Impacts of Non-Lethal High-Temperature Stress on the Development and Reproductive Organs of Bradysia odoriphaga
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bradysia odoriphaga Rearing and Maintenance
2.2. Impacts of High-Temperature Stress on Bradysia odoriphaga Pupae
2.2.1. High-Temperature Impacts Growth and Development of Bradysia odoriphaga Pupae
2.2.2. Impacts of High-Temperature Stress on Egg Laying and Egg Hatching in Bradysia odoriphaga Pupae
2.2.3. Impacts of High-Temperature Stress on Unmated Adult Reproductive Organs Size in Bradysia odoriphaga Pupae
2.3. Impacts of High-Temperature Stress on the Morphology and Structure of Testis and Ovaries in Unmated Bradysia odoriphaga Adults
2.4. Data Analysis
3. Results
3.1. Impacts of High-Temperature Stress on Bradysia odoriphaga Pupae
3.1.1. Impacts of High-Temperature Stress on Bradysia odoriphaga Pupae Emergence Rate
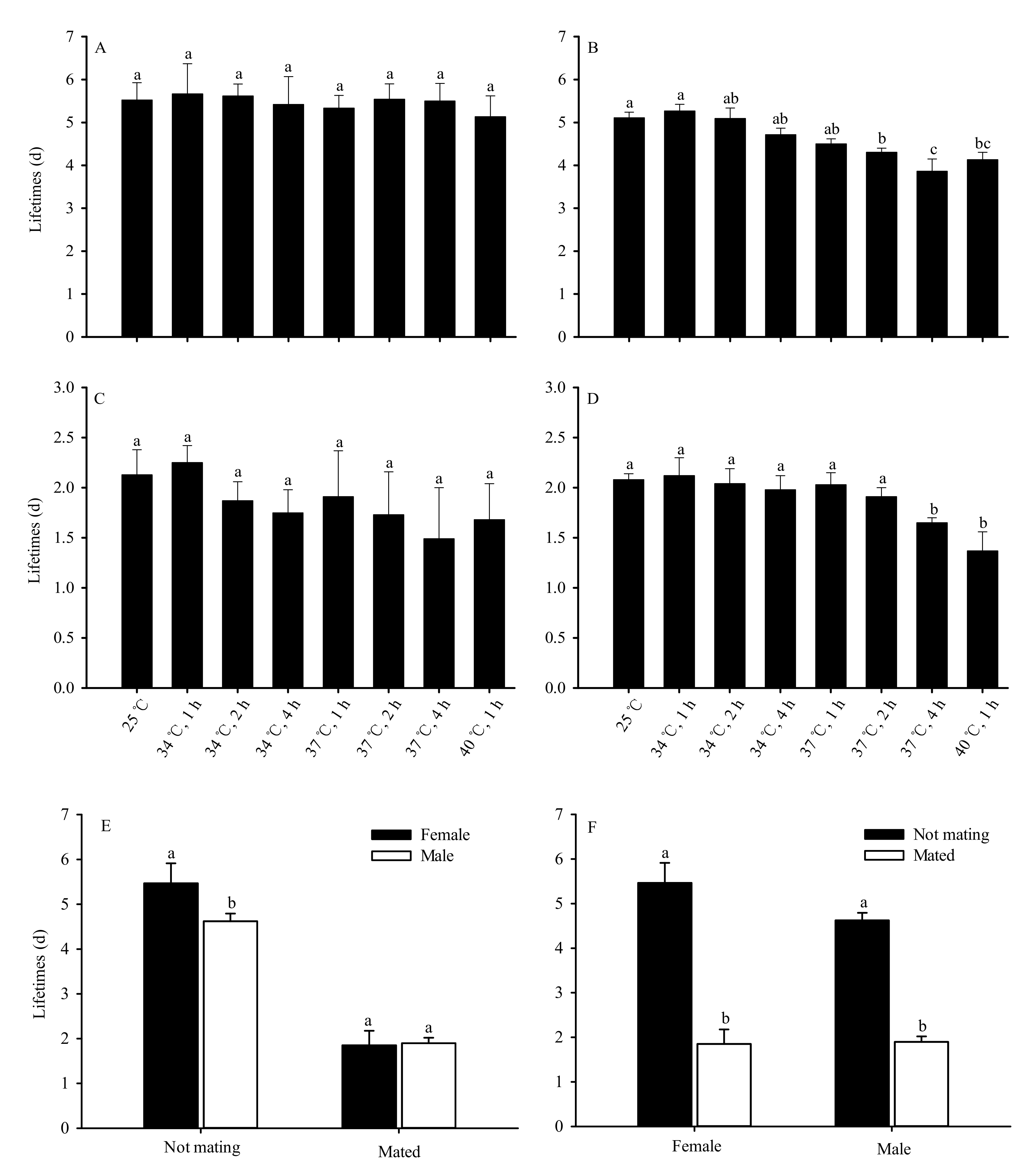
3.1.2. Impacts of High-Temperature Stress on Adults Longevity in Bradysia odoriphaga Pupae
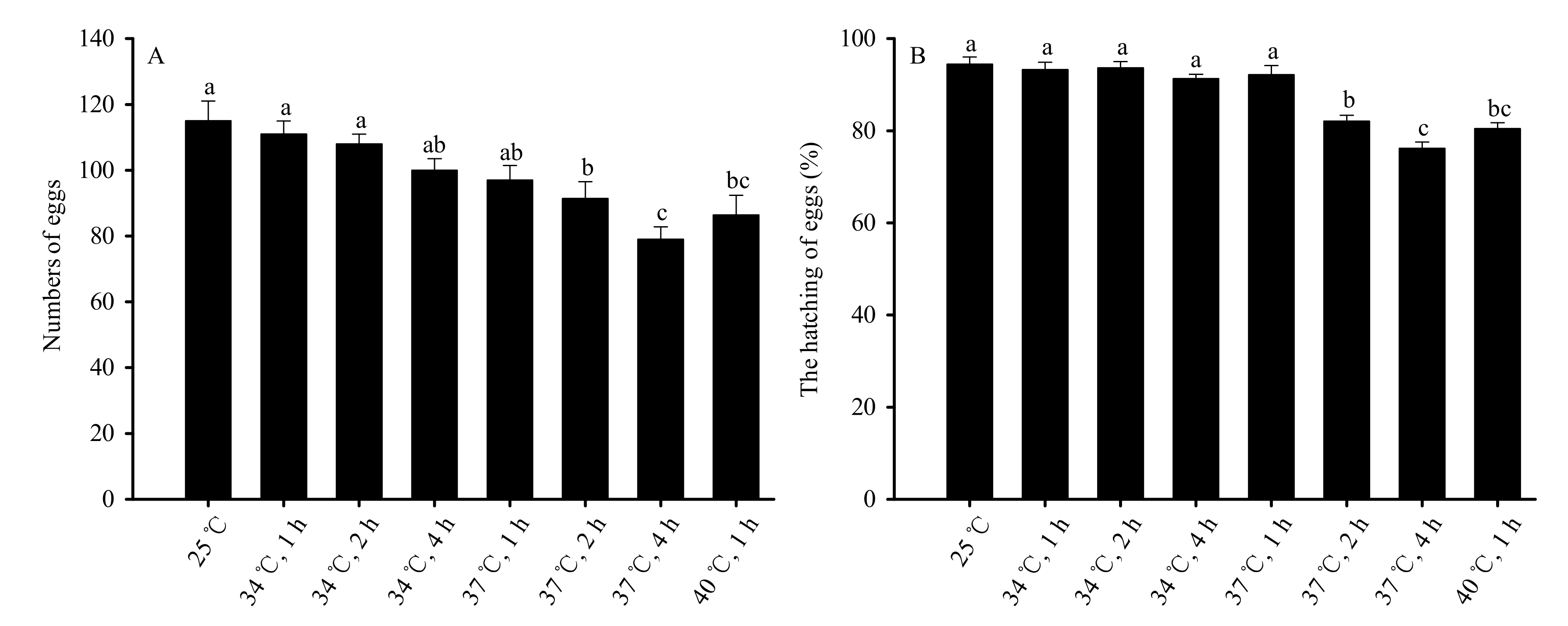
3.1.3. Impacts of High-Temperature Stress on Reproductive Capacity and Egg Hatchability in Bradysia odoriphaga
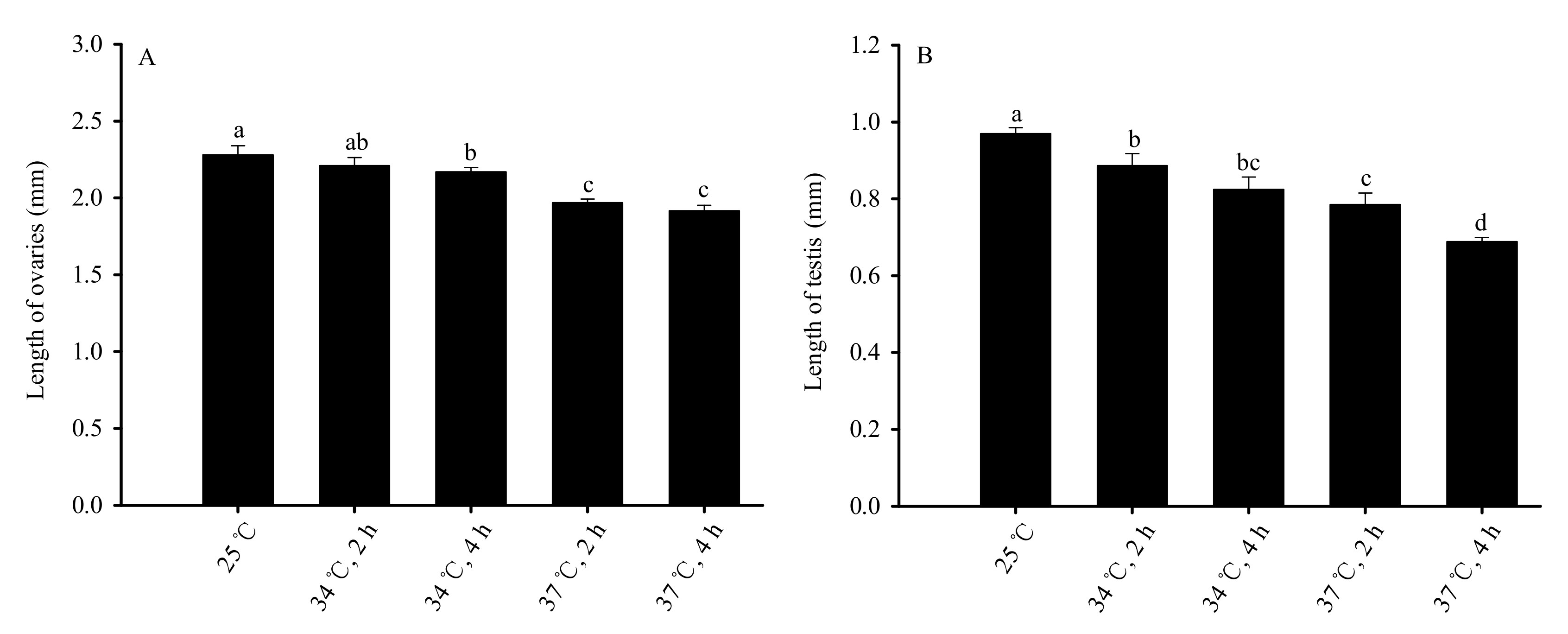
3.1.4. Impacts of High-Temperature Stress on the Reproductive Organs of Unmated Adults in Bradysia odoriphaga Pupae
3.2. Impacts of High-Temperature Stress of Bradysia odoriphaga Unmated Adults on the Size of Reproductive Organs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, J.K.; Zhang, X.M. Notes on the fragrant onion gnats with descriptions of two new species of Bradysia (Diptera: Sciaridae). Acta Agric. Univ. Pekin. 1985, 11, 153–156. [Google Scholar]
