridges. The geography of speciation of Amazonian mammalsl. In Endless Forms. Species and Speciation; Howard, D.J., Berloucher, S.H., Eds.; Oxford University Press: New York, NY, USA, 1998; pp. 202–213. [Google Scholar]
- Vermeij, G.J. Survival during biotic crises: The properties and evolutionary significance of refuges. In Dynamics of Extinction; Elliot, D.K., Ed.; John Wiley & Sons: New York, NY, USA, 1986; pp. 231–246. [Google Scholar]
- Drummond, A.J.; Ho, S.Y.W.; Phillips, M.J.; Rambaut, A. Relaxed phylogenetics and dating with confidence. PLoS Biol. 2006, 4, e88. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Maekawa, K.; Nalepa, C.A. Biogeography and Phylogeny of Wood-feeding Cockroaches in the Genus Cryptocercus. Insects 2011, 2, 354-368. https://doi.org/10.3390/insects2030354
Maekawa K, Nalepa CA. Biogeography and Phylogeny of Wood-feeding Cockroaches in the Genus Cryptocercus. Insects. 2011; 2(3):354-368. https://doi.org/10.3390/insects2030354
Chicago/Turabian StyleMaekawa, Kiyoto, and Christine A. Nalepa. 2011. "Biogeography and Phylogeny of Wood-feeding Cockroaches in the Genus Cryptocercus" Insects 2, no. 3: 354-368. https://doi.org/10.3390/insects2030354
APA StyleMaekawa, K., & Nalepa, C. A. (2011). Biogeography and Phylogeny of Wood-feeding Cockroaches in the Genus Cryptocercus. Insects, 2(3), 354-368. https://doi.org/10.3390/insects2030354