- Yang, Y.T.; Li, W.X.; Xie, W.; Wu, Q.J.; Xu, B.Y.; Wang, S.L.; Zhang, Y.J. Development of Bradysia odoriphaga (Diptera: Sciaridae) as affected by humidity: An age-stage, two-sex, life-table study. Appl. Entomol. Zool. 2015, 50, 3–10. [Google Scholar] [CrossRef]
- Tao, Y.L.; Guo, Y.N.; Wang, J.; Li, L.L.; Yu, Y.; Chu, D. Detection and identification of Wolbachia in Bradysia odoriphaga (Diptera: Sciaridae) populations from Shangdong Province, China. Acta Entomol. Sin. 2015, 58, 454–459. [Google Scholar]
- Shan, T.S.; Chen, C.Y.; Ding, Q.; Chen, X.W.; Zhang, H.H.; Chen, A.Q.; Shi, X.Y.; Gao, X.W. Molecular characterization and expression profiles of nicotinic acetylcholine receptors in Bradysia odoriphaga. Pestic. Biochem. Physiol. 2020, 165, 104563. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.H.; Li, A.H.; Han, R.C.; Cao, L.; Liu, X.L. Factors affecting the control of Bradysia odoriphaga with entomopathogenic nematode Heterorhabditis indica LN2. Nat. Enemies Insects 2004, 26, 150–155. [Google Scholar]
- Ma, J.; Chen, S.L.; Moens, M.; Han, R.C.; Clercq, P.D. Efficacy of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) against the chive gnat, Bradysia odoriphaga. J. Pest Sci. 2013, 86, 551–561. [Google Scholar] [CrossRef]
- Wang, H.T.; Song, C.F.; Wang, Y.Z. The tendency for Bradysia odoriphaga among different color and the luring effect on yellow plate. J. Agr. Sci. Jiangsu 2015, 43, 133–134. [Google Scholar]
- Dang, Z.H.; Dong, J.H.; Gao, Z.L. Biology and injury of Bradysia odoriphaga on leek in different types of cultivation. J. Agric. Univ. Hebei 2002, 24, 65–68. [Google Scholar]
- Ullah, F.; Gul, H.; Desneux, N.; Sai, F.; Gao, X.W.; Song, D.L. Fitness costs in chlorfenapyr-resistant populations of the chive maggot, Bradysia odoriphage. Ecotoxicology 2020, 29, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, F.; Mu, W.; Li, Q.H.; Wang, H.; Chen, C.Y. Life table study of the effects of sublethal concentrations of thiamethoxam on Bradysia odoriphage Yang and Zhang. Pestic. Biochem Phys. 2014, 111, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Boggs, C.L. The fingerprints of global climate change on insect populations. Curr. Opin. Insect Sci. 2016, 17, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Hamby, K.A.; Bellamy, D.E.; Chiu, J.C.; Lee, J.C.; Walton, V.M.; Wiman, N.G.; Biondi, A. Biotic and abiotic factors impacting development, behavior, phenology, and reproductive biology of Drosophila suzukii. J. Pest Sci. 2016, 89, 605–619. [Google Scholar] [CrossRef]
- Cui, X.H.; Wan, F.H.; Xie, M.; Liu, T.X. Effects of heat shock on survival and reproduction of two whitefly species, Trialeurodes vaporariorum and Bemisia tabaci biotype B. J. Insect Sci. 2018, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kobori, Y.; Hanboonsong, Y. Effect of temperature on the development and reproduction of the sugarcane white leaf insect vector, Matsumuratettix hiroglyphicus (Matsumura) (Hemiptera: Cicadellidae). J. Asia-Pac. Entomol. 2017, 20, 281–284. [Google Scholar] [CrossRef]
- Lomeli-Flores, J.R.; Barrera, J.F.; Bernal, J.S. Impacts of weather, shade cover and elevation on coffee leafminer Leucoptera coffeella (Lepidoptera: Lyonetiidae) population dynamics and natural enemies. Crop Prot. 2010, 29, 1039–1048. [Google Scholar] [CrossRef]
- Wang, H.S.; Xu, H.F.; Cui, F. Effect of high temperature on fecundity and ovary development of beet armyworm Spodoptera exigua (Hǜbner). Southwest China J. Agr. Sci. 2006, 19, 916–919. [Google Scholar]
- Zeng, B.; Zhu, W.J.; Fu, Y.G.; Zhou, S.H. Influence of high-temperature exposure on the mating, oviposition and thermotaxis of Bactrocera cucurbitae (Coquillet) (Diptera: Tephritidae). PLoS ONE 2018, 13, e0204065. [Google Scholar] [CrossRef] [PubMed]
- Fields, P.G. The control of stored-product insects and mites with extreme temperatures. J. Stored Prod. Res. 1992, 28, 89–118. [Google Scholar] [CrossRef]
- Shi, C.H.; Hu, J.R.; Zhang, Y.J. The effects of temperature and humidity on a field population of Bradysia odoriphaga (Diptera: Sciaridae). J. Econ. Entomol. 2020, 113, 1927–1932. [Google Scholar] [CrossRef]
- Shi, C.H.; Zhang, S.; Hu, J.R.; Zhang, Y.J. Effects of non-lethal high-temperature stress on Bradysia odoriphaga (Diptera: Sciaridae) larval development and offspring. Insects 2020, 11, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, G.D.; Xue, M.; Luo, Y.; Ji, G.X.; Liu, F.; Zhao, H.; Sun, X. Effect of short-term heat shock and physiological responses to heat stress in two Bradysia adults, Bradysia odoriphaga and Bradysia difformis. Sci. Rep. 2017, 7, 13381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, G.D.; Wilson, L.T.; Henneberry, T.J. Heliothis virescens (Lepidoptera: Noctuidae): Initiation of Summer diapause. J. Econ. Entomol. 1985, 78, 320–324. [Google Scholar] [CrossRef] [Green Version]
- Nibouche, S. High temperature induced diapause in the cotton bollworm shape Helicoverpa armigera. Entomol. Exp. Appl. 1998, 87, 271–274. [Google Scholar] [CrossRef]
- Ma, C.S.; Hau, B.; Poehling, H.M. The effect of heat stress on the survival of the rose grain aphid, Metopolophium dirhodum (Hemiptera: Aphididae). Eur. J. Entomol. 2004, 101, 327–331. [Google Scholar] [CrossRef]
- Mahroof, R.; Subramanyam, B.; Flinn, P. Reproductive performance of Tribolium castaneum (Coleoptera: Tenebrionidae) exposed to the minimum heat treatment temperature as pupae and adults. J. Econ. Entomol. 2005, 98, 626–633. [Google Scholar] [CrossRef]
- Shi, C.H.; Hu, J.R.; Wei, Q.W.; Yang, Y.T.; Cheng, J.X.; Han, H.L.; Wu, Q.J.; Wang, S.L.; Xu, B.Y.; Su, Q.; et al. Control of Bradysia odoriphaga (Diptera: Sciaridae) by soil solarization. Crop Prot. 2018, 114, 76–82. [Google Scholar] [CrossRef]
- Zhang, A.M.; Liu, X.D.; Zhai, B.P.; Gu, X.Y. Influences of temperature on biological characteristics of the small brown planthopper, Laodelphax striatellus (Fallén) (Hemiptera: Delphacidae). Acta Entomol. Sin. 2008, 51, 640–645. [Google Scholar]
- Chiu, M.C.; Kuo, J.J.; Kuo, M.H. Life stage-dependent effects of experimental heat waves on an insect herbivore. Ecol. Entomol. 2015, 40, 175–181. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, Z.; Wang, Q.L.; Zhang, F.; Liu, T.X. Exposing eggs to high temperatures affects the development, survival and reproduction of Harmonia axyridis. J. Therm. Biol. 2014, 39, 40–44. [Google Scholar] [CrossRef]
- Liang, L.; Zhang, S.; Han, H.L.; Xu, B.Y.; Zhang, Y.J. Effects of male age on its mating ability and female reproduction in Bradysia odoriphaga. J. Environ. Entomol. 2019, 41, 657–663. [Google Scholar]
- Li, W.X.; Yang, Y.T.; Xie, W.; Wu, Q.J.; Xu, B.Y.; Wang, S.L.; Zhang, Y.J. Effects of temperature on the age-stage, two-sex life table of Bradysia odoriphaga (Diptera: Sciaridae). J. Econ. Entomol. 2015, 108, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.R.; Xie, C.; Shi, C.H.; Wang, S.L.; Wu, Q.J.; Li, C.R.; Zhang, Y.J. Effect of sex and air temperature on the flight capacity of Bradysia odoriphaga (Diptera: Sciaridae). J. Econ. Entomol. 2019, 112, 2161–2166. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.S.; Ma, G.; Pincebourde, S. Survive a warming climate: Insect responses to extreme high temperatures. Annu. Rev. Entomol. 2021, 66, 163–184. [Google Scholar] [CrossRef]
- Durak, R.; Dampc, J.; Dampc, J. Role of temperature on the interaction between Japanese quince Chaenomeles japonica and herbivorous insect Aphis pomi (Hemiptera: Aphidoidea). Environ. Exp. Bot. 2020, 176, 104100. [Google Scholar] [CrossRef]
- Pelletier, Y. Determination of the lethal high temperature for the Colorado potato beetle (Coleoptera: Chrysomelidae). Can. Agric. Eng. 1998, 40, 185–189. [Google Scholar]
- Uddin, M.D.K.; Yin, X.W.; Zhang, L. Courtship and mating behavior of the Chinese Chive fly, Bradysia odoriphaga (Diptera: Sciaridae) and evidence of female sex pheromone. Pak. J. Zool. 2016, 48, 1543–1548. [Google Scholar]
- Liang, L.N.; Zhang, W.; Ma, G.; Hoffmann, A.A.; Ma, C.S. A single hot event stimulates adult performance but reduces in the oriental fruit moth, Grapholitha molesta. PLoS ONE 2014, 9, e116339. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Medison, R.G.; Zhang, S.; Ma, P.; Shi, C. Impacts of Non-Lethal High-Temperature Stress on the Development and Reproductive Organs of Bradysia odoriphaga. Insects 2022, 13, 74. https://doi.org/10.3390/insects13010074
Hu J, Medison RG, Zhang S, Ma P, Shi C. Impacts of Non-Lethal High-Temperature Stress on the Development and Reproductive Organs of Bradysia odoriphaga. Insects. 2022; 13(1):74. https://doi.org/10.3390/insects13010074
Chicago/Turabian StyleHu, Jingrong, Rudoviko Galileya Medison, Seng Zhang, Peifang Ma, and Caihua Shi. 2022. "Impacts of Non-Lethal High-Temperature Stress on the Development and Reproductive Organs of Bradysia odoriphaga" Insects 13, no. 1: 74. https://doi.org/10.3390/insects13010074




